Abstract
A growing body of evidence is documenting the multidimensional nature of cancer-related fatigue. Although several multidimensional measures of fatigue have been developed, further validation of these scales is needed. To this end, the current study sought to evaluate the factorial and construct validity of the 30-item Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF). A heterogeneous sample of 304 cancer patients (mean age 55 years) completed the MFSI-SF, along with several other measures of psychosocial functioning including the MOS-SF-36 and Fatigue Symptom Inventory, following the fourth cycle of chemotherapy treatment. The results of a confirmatory factor analysis indicated the 5-factor model provided a good fit to the data as evidenced by commonly used goodness of fit indices (CFI 0.90 and IFI 0.90). Additional evidence for the validity of the MFSI-SF was provided via correlations with other relevant instruments (range −0.21 to 0.82). In sum, the current study provides support for the MFSI-SF as a valuable tool for the multidimensional assessment of cancer-related fatigue.
Keywords: Fatigue, assessment, cancer
Introduction
A growing body of evidence has begun to document the complex nature of cancer-related fatigue.1–3 This research has shown that fatigue may manifest in a wide range of symptom domains, including behavioral, cognitive, somatic, and affective.4–6 Despite the trend toward a multidimensional conceptualization of fatigue, many of the instruments frequently used to assess fatigue in cancer patients provide information only about the severity or intensity of the symptom.7,8 Such unidimensional fatigue scales fail to capture the full spectrum of the fatigue symptom profile and are thus of limited research and clinical utility.
More recently, several researchers have developed multidimensional fatigue instruments that assess a wider range of domains in which fatigue may manifest.9–11 Limitations of currently available multidimensional fatigue scales, however, have also been noted in the literature.12 First, due to the more comprehensive assessment of fatigue that they provide, multidimensional scales are often lengthy in terms of the number of items they include and sometimes have complicated response formats. As a result, they may be difficult for already fatigued cancer patients to complete. Second, psychometric evaluation of multidimensional fatigue scales has been limited. Some studies have not employed factor analytic techniques to evaluate scale dimensionality, and others have provided only perfunctory reliability and validity data. In addition, we could identify only one study13 that has performed structural equation modeling (SEM) or confirmatory factor analysis (CFA) to provide confirmation of the scale’s factorial structure. Finally, multidimensional fatigue scales are often disease-specific (cancer) and assume the presence of fatigue. For example, the FACT-An14 is cancer-specific, and the Piper Fatigue Scale15 assumes the respondent is experiencing some measure of fatigue. As a result, the utility of such scales with patients with chronic illness other than cancer is limited, and screening for the presence of fatigue may be necessary prior to scale administration.
In response to the need for a multidimensional fatigue scale that addressed the above-noted limitations, our research group developed the 30-item Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) (Appendix 1). A previous publication described the initial development, factor analysis, and psychometric evaluation of the scale.11 In this earlier study, a pool of 83 items describing the multidimensional nature of fatigue was generated based upon a review of the literature on cancer-related fatigue, discussions with treatment providers, and a survey of available measures of fatigue. Factor analysis was used to reduce the pool of 83 items to a total of 30 items that produced 5 empirically-derived subscales: general fatigue, physical fatigue, emotional fatigue, mental fatigue, and vigor. Results demonstrated the MFSI-SF to be a reliable and valid scale that was sensitive to differences in fatigue between cancer patients and noncancer controls. Moreover, the MFSI-SF is not disease-specific, nor does it assume the presence of fatigue, thus increasing its clinical utility and research appeal. A simple 5-point Likert-scale response format and relatively short length make the MFSI-SF easy to complete for most cancer patients. Despite these advantages, the multidimensional factorial structure of the scale had not been confirmed in a subsequent study. Furthermore, the original validation sample of the MFSI-SF was limited by the fact that it included only women with and without breast cancer. Thus, the first aim of the current study was to apply confirmatory factor analytic techniques to evaluate the stability of the 5-factor structure of the MFSI-SF in a new sample of cancer patients. The second aim was to provide additional information regarding the reliability and validity of the MFSI-SF. The third aim was to examine the performance of the MFSI-SF with a sample that is more heterogeneous in terms of cancer type and gender than the original validation sample.
Methods
Participants
To be eligible, participants had to 1) be diagnosed with cancer; 2) not have received intravenous chemotherapy previously; 3) be scheduled to receive a minimum of four cycles of intravenous chemotherapy with a minimum of seven days between cycles; 4) not be scheduled to receive radiation therapy before the start of the fourth chemotherapy cycle; 5) be capable of speaking and reading English; and 6) be able to give written informed consent before study entry.
Data used in this report are drawn from a larger study of stress management interventions for chemotherapy patients conducted at the Moffitt Cancer Center.16 The sample for this report consists of 304 participants who completed the MFSI-SF and other quality of life measures following the fourth treatment cycle. Prior research has suggested that cancer-related fatigue may develop as a result of cumulative effects of repeated chemotherapy administrations.17 As we wished to examine the performance of the MFSI-SF in a sample of cancer patients expected to be experiencing fatigue, we chose to administer the MFSI-SF after the fourth cycle of chemotherapy. The demographic characteristics of the study participants are presented in Table 1. The 304 participants ranged in age from 28–88 years (mean = 54.9, SD = 11.46). The majority were women (80%), white (89%), currently married (68%), and college graduates (65%). Median household income was in the US$ 40,000–59,999 range. Performance status, as measured by the Eastern Cooperative Oncology Group (ECOG) Performance Status Rating (PSR) scale, was characterized as fully active (58%), restricted in physically strenuous activity (37%), ambulatory but unable to carry out work activities (4%), or capable of carrying out only limited self-care (1%). Although 61% of the participants underwent chemotherapy for breast cancer, a range of cancer diagnoses was sampled in order to allow for heterogeneity in terms of cancer type. Additional common diagnoses were lung cancer (17%),ovariancancer(7%), coloncancer(4%), lymphoma (3%), prostate cancer (2%), and endometrial cancer (2%). Disease and treatment characteristics of the sample are presented in Table 2.
Table 1.
Sample Characteristics (n = 304)
| Mean | (SD) | Range | |
|---|---|---|---|
| Age | 54.9 | (11.46) | 28–88 |
| Sex | n | % | |
| M | 62 | 20.4 | |
| F | 242 | 79.6 | |
| Ethnicitya | |||
| White | 271 | 89.1 | |
| Non-white | 33 | 10.9 | |
| Marital status | |||
| Marriage/marriage-like | 207 | 68.1 | |
| Not married | 97 | 31.9 | |
| Education | |||
| Less than college graduate | 106 | 35.5 | |
| College graduate or higher | 193 | 64.5 | |
| Missing | 5 | ||
| Household yearly income | |||
| <$40,000 | 100 | 37.6 | |
| $40,000+ | 166 | 62.4 | |
| Missing | 38 | ||
| Employment statusa | |||
| Working | 101 | 33.2 | |
| On leave | 70 | 23.0 | |
| Not e mployed | 138 | 45.4 | |
| Retired | 71 | 23.4 | |
| Disabled | 2 | 0.7 | |
| Homemaker | 20 | 6.6 | |
Total is >100% as participants could indicate membership in more than one employment status.
Table 2.
Disease and Treatment Characteristics (n = 304)
| Mean | (SD) | Range | |
|---|---|---|---|
| Average days in treatment since diagnosis | 91.8 | (256.71) | 1–2730 |
| n | % | ||
| Disease stage at treatment | |||
| Stage I | 36 | 11.8 | |
| Stage II | 133 | 43.8 | |
| Stage III | 66 | 21.7 | |
| Stage IV | 69 | 22.7 | |
| Cancer diagnosis | |||
| Breast | 186 | 61.2 | |
| Lung | 51 | 16.8 | |
| Ovary | 21 | 6.9 | |
| Colon | 11 | 3.6 | |
| Non-Hodgkin’s Lymphoma | 10 | 3.2 | |
| Prostate | 6 | 2.0 | |
| Endometrium | 5 | 1.6 | |
| Unknown Primary | 4 | 1.3 | |
| Hodgkin’s Disease | 2 | 0.7 | |
| Bladder | 2 | 0.7 | |
| Other (Melanoma, cervix, testicle, esophagus, merkle cell, retroperitoneal sarcoma) | 6 | 2.0 | |
Procedures
As described previously, participants were recruited as part of a study of stress management interventions. Following consultation with a medical oncologist at which plans for chemotherapy administration were discussed and agreed upon, informed consent was obtained. Of particular interest here are fatigue and quality of life data obtained after the fourth treatment cycle. Please refer to Jacobsen et al.16 for a 220.4 more detailed description of study procedures.
Measures
A self-report measure was used to obtain information regarding the age, marital status, ethnicity, and education level of the participants. Descriptions of other study measures follow.
The Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF)11 consists of 30 statements designed to assess the multidimensional nature of fatigue. Respondents indicate the extent to which they have experienced each symptom during the preceding one-week period (0 = not at all; 4 = extremely). Ratings are summed to obtain scores for 5 subscales (general fatigue, physical fatigue, emotional fatigue, mental fatigue, and vigor) previously identified using factor analysis.11 In addition, the first four MFSI-SF subscales (general, physical, emotional, and mental fatigue) were summed and the vigor scale was subtracted to create a fatigue total score. Reliabilities for the 5 empirically derived scales in the original validation study were 0.96 for the general scale, 0.85 for the physical scale, 0.93 for the emotional scale, 0.90 for the mental scale, and 0.88 for the vigor scale.
The Medical Outcomes Study 36-Item Short Form18 contains 8 multi-item scales: general health perceptions, physical functioning, role limitations due to physical problems, bodily pain, general mental health, vitality, role limitations due to emotional problems, and social functioning. The SF-36 also yields two summary scores that reflect the two-dimensional factor structure underlying the 8 subscales: a physical component summary score and a mental component score. Alpha coefficients for the SF-36 scales in the present study were 0.84 for the general health perceptions scale, 0.93 for the physical functioning scale, 0.89 for the role limitations due to physical problems scale, 0.92 for the bodily pain scale, 0.85 for the general mental health scale, 0.89 for the vitality scale, 0.92 for the role limitations due to emotional problems scale, and 0.91 for the social functioning scale.
The Fatigue Symptom Inventory (FSI)19 assesses the frequency and severity of fatigue as well as its perceived disruptiveness. Frequency is measured as the number of days in the past week (0–7) respondents felt fatigued as well as the percentage of each day on average they felt fatigued (0 = none, 10 = entire day). Perceived severity is measured on 4 separate 11-point scales (0 = not at all fatigued, 10 = as fatigued as I could be) that assess most, least, and average fatigue during the past week as well as current fatigue. Perceived disruptiveness is measured on 7 separate 11-point scales (0 = no interference, 10 = extreme interference) that assess the degree to which fatigue in the past week was judged to interfere with general level of activity, ability to concentrate, relations with others, enjoyment of life, and mood. Interference ratings are also summed to yield a total disruptiveness score. Reliability of the FSI in the present study (alpha) was 0.95 for the disruptiveness scale. As in previous research,20 analyses for the current study focused on the rating of average fatigue severity in the past week.
The ECOG Performance Status Rating (PSR) Scale21 is a single-item scale that provides a rating of the patients’ overall ambulatory ability and physical status. Ratings range from 0 (fully active) to 3 (in bed at least 50% of the time). In the present study, patients rated their own performance status.
Data Analysis
The data were analyzed using SPSS version 11.5. Descriptive statistics (frequencies, percentages, means, and ranges) were generated to characterize the study sample in terms of sociodemographic and medical parameters. The factorial validity of the MFSI-SF was assessed using confirmatory factor analysis (CFA). More specifically, a variance-covariance matrix was generated from the raw data. The initial CFA model (Model 1) included all 30 items from the MFSI-SF and constrained factor loadings such that each item was allowed to load only on the scale that it was associated with based on the exploratory factor analysis reported in Stein et al.11 A number of statistics exist to assess the adequacy of structural models.22 The χ2 goodness of fit statistic assesses the adequacy of the theorized model’s creation of a covariance matrix and estimated coefficients in comparison to the observed covariance matrix. Models that result in a created covariance matrix that significantly deviates from the observed covariance matrix are judged to be inadequate. However, the χ2 test has been criticized as an insufficient test alone to adequately assess model fit due to power estimation problems and sample size.23,24 As such, the comparative fit index (CFI) and the incremental fit index (IFI) were also included as alternative indices of model fit. A general rule of thumb for the acceptability of model fit using these indices is >0.90.25 In addition to examination of the goodness of fit indices, modification indices and standardized residuals are also considered in assessing the fit of the model. Specifically, modification indices provide information about the improvement in model fit that would be obtained if certain variables were allowed to cross-load on a non-intended factor, whereas standardized residuals indicate whether the model over- or underestimates the observed covariances among variables. The reliability of the MFSI-SF was evaluated by calculating the internal consistency (alpha coefficients) for each of the 5 subscales of the instrument. Finally, the validity of the MFSI-SF was evaluated by comparing the association (Pearson correlation coefficients) of the MFSI-SF Total Score as well as each of the five empirically-derived subscale scores with another measure of fatigue and measures of physical functioning.
Results
Confirmatory Factor Analysis
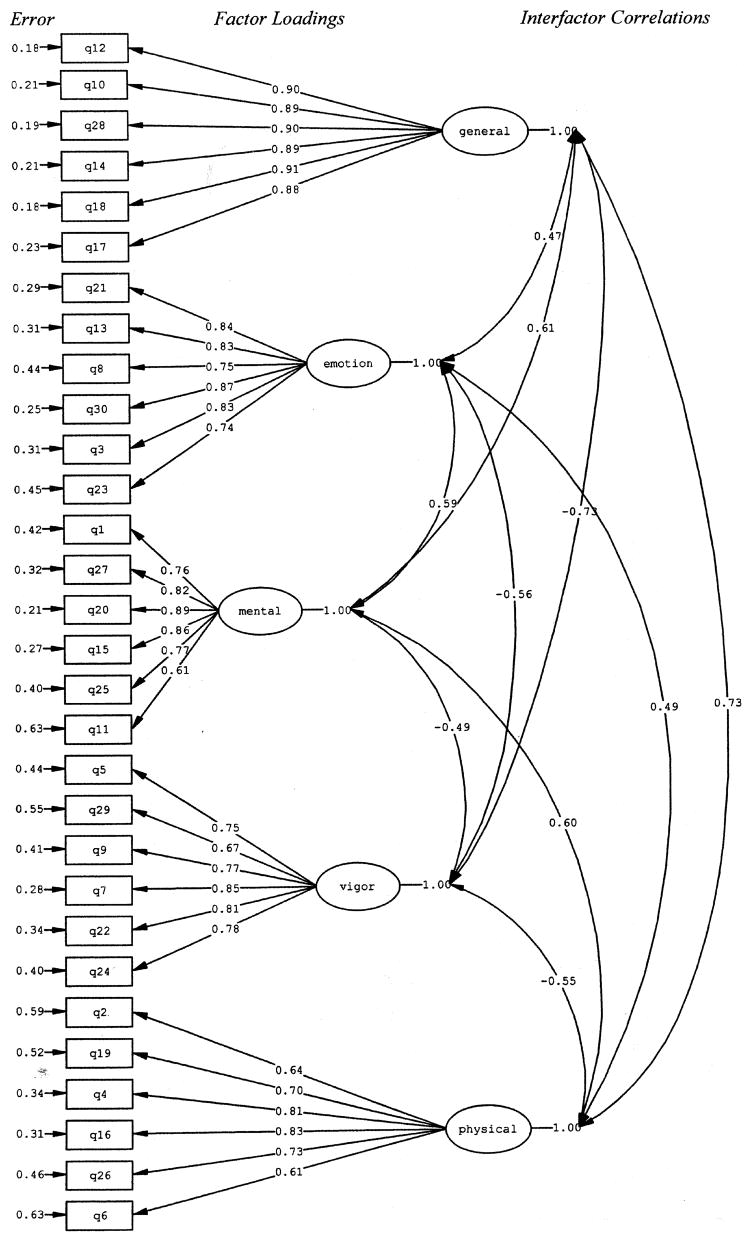
As can be seen from Table 3, results from the confirmatory factor analysis (CFA) suggest that Model 1 was a good fit to the data. The χ2 was significant (P < 0.0001), and the CFI (0.90) and the IFI (0.90) indices suggested a good model fit. In addition, Figure 1 shows that all of the items loaded significantly on their respective factors. Specifically, item loadings ranged from 0.88–0.90 for General Fatigue, 0.74–0.87 for Emotional Fatigue, 0.61–0.89 for Mental Fatigue, 0.67–0.85 for Vigor, and 0.61–0.83 for Physical Fatigue. The error term for each item as well as inter-factor correlations are also represented in Figure 1. I t should be noted that the factor correlations of General Fatigue with Physical Fatigue and Vigor in Model 1 suggest that General Fatigue may not be empirically distinct from the latter two factors. Further examination of the modification indices and standardized residuals indicated that 7 items (1, 9, 10, 18, 24, 26, and 29) would have loaded on more than one factor had the paths not been constrained. As a result, we constructed a second CFA model (Model 2) that removed these items and computed new goodness of fit indices. As can be seen from Table 3, the fit indices did not improve significantly from Model 1 t o Model 2. In fact, they increased by only 0.01 (from 0.90 to 0.91), suggesting deletion of the 7 items above was not warranted. In further support of this conclusion, the factor correlations among the General Fatigue, Physical Fatigue, and Vigor factors were not significantly decreased when Model 2 was compared to Model 1.
Table 3.
Confirmatory Factor Analysis of the 5-Factor Models of the MFSI-SF
| Model | χ2 | Df | χ2/df | CFI | IFI |
|---|---|---|---|---|---|
| Model 1 | 1,371.15 | 395 | 3.47 | 0.90 | 0.90 |
| Model 2 | 660.08 | 220 | 3.00 | 0.91 | 0.91 |
Fig. 1.
Confirmatory factor analysis of the MFSI-SF (Model 1).
Reliability and Validity
The reliability of the MFSI-SF was evaluated by computing the internal consistency of each of the 5 empirically derived subscales. Alpha coefficients for the General Fatigue (0.96), Emotional Fatigue (0.92), Physical Fatigue (0.87), Mental Fatigue (0.91), and Vigor (0.90) subscales were all withinacceptable limits. These findings are consistent with previous estimates of internal consistency reported in the original validation study.11
The construct validity of the MFSI-SF was evaluated through comparisons of the MFSI-SF with other instruments administered in the protocol. It was predicted that the MFSI-SF would be moderately to highly correlated with other measures of fatigue (concurrent validity) and moderately correlated with instruments that measured concepts that are related to fatigue (convergent validity). Concurrent validity was examined by computing correlations between the MFSI-SF and two established measures of fatigue, the FSI average fatigue severity rating and the SF-36 Vitality Scale score. As shown in Table 4, results demonstrated moderate to high correlations, indicating that the five empirically-derived subscales are measuring constructs similar to those measured by the FSI average fatigue rating and the SF-36 Vitality Scale. Convergent validity was examined by computing correlations between the MFSI-SF and the SF-36 Physical Composite Score, a measure of physical health. Table 4 demonstrates that, as expected, the correlations were in the moderate range but somewhat lower than the correlations between the MFSI-SF and other measures of fatigue. The only exception to this pattern was the MFSI-SF Physical Fatigue Score, which, not surprisingly, yielded correlations with the SF-36 Physical Component Score similar to this with other measures of fatigue.
Table 4.
MFSI-SF Validity
| Correlation Coefficient (r)
|
|||
|---|---|---|---|
| FSI Average Fatigue | SF-36 Vitality | SF-36 Physical Component Score | |
| MFSI-SF–General | 0.82 | −0.82 | −0.55 |
| MFSI-SF–Emotional | 0.36 | −0.43 | −0.21 |
| MFSI-SF–Physical | 0.58 | −0.53 | −0.56 |
| MFSI-SF–Mental | 0.50 | −0.48 | −0.34 |
| MFSI-SF–Vigor | −0.60 | 0.74 | 0.48 |
| MFSI-SF–Total | 0.74 | −0.78 | −0.55 |
All P < 0.001.
Discussion
The present study extends previous research with the MFSI-SF in several ways. First, the results of the confirmatory factor analysis of the instrument with a new sample of cancer patients provide additional support for the 5-factor structure of the MFSI-SF that was identified via exploratory factor analysis of the original 83-item version of the instrument.11 As previously noted, however, some of the factors are highly correlated, and several items would have loaded on more than one factor had the paths not been constrained. This finding suggests that the factors of the MFSI-SF share variance and are not completely distinct dimensions. Such factor correlations are perhaps not unexpected given that the MFSI-SF subscales are all considered to be somewhat different and yet related aspects of the overall fatigue symptom profile. We did evaluate an alternative model (Model 2) that excluded several items from the MFSI-SF. However, Model 2 did not result in significant reductions in the correlations between factors, nor did it significantly improve the overall fit to the data, as compared to Model 1. Therefore, it did not appear justified from a statistical standpoint to remove these items from the scale. Furthermore, removal of these items is not desirable from a research or clinical perspective as they still impart important information about the symptom profile of fatigue in the cancer patient. Nonetheless, we recognize that particular factors are correlated, and additional studies with larger sample sizes are needed to continue assessing the factorial validity of the scale.
The second aim of the study was to further examine the reliability and validity of the MFSI-SF. With respect to reliability, estimates of internal consistency for the MFSI-SF subscales were all in the acceptable-to-good range, which is consistent with the results from the original validation study. As the instrument was administered only once to the patients, we were unable to compute test–retest reliability coefficients in the current study. However, test–retest reliability estimates between 3 separate administrations of the instrument were found to be acceptable in the Stein et al. study.11 Future studies should evaluate the test–retest reliability of the MFSI-SF in a heterogeneous sample that includes patients with cancers other than those of the breast. In terms of validity, correlations between the 5 subscales of the MFSI-SF and 2 measures of fatigue demonstrated excellent concurrent validity, whereas correlations with a measure of physical well-being provided support for convergent validity. Additional support for the validity of the MFSI-SF subscales is provided by the fact that the lowest correlations were between the SF-36 Physical Composite Score and the MFSI-SF Emotional and Mental Fatigue subscales. Finally, with the exception of the Vigor subscale of the MFSI-SF, all correlations between the SF-36 Physical Composite score and the MFSI-SF subscales were in the negative direction. This finding provides yet additional evidence for the validity of these scales as higher scores of the MFSI-SF subscales other than Vigor are indicative of more fatigue, whereas higher scores on the SF-36 scales are indicative of better functioning.
The final aim of the study was to examine the performance of the MFSI-SF with a sample that included both male and female cancer patients diagnosed with a wide range of cancers. In general, the results of the present study provide greater confidence that the MFSI-SF is a valuable and useful tool for the assessment of fatigue in cancer patients of different diagnoses and sexes. However, due to small numbers of patients within certain diagnoses, specific comparisons of the MFSI-SF among cancer types could not be made with the current sample. Likewise, about 80% of the patients in our sample were women, and almost 90% identified themselves as white, precluding adequate numbers to make either gender or racial/ethnic comparisons of score on the MFSI-SF. Future studies should attempt to include greater numbers of male and female cancer patients from varied racial/ethnic backgrounds as well as patients who have been diagnosed with cancers other than of the breast to allow for comparisons of the MFSI-SF across these groups. Another potential limitation of this study is that the participants were part of a study investigating the efficacy of stress management training. It is possible that selection bias may exist or that participation in the stress management study may have had an impact on respondents’ fatigue symptom profile.
Despite these limitations, the MFSI-SF appears to be a valuable tool for the multidimensional assessment of cancer-related fatigue. In addition to its valid factor structure and sound psychometric properties, the scale has several advantages over other multidimensional fatigue scales. One of the most useful characteristics of the MFSI-SF is that it is not disease-specific. That is, the items make no reference to a person’s particular illness. Thus it may be used with patients of other chronic illnesses associated with increased fatigue, such as chronic fatigue syndrome, HIV/AIDS, and fibromyalgia. Future research should investigate the performance of the MFSI-SF in populations with chronic illnesses other than cancer. Also, because it does not assume the presence of fatigue, the MFSI-SF can be administered to healthy individuals, allowing for comparisons of fatigue between healthy persons and the physically ill. The utility of the MFSI-SF is further increased by the use of a single response format for all 30 items (i.e., all items are answered on the same 5-point scale) and the brevity of most items. As a result, the MFSI-SF may be easier to complete and less burdensome on fatigued patients than other multidimensional fatigue scales. This statement is tempered by the acknowledgment that no empirical data are available to support this claim. Nevertheless, the MFSI-SF may be an attractive option for researchers and clinicians seeking toassessthe multidimensionalaspectsof fatigue in cancer patients.
In summary, the MFSI-SF appears to be a valid and reliable measure of cancer-related fatigue with a stable multidimensional factorial structure. The scale provides information not only about the overall level of fatigue, but also about the extent to which the respondent is experiencing fatigue across five important domains in which fatigue may manifest. Future research should continue to examine the utility of the scale with cancer patients who are diverse in terms of their sex, ethnicity, and cancer diagnosis as well as patients with other chronic illness and the general population.
Acknowledgments
We wish to thank Corinne Crammer and Donoria Wilkerson for their contributions to the preparation of this manuscript and Mary Coffeen, Lora Azzarello, and Heidi Grenke for their assistance in carrying out the study. This study was supported by research grants from the National Cancer Institute (R01 CA70875) and the American Cancer Society (PBR-99).
Appendix 1
The Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF).
Below is a list of statements that describe how people sometimes feel. Please read each item carefully, then circle the one number next to each item which best describes how true each statement has been for you in the past seven days.
| Not at all | A little | Moderately | Quite a bit | Extremely | ||
|---|---|---|---|---|---|---|
| 1. | I have trouble remembering things | 0 | 1 | 2 | 3 | 4 |
| 2. | My muscles ache | 0 | 1 | 2 | 3 | 4 |
| 3. | I feel upset | 0 | 1 | 2 | 3 | 4 |
| 4. | My legs feel weak | 0 | 1 | 2 | 3 | 4 |
| 5. | I feel cheerful | 0 | 1 | 2 | 3 | 4 |
| 6. | My head feels heavy | 0 | 1 | 2 | 3 | 4 |
| 7. | I feel lively | 0 | 1 | 2 | 3 | 4 |
| 8. | I feel nervous | 0 | 1 | 2 | 3 | 4 |
| 9. | I feel relaxed | 0 | 1 | 2 | 3 | 4 |
| 10. | I feel pooped | 0 | 1 | 2 | 3 | 4 |
| 11. | I am confused | 0 | 1 | 2 | 3 | 4 |
| 12. | I am worn out | 0 | 1 | 2 | 3 | 4 |
| 13. | I feel sad | 0 | 1 | 2 | 3 | 4 |
| 14. | I feel fatigued | 0 | 1 | 2 | 3 | 4 |
| 15. | I have trouble paying attention | 0 | 1 | 2 | 3 | 4 |
| 16. | My arms feel weak | 0 | 1 | 2 | 3 | 4 |
| 17. | I feel sluggish | 0 | 1 | 2 | 3 | 4 |
| 18. | I feel run down | 0 | 1 | 2 | 3 | 4 |
| 19. | I ache all over | 0 | 1 | 2 | 3 | 4 |
| 20. | I am unable to concentrate | 0 | 1 | 2 | 3 | 4 |
| 21. | I feel depressed | 0 | 1 | 2 | 3 | 4 |
| 22. | I feel refreshed | 0 | 1 | 2 | 3 | 4 |
| 23. | I feel tense | 0 | 1 | 2 | 3 | 4 |
| 24. | I feel energetic | 0 | 1 | 2 | 3 | 4 |
| 25. | I make more mistakes than usual | 0 | 1 | 2 | 3 | 4 |
| 26. | My body feels heavy all over | 0 | 1 | 2 | 3 | 4 |
| 27. | I am forgetful | 0 | 1 | 2 | 3 | 4 |
| 28. | I feel tired | 0 | 1 | 2 | 3 | 4 |
| 29. | I feel calm | 0 | 1 | 2 | 3 | 4 |
| 30. | I am distressed | 0 | 1 | 2 | 3 | 4 |
Administration and Scoring
The MFSI-SF can be completed in a wide variety of settings in about 5 minutes. Items are rated on a 5-point scale indicating how true each statement was for the respondent during the last week (0 = Not all; 4 = Extremely). Scoring instructions for the MFSI-SF are as follows:
General scale score = sum of items 10, 12, 14, 17, 18, and 28
Physical scale score = sum of items 2, 4, 6, 16, 19, and 26
Emotional scale score = sum of items 3, 8, 13, 21, 23, and 30
Mental scale score = sum of items 1, 11, 15, 20, 25, and 27
Vigor scale score = sum of items 5, 7, 9, 22, 24, and 29
Total scale score = sum of scales 1–4 minus the Vigor scale score
References
- 1.Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist. 1999;4:1–10. [PubMed] [Google Scholar]
- 2.Cella DF, Peterman A, Passik S, et al. Progress toward guidelines for the management of fatigue. Oncology (Huntingt) 1998;12(11A):369–377. [PubMed] [Google Scholar]
- 3.Cella DF. Factors influencing quality of life in cancer patients: anemia and fatigue. Semin Oncol. 1998;25(3Suppl 7):43–46. [PubMed] [Google Scholar]
- 4.Cimprich B. Development of an intervention to restore attention in cancer patients. Cancer Nurs. 1993;16(2):83–92. [PubMed] [Google Scholar]
- 5.Rhodes VA, Watson PM, Hanson BM. Patients’ descriptions of the influence of tiredness and weakness on self-care abilities. Cancer Nurs. 1988;11(3):186–194. [PubMed] [Google Scholar]
- 6.Knobf MT. Physical and psychologic distress associated with adjuvant chemotherapy in women with breast cancer. J Clin Oncol. 1986;4(5):678–684. doi: 10.1200/JCO.1986.4.5.678. [DOI] [PubMed] [Google Scholar]
- 7.Pearson PG, Byars GE. The development and validation of a checklist measuring subjective fatigue. Randolf AFB, TX: School of Aviation, USAF; 1956. pp. 56–115. [Google Scholar]
- 8.McNair DM, Lorr M, Droppleman LF. Profile of Mood States, revised. San Diego, CA: EdITS/Educational and Industrial Testing Service; 1992. [Google Scholar]
- 9.Smets E, Garssen B, Bonke B, Haes JD. The multidimensional fatigue inventory: psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–329. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 10.Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- 11.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6(3):143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS. Cancer-related fatigue: new directions for research. Introduction Cancer. 2001;92(6 Suppl):1657–1661. doi: 10.1002/1097-0142(20010915)92:6+<1657::aid-cncr1492>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz A, Meek P. Additional construct validity of the Schwartz Cancer Fatigue Scale. J Nurs Meas. 1999;7(1):35–45. [PubMed] [Google Scholar]
- 14.Cella DF. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13–19. [PubMed] [Google Scholar]
- 15.Piper BG, Lindsey AM, Dodd MJ, et al. The development of an instrument to measure the subjective dimension of fatigue. In: Funk S, Tournquist E, Champagne M, et al., editors. Key aspects of comfort: management of pain, fatigue and nausea. New York: Springer; 1989. pp. 199–220. [Google Scholar]
- 16.Jacobsen PB, Meade CD, Stein KD, et al. Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. J Clin Oncol. 2002;20(12):2851–2862. doi: 10.1200/JCO.2002.08.301. [DOI] [PubMed] [Google Scholar]
- 17.Hickok JT, Morrow GR, McDonald S, Bellg AJ. Frequency and correlates of fatigue in lung cancer patients receiving radiation therapy: implications for management. J Pain Symptom Manage. 1996;11(6):370–377. doi: 10.1016/0885-3924(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 20.Broeckel JA, Jacobsen PB, Horton J, et al. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998;16(5):1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- 21.Zubrod CG, Schneiderman M, Frei E, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustand and triethylene thiophosphoramide. J Chronic Dis. 1960;11:7–33. [Google Scholar]
- 22.Bollen KA. Structural equations with latent variables. New York: John Wiley&Sons; 1989. [Google Scholar]
- 23.Keller SD, Ware JE, Jr, Bentler PM, et al. Use of structural equation modeling to test the construct validity of the SF-36 Health survey in ten countries: results from the IQOLA Project. International Quality of Life Assessment J Clin Epidemiol. 1998;51(11):1179–1188. doi: 10.1016/s0895-4356(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 24.Hu L, Bentler PM. Evaluating model fit. In: Hoyle RH, editor. Structural equation modeling: concepts, issues, and applications. Thousand Oaks, CA: Sage; 1995. pp. 76–99. [Google Scholar]
- 25.Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]