Abstract
Tumor formation involves epigenetic modifications and microenvironmental changes as well as cumulative genetic alterations encompassing somatic mutations, loss of heterozygosity, and aneuploidy. Here, we show that conditional targeting of p120 catenin in mice leads to progressive development of skin neoplasias associated with intrinsic NF-κB activation. We find that, similarly, squamous cell carcinomas in humans display altered p120 and activated NF-κB. We show that epidermal hyperproliferation arising from p120 loss can be abrogated by IκB kinase 2 inhibitors. Although this underscores the importance of this pathway, the role of NF-κB in hyperproliferation appears rooted in its impact on epidermal microenvironment because as p120-null keratinocytes display a growth-arrested phenotype in culture. We trace this to a mitotic defect, resulting in unstable, binucleated cells in vitro and in vivo. We show that the abnormal mitoses can be ameliorated by inhibiting RhoA, the activity of which is abnormally high. Conversely, we can elicit such mitotic defects in control keratinocytes by elevating RhoA activity. The ability of p120 deficiency to elicit mitotic alterations and chronic inflammatory responses, that together may facilitate the development of genetic instability in vivo, provides insights into why it figures so prominently in skin cancer progression.
Keywords: adherens junctions, NF-κB, squamous cell carcinoma, RhoA, cadherin
p120 catenin (p120) is a component of adherens junctions (AJs) and binds directly to the cytoplasmic domain of cadherins (1). This interaction is required for retaining and stabilizing AJs at the plasma membrane (2, 3). Because reductions in expression and/or functional activity of AJs correlate with acquisition of malignancy, it has been postulated that loss of p120 might have a causal role during this process (4, 5). Indeed, p120 expression is frequently diminished in human cancers of diverse etiologies, and a correlation has been noted between p120 down-regulation and poor patient prognosis (6).
Various models have been proposed for how alterations in p120 might contribute to malignancy (7). The most widely held view is that p120 mutations destabilize AJs and disrupt intercellular adhesion, which in turn enhances migratory and invasive features of cancer-poised cells (5, 7, 8). This notion is consistent with some human cancers in which loss-of-function mutations occur in other AJ components, e.g., epithelial cadherin (E-cadherin) and α-catenin, and with the more broadly observed reductions in membrane immunolocalization of AJ components (9, 10). That said, it has also been posited that, when E-cadherin is lost, p120 may redistribute and interact with and activate its nuclear transcription cofactor Kaiso or its functional binding partner RhoA GTPase, thereby triggering downstream cellular responses (11, 12). Adding to the complexity involved is the existence of different p120 isoforms, some of which are associated with acquisition of migratory and invasive capabilities (13).
Conditional ablation of p120 in salivary glands has recently uncovered a causal role of p120 loss in promoting tumor formation in mice (14). p120 ablation in submandibular glands induced rapid dysplasia, cell shedding, and epithelial masses that proliferated and displayed reduced apoptosis. In combination with defects in epithelial morphology and adhesion, these manifestations were indistinguishable from high-grade intraepithelial neoplasia. In contrast, conditional ablation of p120 in skin epidermis elicited a lethal hyperproliferative disorder associated with chronic inflammation and hyperactive NF-κB signaling (15). In the present study, we engrafted skins of p120 conditional KO (cKO) and WT mice, enabling us to further dissect underlying relations among loss of p120, hyperactivation of NF-κB, inflammation, and tumorigenesis. Our findings unveil an unappreciated role for p120 loss in skin cancer. We also demonstrate an inflammation-independent role for p120 deficiency in mitosis and show that it is linked to an aberrant superactivation in RhoA GTPase.
Results
Loss of p120 Promotes Tumor Formation.
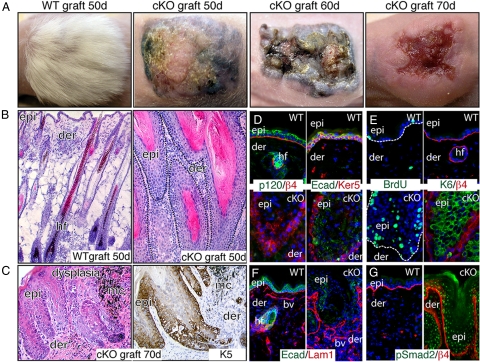
We discovered that mice conditionally targeted for p120 ablation in skin and oral epithelia suffer from a lethal chronic inflammatory disease as a result of markedly increased NF-κB signaling (15). To explore the possible role of p120 deficiency and cancer, we performed skin engraftments onto nude mice (Fig. 1). Within 15 to 50 d after engraftment, 100% of cKO grafts displayed signs of epidermal hyperkeratosis compared with their WT counterparts. By 50 d, cKO grafts developed raised nodules that soon began to ulcerate and adopt an erythematous crateriform appearance (Fig. 1A). At the histological level, epithelial undulations with eosinophilic keratinized debris accompanied the papilloma-like protuberances (Fig. 1B), and by 70 d, dysplastic keratinocytes had populated the invaginations (Fig. 1C). Aberrant clusters of pigment-filled melanocytes, typically confined to skin epithelium, were frequent in the dermis surrounding p120-null epidermal invaginations (Fig. 1C).
Fig. 1.
Absence of p120 in skin promotes tumor formation. WT and p120 cKO newborn back skins were engrafted onto nude mice. (A) Phenotypic appearance of skin grafts. Note that 50-d cKO grafts display hyperkeratosis and a paucity of hair. At later stages, grafts appear as a group of papules or nodules with various degrees of hyperkeratosis and ulceration. (B and C) Histological analyses show that 50-d cKO grafts exhibit well circumscribed, keratin-filled, epidermal invaginations that extend into the dermis. Signs of hyperkeratosis and acanthosis with atypical cells are evident. By 70 d, cKO grafts display some signs of dysplasia and early neoplasia. Immunohistochemistry reveals expansion of K5 expression. (D–G) Immunofluorescence was conducted on frozen sections of 50-d (D and E) and 70-d (F and G) grafts with the indicated Abs. Notes on cKO grafts: AJ proteins no longer localize to cell borders, cKO epidermis is hyperproliferative, the basement membrane is largely intact, and the TGFβ effector nuclear pSmad2 is prominent. Abbreviations: Epi, epidermis; der, dermis; hf, hair follicles; E-cad, E-cadherin; β4, β4 integrin; Ker, keratin; Lam, Laminin; bv, blood vessel.
Immunohistological analyses confirming the absence of p120 showed the expected reductions in all AJ components, including E-cadherin, α-catenin, and β-catenin [Fig. 1D and supporting information (SI) Fig. S1] (15). However, despite these data and the well documented role of p120 in stabilizing AJs, no indications of perturbed intercellular junctions were observed ultrastructurally (Fig. S1B). These findings agreed with our earlier assays on neonatal skin that suggested that the Armadillo Repeat gene deleted in Velo-Cardio-Facial syndrome may partially compensate for loss of p120 (15).
In contrast, signs of epidermal hyperproliferation were prevalent histologically and biochemically. BrdU labeling revealed enhancement of actively cycling cells, both basally and suprabasally (Fig. 1E). This was further reflected by suprabasal expression of keratin 6, which is typically expressed anomalously in hyperproliferative states (Fig. 1E), and suprabasal expansion of basal keratin 5 (K5) (Fig. 1C). Additionally, rather than the standard confinement of transmembrane β4 integrin to hemidesmosomes at the basement membrane–epidermal border, β4 was more uniformly distributed and often suprabasal (Fig. 1D). The basal lamina appeared to be largely intact, albeit aberrant (Figs. 1F and S1C).
These collective changes are associated with neoplasia and progression to malignancy. That said, p120-deficient grafts lacked certain key features of invasive squamous cell carcinoma (SCC). In this regard, most skin keratinocytes were epithelioid-shaped, K5/K14-expressing, and adherent, and many of their nuclei displayed phosphorylated (i.e., TGFβ-active) Smad2 (Fig. 1G). In contrast, invasive SCCs are typified by widespread discontinuities in the basement membrane, a reduction/absence of nuclear phospho-Smad2, and an epithelial–mesenchymal transition marked by loosely adherent, spindle-shaped cells that coexpress vimentin and simple epithelial keratins (i.e., K8/K18) rather than K5/K14 (16).
Loss of p120 and NF-κB Activation Are Common Features of Human SCCs.
Regional loss or down-regulation of p120 gene expression and/or alterations in its cellular distribution occur frequently in a human cancers, including SCCs (6, 7). Recently, we showed that NF-κB activation is common in human skin SCCs under conditions in which α-catenin is absent (17), and that conditional ablation of either α-catenin or p120 in mice is associated with NF-κB activation (15, 17).
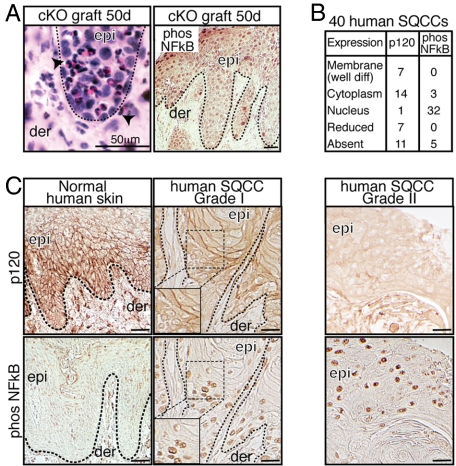
To further explore the possible link between loss of p120 and NF-κB signaling pathways as conjunctive factors of skin malignancies, we first corroborated the presence of dense inflammatory infiltrates in p120 cKO skin grafts (Fig. 2A, arrowheads) and the presence of nuclear phospho-(active) NF-κB (phospho-NF-κB) (Fig. 2A) (15). Analyses of the presence and distribution of p120 and phospho-NF-κB in serial human SCC sections from 40 patients revealed a similar correlation (Fig. 2 B and C). In normal human skin, p120 localized to intercellular boundaries, and nuclear phospho-NF-κB was below detection limits. In contrast, most of the tumors showed nuclear phospho-NF-κB and perturbations in p120 expression and/or localization. Typically, poorly differentiated tumors (i.e., grade II) stained for nuclear phospho-NF-κB but not p120 (Fig. 2C). This inverse correlation was also found in poorly differentiated areas (Fig. 2C Insets) of well differentiated tumors (grade I). Reductions of other AJ constituents accompanied p120 loss (data not shown), consistent with the findings in ref. 17 and with the known role of p120 catenin in AJ stabilization (3). That said, these correlations were not perfect, and NF-κB is known to be activated by means other than down-regulation of AJ proteins.
Fig. 2.
p120 loss, NF-κB activation, and relationship with human skin cancer. (A) Histologic and immunohistochemistry analyses of skin grafts reveal an association of p120 loss with chronic inflammatory infiltrates (arrowheads) and NF-κB phosphorylation/activation. (B and C) Immunohistochemical analyses of p120 and NF-κB in human SCCs of different grades. Table summarizes expression and distribution for 40 human SCCs (the two analyses are presented as independent datasets). Shown are representative examples of the data. Insets denote magnified areas showing well and poorly differentiated regions of a grade I tumor. Note that, in areas where p120 is not detected, NF-κB is nuclear and activated. Abbreviations: Phos, phosphorylated; SQCC, squamous cell carcinoma.
Proliferation Defects in the Absence of p120.
In recent years, connections between inflammation and tumor progression have become increasingly apparent (18). To address the extent to which abnormalities in p120-deficient skin arise from associated NF-κB activity, we blocked NF-κB activation specifically with TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophene carboxamide) (19), a small-molecule inhibitor of IκB kinase 2 (IKK2) kinase (20). We first tested the efficacy of TPCA-1 in primary p120fl/fl keratinocytes (mKers) after Cre-mediated targeting in vitro (Fig. S2A). The inhibitor was effective at blocking NF-κB phosphorylation not only in p120-null mKers but also in mKers potently stimulated with TNFα (Fig. S2B). Additionally, in luciferase reporter assays, NF-κB activity was significantly diminished by the IKK2 inhibitor (Fig. S2C).
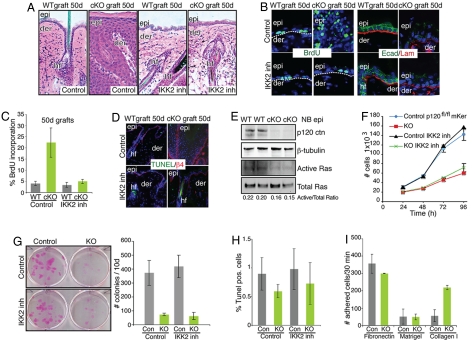
In vivo, IKK2 inhibition corrected many of the morphological anomalies of p120 cKO skin grafts (Figs. 3A and S2D). Hair growth was significantly improved (Fig. S2D), and morphological and biochemical signs of epidermal hyperplasia waned (Fig. 3 A–C). However, IKK2 inhibitors failed to rescue cadherin distribution at intercellular contacts (Fig. 3B). These findings were consistent with and extended our old observations with the nonspecific, anti-inflammatory agent dexamethasone (15). Our current studies pointed specifically to IKK2 and its downstream target NF-κB as the underlying cause of the chronic inflammatory response in p120 cKO skin.
Fig. 3.
Inflammatory responses are causative of hyperproliferation features in vivo, whereas in vitro loss of p120 results in hypoproliferation. (A) Histological analyses of skin grafts with and without treatment with an IKK2 inhibitor that specifically prevents NF-κB phosphorylation and activation (n = 5). (B) Blocking NF-κB activation and inflammation rescues hyperproliferation but not cadherin distribution at cell–cell boundaries. (C) Quantification of BrdU incorporation. Error bars indicate SD. (D) TUNEL assay. Note that IKK2 inhibition shows no increase in apoptosis. (E) Ras pull-down assays. Note the small decrease on the levels of active Ras in p120 cKO neonatal epidermis. (F–I). In vitro studies. (F) Proliferation curves of p120-null mKer with and without IKK2 inhibitor treatment. Note that p120 loss, but not IKK2 inhibition, affects mKer proliferation in vitro. (G) Colony formation efficiency after 10 d of plating. (H) TUNEL assay with and without IKK2 inhibitor. (I) Adhesion assays on different extracellular matrices.
To probe more deeply into the hyperproliferative defects in our cKO skin grafts, we tested for signs of altered apoptosis or activation of Ras-MAPK. As judged by TUNEL analyses, neither loss of p120 nor IKK2 inhibition affected apoptosis in skin (Fig. 3D). Moreover, unexpectedly, we detected a small decrease, rather than an increase, in Ras activity levels in newborn cKO versus control epidermis (Fig. 3E). At this stage in development, phenotypic signs of inflammation and hyperproliferation were absent in the skin (15).
To dissect the cell-autonomous effects of p120 loss on epidermal proliferation, we turned to in vitro studies. Interestingly, p120-null mKers cultures grew significantly more slowly and formed fewer colonies than their WT counterparts (Fig. 3 F and G). Moreover, IKK2 inhibition had no affect on the growth-related defects observed in colony initiation. As judged by TUNEL and adhesion assays, KO mKers also showed no increase in apoptosis and were not significantly less adherent to different substrates (Fig. 3 H and I). Thus, although loss of p120 in vivo led to NF-κB activation, immune infiltration, and proinflammatory-induced hyperproliferation in vitro, it led to impaired colony growth that appeared to be at least partially independent of NF-κB, apoptosis, and cell adhesion.
Loss of p120 Promotes Generation of Binucleate Cells.
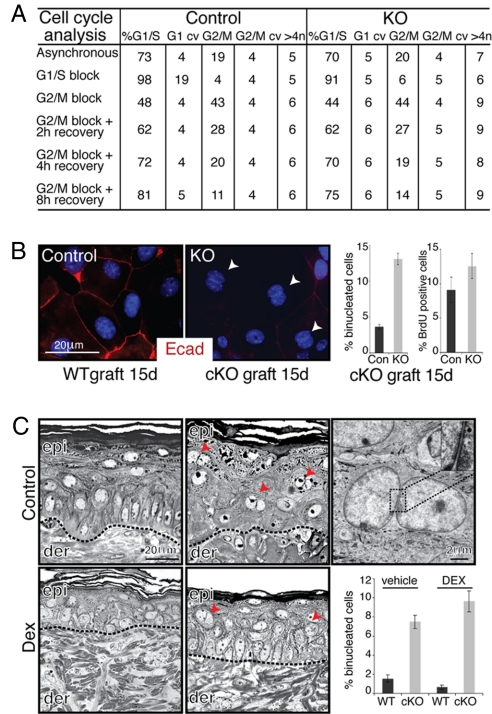
To better understand the impairment in p120-null mKer colony formation and/or growth, we first performed cell cycle analysis in asynchronized and synchronized growing cultures. Most notably, the number of cells with at least 4N DNA content was consistently higher in p120-null cultures recovering after a G2/M block with nocodazole (Fig. 4A). Analogous results were obtained by karyotyping analysis in which p120-null cells displayed at least 4N DNA content (57.5 ± 7.5%) compared with controls (22.5 ± 7.5%). This included chromosomal breaks, end-to-end fusions, and fragments. These differences were largely masked in asynchronous cultures and in synchronized cultures blocked in G1/S by thymidine. When coupled with the reduced rates of proliferation (Fig. 3F), these results were suggestive of a mitotic cell cycle defect.
Fig. 4.
Loss of p120 leads to the generation of binucleated cells in vivo and in vitro. (A) FACS analysis of DNA content profile of asynchronously growing mKers and of G1/S and G2/M synchronized cultures (see Methods for details). (B Left) Examples of binucleate cells in p120-null mKers (arrowheads; red, E-cad; blue, DAPI). (Right) Quantification of binucleate cells and S-phase (i.e., BrdU-labeled) cells in control and p120-null cultures. (C) Morphological analyses of skin grafts processed for Epon embedding and sectioning. Note that binucleated epidermal cells (arrowheads) appear in p120 cKO skin even when inflammation was blocked with dexamethasone. Inset shows magnified view of boxed area. Abbreviations: cv, coefficient of variation; Dex, dexamethasone.
In characterizing the defect, we discovered an increase in binucleate cells in our p120-null cultures compared with their WT counterparts (Fig. 4B). Closer inspection revealed the presence of binucleate cells in vivo, even in grafted skin samples analyzed shortly after removing the wound bandages (Fig. 4C). This defect was not eliminated by dexamethasone, suggesting that the defects are not caused solely by epidermal alterations resulting secondarily from inflammation (Fig. 4C).
Notably, binucleate cells were often detected in differentiating layers, suggesting that basal cells acquiring this defect terminally differentiated and were shed from the skin or died and became engulfed by other cells within the tissue. We posit that the later appearance of dysplastic lesions may arise from a combination of the hyperproliferative response induced by subsequent chronic inflammation and a small number of cells that overcome the mitotic cell cycle arrest checkpoint and survive. If so, the genetic instability resulting from this defect might render cells prone to accumulating further alterations, possibly explaining the progressive alterations in p120 cKO skin morphology with age.
Extended M-Phase and Mitotic Defects in p120-null Keratinocytes.
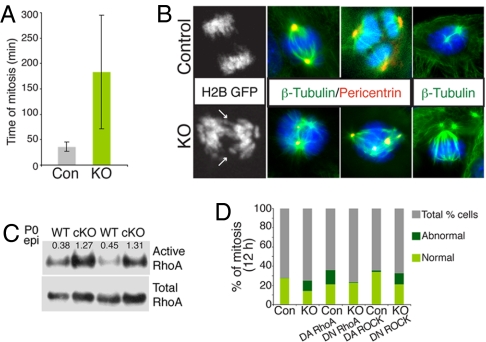
To pinpoint the mitotic defects in p120-null mKers, cells were transfected with histone 2B–GFP and imaged by videomicroscopy. Remarkably, whereas normal control mKers completed mitosis in ≈30 min, some p120-null cells took an unusually long time to finish mitosis (≈4 h) and displayed marked cytokinesis defects (Fig. 5A, and Movies S1 and S3). These defects resulted in furrow regression and generation of binucleated cells, confirming our findings of tetraploid cells (Movies S2 and S4). We also observed cells with lagging chromosomes or chromosome bridges (Fig. 5B, arrows). This appeared to result from alterations in spindle organization and/or lack of microtubule (MT) tension because p120-null cells presented a diverse array of abnormal spindle phenotypes and often, an increased number of centrosomes and altered MT dynamics (Fig. 5B and data not shown).
Fig. 5.
p120-null cells exhibit abnormal mitosis in a RhoA GTPase-dependent fashion. (A) Quantification of the extended length of M-phase in KO mKers. Mitoses were monitored by bright-field videoimaging. Shown are control (n = 60) and p120-null mKers (n = 58). Mean times of total mitoses are shown. (B) Immunofluorescence microscopy of mKers labeled with Abs is indicated (color coding according to the secondary). Note the presence of abnormal spindles and centrosomes in KO cells. Black-and-white images depict a representative example of lagging chromosomes and chromosomal bridges in p120-null cells (arrows). (C) RhoA activity is increased in newborn p120 cKO epidermis. (D) Percentage of cells with normal and abnormal mitosis (i.e., binucleated cells, furrow regression, and mitotic catastrophe) and dependency on RhoA activity. Abbreviations: H2B, histone 2B; DA, dominant active; DN, dominant negative.
Cytokinesis depends on RhoA signaling whereas cell rounding and cleavage furrow ingression are reliant on active RhoA, and abscission requires RhoA deactivation (21). p120 is an established regulator of RhoA GTPase activity (12, 22), and as confirmed by pull-down assays, RhoA activity in vivo was markedly up-regulated in p120-null epidermal cells from newborn skin (Fig. 5C). RhoA elevation appeared to occur well in advance of signs of inflammation and hyperproliferation (15).
To test whether elevated RhoA activity might be at the root of the observed mitotic abnormalities, we infected our mKer cultures with adenoviruses harboring dominant negative and dominant active RhoA transgenes. Under the conditions used, control cells displayed enhanced aberrations in cytokinesis when RhoA was overactivated, whereas binucleation diminished markedly within p120-null cultures when RhoA activity was reduced (Fig. 5E, and Movies S5 and S6). In contrast, the cytokinesis defects in p120-null cultures were not rectified by inhibiting the RhoA effector ROCK (Fig. 5E), suggesting that a different RhoA effector and/or contributing factors are involved.
Discussion
The tumor suppressive effects of p120 have been surmised to arise primarily through stabilizing E-cadherin and controlling cell adhesion (5, 14). Because reduced cadherins are commonly associated with epithelial-to-mesenchymal transitions (23), this would place the role of p120 deficiency relatively late in malignant progression. Thus, it was surprising that, in skin, even though loss of p120 resulted in dysplasia, early neoplasia, and reduced E-cadherin at cell–cell borders, intercellular adhesion appeared intact, with no obvious signs of epithelial-to-mesenchymal transitions. We posit that a relative of p120, Armadillo Repeat gene deleted in Velo-Cardio-Facial syndrome, might partially compensate for p120 loss in skin (15) because p120 loss was more severe in the salivary gland, leading to high-grade submandibular gland neoplasia associated with marked defects in adhesion and epithelioid morphology (14).
p120 cKO skin is associated with a robust inflammatory response initiated by increased IKK2 and NF-κB activity within the mutant epidermal cells (15). The impact of NF-κB activity and chronic inflammation in malignancy is well established (18), and by using IKK2 inhibitors, we showed that the epidermal hyperplasia in p120 cKO skin grafts relies on secondary NF-κB–mediated inflammatory responses. Surprisingly, absence of p120 in vitro led to impaired colony growth independently of NF-κB, apoptosis, or cell adhesion. Hence, removal of keratinocytes from their microenvironment may explain a shift from a proliferative to a growth-arrested phenotype. We posit that, in vivo, the epithelial–stromal interactions may prompt genetically unstable p120-null epithelial cells to divide and accumulate genetic alterations.
That said, many notable skin disorders, including eczema and psoriasis, are associated with chronic inflammation and yet do not progress to cancer. Our finding that early mitotic defects occur in p120 cKO skin offers insights into how NF-κB-related changes in hyperproliferation might exacerbate genetic instability and a tumorigenic state when this critical gene is mutated. Our findings support the long-standing view that if a cell containing an aberrant number of centrosomes and/or tetraploidy overcomes a checkpoint control and executes an abnormal mitosis, this may lead to genomic instability and cancer (24–26).
Our findings are intriguing in light of earlier studies that showed that p120 can associate with MTs (27, 28), regulates MT dynamics (28), and participate in the centrosomal checkpoint (29). In Drosophila melanogaster, p120 has been detected at centrosomes (30), whereas in Caenorhabditis elegans, loss of p120 causes germ line cytokinesis defects (31). Although we did not observe localization of endogenous p120 at centrosomes or MT, we did find that p120 overexpression caused its accumulation at these sites (data not shown). Because centrosomal location has also been observed in some cancer cell lines (27–29), it seems likely that the degree to which p120 associates with AJs will impact centrosomal dynamics and mitosis. Whether the defects we observe in MT dynamics and centrosomes arise from specific loss of p120 or from consequential reduction in other cadherin–catenin components in mKers remains unclear. In this regard, it may be relevant that adenomatous polyposis coli is often an associate of AJs because loss of adenomatous polyposis coli function appears to result in genomic instability in colon cancer cells (32, 33).
Irrespective of possible roles of other AJ components, elevated RhoA activity appeared to be critical in causing the mitotic defects in p120-null mKers. Although ROCK inhibition did not ameliorate the cytokinesis defects, the RhoA effector citron kinase has been implicated in completion of cytokinesis (21, 34) and thus requires additional study. Interestingly, multinucleation has also been observed in α-catenin-null mKer cultures (35), but in this case, Rac and not RhoA appeared to be overactive (17). In face of this finding, there may be multiple molecular circuits involved.
Finally, it is worthy of mention that a second relative of p120, p0071, has been correlated with RhoA-mediated cytokinesis defects in some human cancer cell lines (36, 37). In this study, p0071, but not p120, was localized to the cell division furrow, and the shRNA knockdown of p0071 expression appeared to reduce rather than increase RhoA activity. Based on these collective studies, it is tempting to speculate that, through orchestrating localized activities of Rho GTPases, catenins function in coordinating the progression through mitosis.
In closing, our studies point to key roles for p120 catenin in regulating mitosis and IKK2-dependent NF-κB activity in skin keratinocytes. Along with the well established role of p120 in stabilizing AJ components, these facets provide important insights into how this protein may function in regulating tissue homeostasis and how the process goes awry when the gene is defective.
Materials and Methods
Animal Husbandry, Genotyping, and Skin Grafts.
Generation of K14-Cre/p120fl/fl mice was described in ref. 15. For genotyping, the following primers were used: CreFW 5′ TGCTGTTTCACTGGTTATGCGG 3′, CreRW 5′ TTGCCCCTGTTTCACTATCCAG 3′, p120FW 5′ TTTTAGAGCCTCCCACATACAAGC 3′, and p120RW 5′ TCAGCACCCACACAAAGGTTG 3′. P0 back skins were dissected from 30 WT and 30 p120 cKO mice and grafted onto recipient nu/nu mice (15). After 5 d, mice were given an i.p. daily injection of 20 mg/kg IKK2 inhibitor IV (Calbiochem). Control mice received carrier (PBS solution/DMSO). After an additional 50 d, mice were killed and engrafted skins were removed and processed.
Supplementary Material
Acknowledgments.
We thank A.B. Reynolds (Vanderbilt University) for the p120fl/fl mice, M. Schober, C. Tinkle, and other members of the Fuchs lab for their helpful suggestions and critical advice, L. Polak and N. Stokes for assistance in the LARC animal facility, Julie White and Margaret Leversha for their assistance with pathology and karyotype analysis, respectively, and Alison North and Shivaprasad Bhuvandendran for their assistance. E.F is an Investigator of the Howard Hughes Medical Institute and supported by National Institutes of Health Grant AR27883. S.E.W. is an American Cancer Society postdoctoral fellow.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 15225.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807301105/DCSupplemental.
References
- 1.Reynolds AB. p120-catenin: Past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ireton RC, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birchmeier W, Behrens J. Cadherin expression in carcinomas: Role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 6.van Hengel J, van Roy F. Diverse functions of p120ctn in tumors. Biochim Biophys Acta. 2007;1773:78–88. doi: 10.1016/j.bbamcr.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: Implications for tumor progression. Differentiation. 2002;70:583–589. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 8.Macpherson IR, et al. p120-catenin is required for the collective invasion of squamous cell carcinoma cells via a phosphorylation-independent mechanism. Oncogene. 2007;26:5214–5228. doi: 10.1038/sj.onc.1210334. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin JM, Nelson WJ. Bench to bedside and back again: Molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin Cancer Biol. 2008;18:53–64. doi: 10.1016/j.semcancer.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 11.van Roy FM, McCrea PD. A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer. 2005;5:956–964. doi: 10.1038/nrc1752. [DOI] [PubMed] [Google Scholar]
- 12.Anastasiadis PZ. p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa M, et al. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion and predicts metastatic disease. J Biol Chem. 2008;283:18344–18354. doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell. 2006;10:21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Moreno M, et al. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guasch G, et al. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–327. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobielak A, Fuchs E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci USA. 2006;103:2322–2327. doi: 10.1073/pnas.0510422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podolin PL, et al. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell proliferation. J Pharmacol Exp Ther. 2005;312:373–381. doi: 10.1124/jpet.104.074484. [DOI] [PubMed] [Google Scholar]
- 20.Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 21.Narumiya S, Yasuda S. Rho GTPases in animal cell mitosis. Curr Opin Cell Biol. 2006;18:199–205. doi: 10.1016/j.ceb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Wildenberg GA, et al. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 23.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 24.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara T, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 26.Nigg EA. Origins and consequences of centrosome aberrations in human cancers. Int J Cancer. 2006;119:2717–2723. doi: 10.1002/ijc.22245. [DOI] [PubMed] [Google Scholar]
- 27.Franz CM, Ridley AJ. p120 catenin associates with microtubules: Inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279:6588–6594. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 28.Ichii T, Takeichi M. p120-catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes Cells. 2007;12:827–839. doi: 10.1111/j.1365-2443.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 29.Chartier NT, et al. Cyclin-dependent kinase 2/cyclin E complex is involved in p120 catenin (p120ctn)-dependent cell growth control: A new role for p120ctn in cancer. Cancer Res. 2007;67:9781–9790. doi: 10.1158/0008-5472.CAN-07-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol. 2003;160:433–449. doi: 10.1083/jcb.200211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol. 2003;162:15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rusan NM, Peifer M. Original CIN: Reviewing roles for APC in chromosome instability. J Cell Biol. 2008;181:719–726. doi: 10.1083/jcb.200802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caldwell CM, Green RA, Kaplan KB. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J Cell Biol. 2007;178:1109–1120. doi: 10.1083/jcb.200703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naim V, Imarisio S, Di Cunto F, Gatti M, Bonaccorsi S. Drosophila citron kinase is required for the final steps of cytokinesis. Mol Biol Cell. 2004;15:5053–5063. doi: 10.1091/mbc.E04-06-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 36.Wolf A, et al. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat Cell Biol. 2006;8:1432–1440. doi: 10.1038/ncb1504. [DOI] [PubMed] [Google Scholar]
- 37.Keil R, Wolf A, Huttelmaier S, Hatzfeld M. Beyond regulation of cell adhesion: Local control of RhoA at the cleavage furrow by the p0071 catenin. Cell Cycle. 2007;6:122–127. doi: 10.4161/cc.6.2.3741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.