Abstract
Black cohosh is a commonly used botanical dietary supplement for the treatment of climacteric complaints. Since the opiate system in the brain is intimately associated with mood, temperature and sex hormonal levels, we investigated the activity of black cohosh extracts at the human μ opiate receptor (hMOR) expressed in Chinese hamster ovary cells. The 100% methanol-, 75% ethanol- and 40% 2-propanol- extracts of black cohosh effectively displaced the specific binding of [3H]DAMGO to hMOR. Further studies of the clinically used ethanol extract indicated that black cohosh acted as a mixed competitive ligand, displacing 77 ± 4% [3H]DAMGO to hMOR (Ki = 62.9 μg/ml). Using the [35S]GTPγS assay, the action of black cohosh was found to be consistent with an agonist, with an EC50 of 68.8 ± 7.7 μg/ml. These results demonstrate for the first time that black cohosh contains active principle(s) that activate hMOR, supporting its beneficial role in alleviating menopausal symptoms.
Keywords: black cohosh, menopause, hot flashes, opiate, botanical dietary supplement
INTRODUCTION
Actaea racemosa L. or Cimicifuga racemosa (L.) Nutt. (Ranunculaceae), commonly known as black cohosh, has been traditionally used for gynecologic and pain disorders. The first medicinal use of black cohosh is generally attributed to Native Americans, who used black cohosh for the treatment of a variety of disorders, including various conditions unique to women such as amenorrhea and menopause, rheumatism, kidney disorders, malaise, and pain during menses and childbirth (1,2). Use of black cohosh was accepted and popularized by eclectic medical practitioners in the late 19th and early 20th centuries. Dr. John King made a significant contribution publicizing the use of black cohosh, or as he referred to it, Macrotys. King claimed that Macrotys was a remedy of “abnormal conditions of the principal organs of reproduction in the female”, and the roots “very efficacious in the treatment of chronic ovaritis, endometriosis, and menstrual derangements, such as amenorrhea, dysmenorrhea” (1). Black cohosh root was an official drug (under the name “black snakeroot”) in the United States Pharmacopoeia (USP) from 1820 to 1926.
With a shift toward evidence-based modern medicine, the use of black cohosh in America faded by the 1930’s. However, its medicinal use gained popularity in Europe, especially in Germany, since the 1950’s. Black Cohosh is one of the main herbs recommended in Germany for menopause, PMS and secondary amenorrhoea (3). In Europe, black cohosh is commonly prescribed as an alternative to hormone replacement therapy for menopause (4). Over the past decade or so, black cohosh also regained its popularity for alleviating menopausal symptoms in the United States. The North American Menopause Society (NAMS) recommends the short term (less than 6 months) use of a black cohosh supplement for menopause (5). A number of clinical trials have been carried out to examine the efficacy and safety of black cohosh (6–11). Black cohosh appears to be effective in reducing hot flashes and depression, although more clinical evidence is still needed (12–17).
The mechanism for the effectiveness of black cohosh in treating menopausal symptoms is not entirely clear. Black cohosh was initially thought to contain formononetin, an estrogenic isoflavone (18–20). However, more recent studies have not found significant estrogenic constituents or activity (21–25). Since there are significant CNS manifestations in menopause and a tight connection between sex hormones and CNS receptors (26–28), it is plausible that black cohosh extracts may interact with CNS systems for at least some of the beneficial effects in treating menopausal symptoms. Indeed, it has been reported that black cohosh acted as an agonist at the 5HT1 A, ID and 7 receptors (29), and may also have affinity to the dopamine D2 receptor (20). Since the opioid system, especially the μ opiate receptor, is essential for temperature and hormone homeostasis, this may be one of the mechanisms for the action of black cohosh in menopause. In this study, we examined three different black cohosh extracts in μ opiate receptor binding and functional assays.
MATERIALS AND METHODS
Chemicals
Guanosine 5′-diphosphate (GDP), guanosine 5′-[γ-thio] triphosphate (GTPγS), bovine serum albumin (BSA), dithiothreitol (DTT), disodium ethylenediamine tetraacetate (EDTA), 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid N-(2-Hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (HEPES) were obtained from Sigma (St. Louis, MO). [D-Ala2,N-MePhe4-Gly-ol5] enkephalin (DAMGO), and [3H]DAMGO were from Multiple Peptide Systems (San Diego, CA). [35S]GTPγS was purchased from Amersham Biosciences (Piscataway, NJ). All other chemicals were purchased from Sigma (St. Louis, MO).
Plant materials
Black cohosh extracts were prepared and characterized as previously described (29–31). Briefly, black cohosh rhizomes/roots were provided by PureWorld Botanicals, Inc. (South Hackensack, NJ; lot number: 9-1744) (29), and were botanically verified by the UIC/NIH Center for Botanical Dietary Supplements Research and characterized by PCR (30). A voucher specimen (BC001) has been deposited at the Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS), University of Illinois at Chicago. Milled roots/rhizomes of black cohosh were separately extracted by percolation with 75% ethanol (CR-ethanol), 100% methanol (CR-methanol), or 40% 2-propanol (CR-isopropanol) and then dried to a powder in vacuo. These extracts were chemically characterized by HPLC and standardized to active triterpene glycosides (31). The HPLC method has since been adopted by the USP-NF. The 75% ethanol extract, chemically standardized to 5.6% active triterpene glycosides and biologically standardized to 5HT7 receptor, is currently being used in a four arm randomized double-blind and placebo-controlled Phase II clinical trial that is being conducted by the UIC/NIH Center for Botanical Dietary Supplements Research. Extensive effort has been carried out to identify the active principles in black cohosh extracts, which have so far yielded a number of new compounds (32–37). For pharmacological assays, the dried extracts were dissolved in DMSO. The final DMSO concentrations in all assays were below 0.5%, which was found not to interfere with either the receptor binding or GTPγS binding assay.
Cell culture
Chinese hamster ovary (CHO) cells stably transfected with human mu opioid receptors (CHO-hMOR) were established as described previously (38). CHO-hMOR cells were cultured in Dulbecco’s modified eagle medium (DMEM) and Ham’s F-12 medium (1:1) supplemented with 10% newborn calf serum, 100 IU/ml penicillin and 100 (μg/ml streptomycin. To maintain stable selection, 200 (μg/ml G418 was added to the growth medium. Cells were cultured in an incubator maintained at 37°C with 5% CO2 in humidified air.
Receptor binding assay
Receptor binding assay was carried out as previously described (38–40). Briefly, membranes from CHO-hMOR cells were prepared by Polytron homogenization at setting 6 for 2 min on ice, followed by centrifugation at 20,000 g for 30 min at 4°C. Protein content was determined by the Coomassie protein assay method (Pierce Biotechnology, Rockford, IL) using bovine serum albumin as the standard. For receptor binding assay, the receptor membranes (50 μg protein/reaction) were incubated with a series of concentrations of [3H]D-Ala2,N-Me-Phe4-Gly5-ol]enkephalin (DAMGO, 1 nM) and different concentrations of black cohosh extracts in 50 mM Tris buffer (pH 7.4) at 30°C for 1 h. Nonspecific binding was determined in the presence of 20 μM unlabelled DAMGO. Reactions were terminated by rapid vacuum filtration through GF/B filters presoaked with 0.2% polyethylenimine. Filter-bound radioactivity was determined by liquid scintillation counting (Beckman Coulter Inc., Fullerton, CA). Results, expressed in Mean ± SD, were analyzed using the Prism program (GraphPad Software, San Diego, CA).
Kinetics of receptor binding
The mode of receptor binding by black cohosh extract was further characterized to determine full vs partial, competitive, uncompetitive, vs noncompetitive binding to the (μ opiate receptor. To obtain Kd and Ki values, [3H]DAMGO ranging from 0.1~4 nM and black cohosh ethanol extract ranging from 0 ~ 200 μg/ml were used in the receptor binding assay as described above. Data were further transformed into a double-reciprocal plot to determine the mechanism of receptor-ligand interaction.
[35S]Guanosine 5′-[γ-thio]triphosphate (GTPγS) binding assay
Since the μ-opiate receptor is a G protein coupled receptor, we employed the GTPγS binding assay to determine the activation of the receptor. The [35S]GTPγS binding assay was performed based on the method previously described (38,39). Briefly, cell membranes (40 μg protein) were incubated with 0.1 nM [35S]GTPγS in the reaction buffer (50 mM HEPES, 100 mM sodium chloride, 1 mM EDTA, 5 mM magnesium chloride, 30 μM GDP, 1 mM DTT, and 0.1% BSA, pH 7.4) in the presence or absence of black cohosh extracts or DAMGO, at 30°C for 1h. The basal level was defined as the amount of [35S]GTPγS bound in the absence of any agonist. Non-specific binding was determined in the presence of 10 μM unlabeled GTPγS. Reactions were terminated by rapid filtration and filter-bound radioactivity was determined as described above.
Data and statistical analysis
Data, expressed as mean ± SD, were analyzed using the GraphPad Prism program to obtain IC50, maximum inhibition, EC50, and Emax values. The dissociation constant Ki was determined using the method of Cheng and Prusoff (41). Differences in responses between groups were determined using ANOVA followed by Student’s t (two groups) or Dunnett’s t (multiple groups) tests.
RESULTS
Affinity of black cohosh extracts to the μ opiate receptor
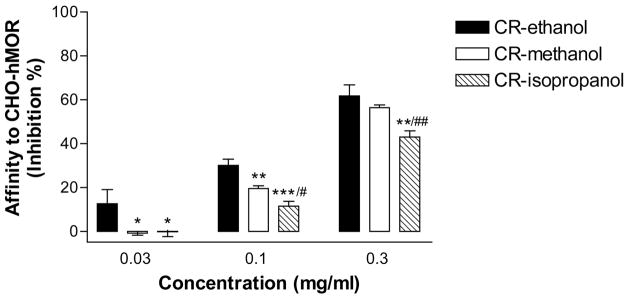
Three different black cohosh extracts, a 75% ethanol extract, a 100% methanol extract, and a 75% isopropanol extract, were subject to a receptor binding assay to determine their affinity to the human μ opiate receptor (hMOR). [3H]DAMGO, a μ opiate receptor-specific radioligand that binds to the receptor with a high affinity (38), was used in the assay. All three extracts were able to displace the specific binding of [3H]DAMGO to hMOR (Fig. 1), indicating that black cohosh contains constituents with an affinity to the receptor. As expected, the affinity of black cohosh extracts is concentration-dependent. The 75% ethanol extract of black cohosh (CR-ethanol) appeared to be most effective. This is also the extract that is used in a Phase II clinical trial that is being conducted by the UIC/NIH Center for Botanical Dietary Supplements Research. We further characterized CR-ethanol in receptor binding and functional assays.
Figure 1. Affinity of black cohosh extracts to the human μ opiate receptor (hMOR) expressed in Chinese hamster ovary (CHO) cells.
Three black cohosh extracts, 100% methanol (CR-methanol), 75% ethanol (CR-ethanol), or 40% 2-propanol (CR-isopropanol), were used to displace the binding of [3H]DAMGO, a selective hMOR agonist, to CHO-hMOR cells. The affinities of these extracts, at three different concentrations (0.03, 0.1, and 0.3 mg/ml), were compared. The percentage inhibition of the specific radioactivity of [3H]DAMGO bound to the CHO-hMOR cell membranes was shown. Data are the mean ± SD (N = 3). * P < 0.05, ** P < 0.01, *** P < 0.001, compared with CR-ethanol; #P < 0.05, ** P < 0.01, comparison between CR-methanol and CR-isopropanol.
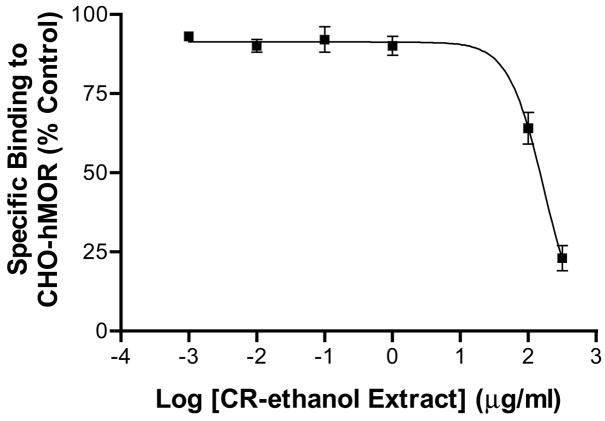
To obtain the dissociation rate constant (Ki), different concentrations of CR-ethanol, ranging from 0.001 – 300 μ/ml, were tested in the competition binding assay against [3H]DAMGO (1 nM). The extract dose-dependently displaced the specific binding of [3H]DAMGO, with a Ki value of 62.9 μg/ml (IC50: 165.9 μg/ml) (Fig 2). At the highest concentration used, the extract displaced 77 ± 4% of [3H]DAMGO binding, indicating the black cohosh extract had strong affinity to hMOR.
Figure 2.
Displacement of [3H]DAMGO binding by a clinically used 75% ethanol extract of black cohosh (CR-ethanol). CR-ethanol (0.01 – 300 μg/ml) was used to dose-dependently compete for the binding of [3H]DAMGO (1 nM) to the CHO-hMOR cell membranes. The specific radioactivity of [3H]DAMGO bound to hMOR in the absence of black cohosh was set to 100%. Each point represents the mean ± SD (N = 3). The Ki and IC50 values were estimated to be 62.9 μg/ml and 165.9 μg/ml, respectively.
Mechanism of receptor binding
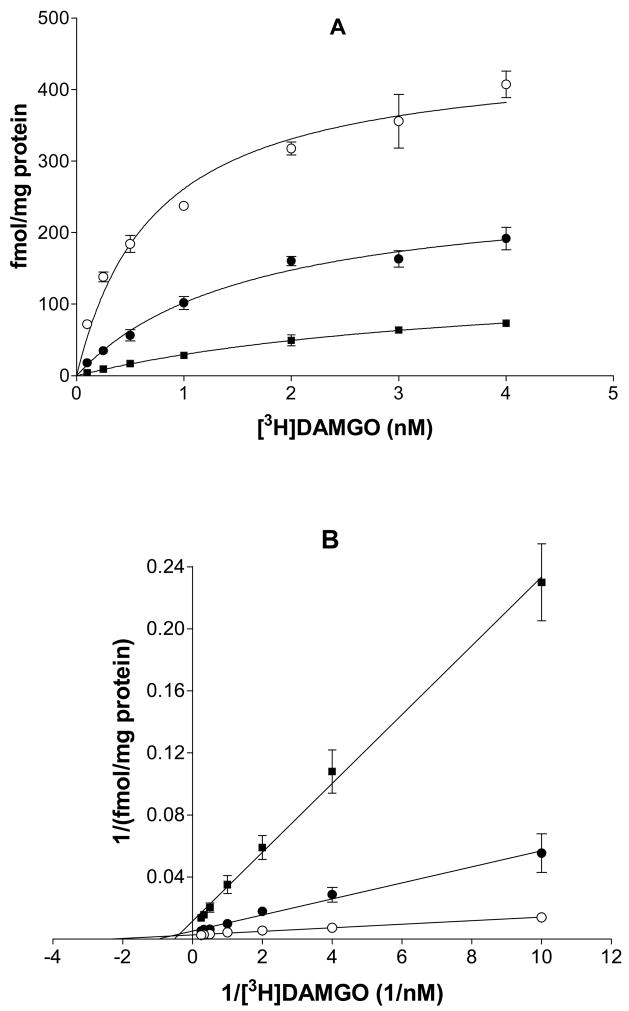
To investigate whether the black cohosh extract functions as a competitive, noncompetitive, uncompetitive, or mixed inhibitor of ligand binding to hMOR, radioligand receptor saturation binding assay was carried out using [3H]DAMGO (0.1–4 nM) in the absence or presence of 100 and 200 μg/ml of CR-ethanol. Black cohosh significantly inhibited the saturation binding of DAMGO to hMOR, and as a result, the maximum binding Bmax was decreased by 41% and 68% with 100 and 200 μg/ml CR-ethanol respectively (Fig 3A). The apparent Kd of DAMGO for hMOR was increased 1.2 and 4.4 fold in the presence 100 and 200 μg/ml of CR-ethanol, respectively (Table 1). Further analysis using a double-reciprocal plot indicated that the data fit best with a mixed competition mode where the hMOR-bound constitutent(s) in black cohosh not only bind to the receptor binding site of hMOR alone but can also bind in the presence of DAMGO. A schematic model for black cohosh acting as a mixed competitive ligand (I) for the binding of DAMGO to hMOR is illustrated in Fig. 4.
Figure 3.
Inhibition of black cohosh on the specific binding of [3H]DAMGO to hMOR. (A) Saturation binding of [3H]DAMGO to hMOR in the absence or presence of 100 μg/ml or 200 μg/ml black cohosh. (B) Reciprocal plots of the binding data of [3H]DAMGO to hMOR. (○) no black cohosh; (●) 100 μg/ml black cohosh; (▪) 200 μg/ml black cohosh. Each point represents the mean ± SD (N = 3).
Table 1.
[3H]DAMGO binding to the human μ opiate receptor in the absence or presence of black cohosh 75% ethanol extract
| black cohosh (μg/ml)
|
|||
|---|---|---|---|
| 0 | 100 | 200 | |
| Kd(nM) | 0.73 | 1.62 | 3.94 |
| Bmax(fmol/mg protein) | 451.4 | 267.2 | 146.5 |
Figure 4.
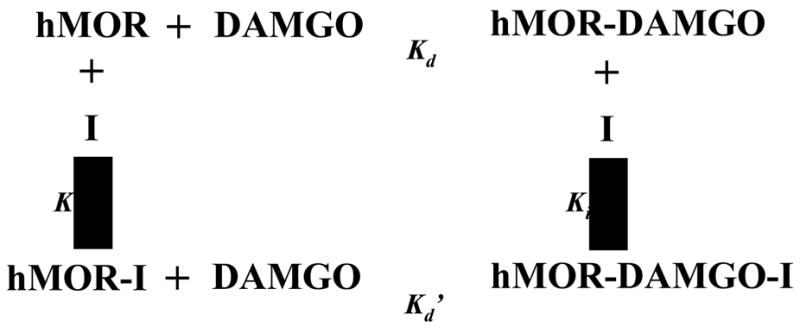
A scheme illustrating the mixed competitive action of black cohosh (I) on the binding of DAMGO to the human μ opiate receptor (hMOR). Kd, Kd′, Ki and Ki′ are dissociation rate constants.
Activation of the human μ opiate receptor by black cohosh extract
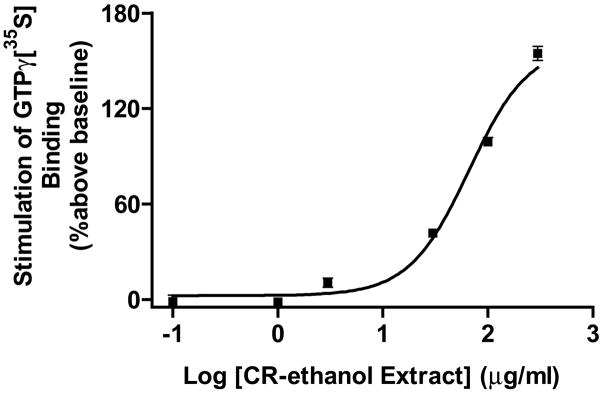
It is critical to ascertain if the black cohosh extracts contain agonistic or antagonistic opiate activity at the receptor. The μ opiate receptor belongs to the superfamily of G protein-coupled receptors. Activation of hMOR leads to the activation of G proteins and subsequent cellular signaling. The efficacy of a hMOR agonist in activating G-proteins, which can be measured by [35S]GTPγS binding to the cytoplasmic membranes (39), can be used to determine the agonist’s activity at the receptor. Therefore, we employed the GTPγS binding assay to determine the mode of action of CR-ethanol. The extract dose-dependently activated hMOR as revealed by the significant stimulation of GTPγS binding to CHO-hMOR (Fig. 5). The maximum effect was 162.2 ± 14.7%, which was lower than that of DAMGO, a full agonist of hMOR (p < 0.05). The EC50 of CR-ethanol was estimated to be 68.8 ± 7.7 μg/ml (N = 3). These data suggest that the black cohosh extract contains active constituent(s) that act as an agonist at the human μ opiate receptor, which is consistent with its beneficial action in relieving at least some of the menopausal symptoms.
Figure 5.
Stimulation of [35S]GTPγS binding by a clinically used 75% ethanol extract of black cohosh (CR-ethanol). CR-ethanol stimulated [35S]GTPγS binding by 166 ± 15%. Each point represents the mean ± SD (N = 3). The EC50 value was estimated to be 78.1 ± 18.8μg/ml.
DISCUSSION
A large segment of the female population is affected by menopausal symptoms. Limited treatment options are available. Hormone replacement therapy is effective in treating hot flashes and other symptoms. However, the Women’s Health Initiative and long-term follow-up from the Heart and Estrogen/progestin Replacement Study found an increased risk of cardiovascular disease, breast cancer, and stroke among women randomized to hormone therapy (42–45). Many women have turned to botanical dietary supplements for the relief of menopausal symptoms. Black cohosh is one of the most commonly used botanical dietary supplements for alleviating menopausal symptoms. Despite the long history and wide use, its mechanisms of action are still not entirely understood.
Early studies have examined the phytoestrogens contained in black cohosh (18–20). However, more recent studies did not find significant estrogenic constituents or activity (21–25,29). Since there are significant CNS manifestations in menopause and tight connection between sex hormone and the CNS receptors (26–28), it has been proposed that black cohosh may act centrally (20,29).
The current study demonstrated for the first time that black cohosh extracts contained constituents that have significant affinity to the human μ opiate receptor. Moreover, a clinically used 75% ethanol extract of black cohosh acted as a partial agonist at the receptor.
The opiate receptor system affects several aspects of female reproductive neuroendocrinology, such as the control of sex hormones and the display of lordosis behavior (28,46–51). In fact, a decreased β-endorphin level is considered a hormonal marker for menopause (27). Hot flashes, a major complaint of menopausal women, are hypothesized to be caused by an erroneous setting of body core temperature (52). The CNS temperature control center is regulated by the endogenous opiate system as well as catecholamines (26,27,53). Opiates can therefore alter core temperature setting directly or indirectly by affecting the release and levels of catecholamines (27,54,55). Striking similarities exist between opiate withdrawal and menopausal hot flashes (56). Not surprisingly, opiate dependence withdrawal has been used as an animal model of menopausal hot flashes (57).
Therefore, botanical dietary supplements containing opiate activity are expected to have beneficial effects in relieving menopausal symptoms, including reducing hot flashes. The opiate agonistic activity of black cohosh may explain at least in part its efficacy in alleviating menopausal symptoms.
Acknowledgments
This publication was funded by the following grants: AT003476 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), AT000155 jointly provided to the UIC/NIH Center for Botanical Dietary Supplements Research by the ODS, the National Institute for General Medical Sciences, the Office for Research on Women’s Health, and the NCCAM, and DA005050 from the National Institute on Drug Abuse grant. Donna Webster is supported by an NIH predoctoral fellowship (F31AT002669). Jian Lu is a University Fellow. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, NIDA or the National Institutes of Health.
Abbreviations used
- CHO
Chinese hamster ovary
- CR-ethanol
a 75% ethanol extract of black cohosh
- CR-methanol
a 100% methanol extract of black cohosh
- CR-isopropanol
a 40% 2-propanol extract of black cohosh
- DAMGO
[D-Ala2,N-Me-Phe4-Gly5-ol]enkephalin
- DTT
dithiothreitol
- HEPES
4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid N-(2-Hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid)
- hMOR
human μ-opiate receptor
Literature Cited
- 1.Foster S. Black cohosh: Cimicifuga racemosa. A literature review. HerbalGram. 1999;45:35–50. [Google Scholar]
- 2.Borrelli F, Ernst E. Cimicifuga racemosa: a systematic review of its clinical efficacy. Eur J Clin Pharmacol. 2002;58:235–241. doi: 10.1007/s00228-002-0457-2. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal M, et al. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. American Botanical Council: Integrative Medicine Communications; Austin, Texas: 1998. p. 90. [Google Scholar]
- 4.McKenna DJ, Jones K, Humphrey S, Hughes K. Black cohosh: efficacy, safety, and use in clinical and preclinical applications. Altern Ther Health Med. 2001;7:93–100. [PubMed] [Google Scholar]
- 5.NAMS. Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11:11–33. doi: 10.1097/01.GME.0000108177.85442.71. [DOI] [PubMed] [Google Scholar]
- 6.Warnecke G. Influence of a phytopharmaceutical on climacteric complaints. Die Meizinische Welt. 1985;36:871–874. [Google Scholar]
- 7.Stoll W. Phytopharmacon influences atrophic vaginal epithelium: double-blind study-Cimicifuga vs estrogenic substacnes. Therapeuticum. 1987;1:23–31. [Google Scholar]
- 8.Lehmann-Willenbrock E, Riedel HH. Clinical and endocrinologic studies of the treatment of ovarian insufficiency manifestations following hysterectomy with intact adnexa. Zentralbl Gynakol. 1988;110:611–618. [PubMed] [Google Scholar]
- 9.Wuttke W, Seidlova-Wuttke D, Gorkow C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: effects on menopause symptoms and bone markers. Maturitas. 2003;44(Suppl 1):S67–77. doi: 10.1016/s0378-5122(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 10.Osmers R, Friede M, Liske E, Schnitker J, Freudenstein J, et al. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. Obstet Gynecol. 2005;105:1074–1083. doi: 10.1097/01.AOG.0000158865.98070.89. [DOI] [PubMed] [Google Scholar]
- 11.Uebelhack R, Blohmer JU, Graubaum HJ, Busch R, Gruenwald J, et al. Black cohosh and St. John’s wort for climacteric complaints: a randomized trial. Obstet Gynecol. 2006;107:247–255. doi: 10.1097/01.AOG.0000196504.49378.83. [DOI] [PubMed] [Google Scholar]
- 12.Hardy ML. Herbs of special interest to women. J Am Pharm Assoc (Wash) 2000;40:234–242. doi: 10.1016/s1086-5802(16)31064-6. quiz 327–329. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson JS, Troxel AB, Evans J, Klaus L, Vahdat L, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001;19:2739–2745. doi: 10.1200/JCO.2001.19.10.2739. [DOI] [PubMed] [Google Scholar]
- 14.Kang HJ, Ansbacher R, Hammoud MM. Use of alternative and complementary medicine in menopause. Int J Gynaecol Obstet. 2002;79:195–207. doi: 10.1016/s0020-7292(02)00297-7. [DOI] [PubMed] [Google Scholar]
- 15.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized controlled trials. Ann Intern Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 16.Pockaj BA, Loprinzi CL, Sloan JA, Novotny PJ, Barton DL, et al. Pilot evaluation of black cohosh for the treatment of hot flashes in women. Cancer Invest. 2004;22:515–521. doi: 10.1081/cnv-200026394. [DOI] [PubMed] [Google Scholar]
- 17.Nappi RE, Malavasi B, Brundu B, Facchinetti F. Efficacy of Cimicifuga racemosa on climacteric complaints: a randomized study versus low-dose transdermal estradiol. Gynecol Endocrinol. 2005;20:30–35. doi: 10.1080/09513590400020922. [DOI] [PubMed] [Google Scholar]
- 18.Jarry H, Harnischfeger G, Duker E. The endocrine effects of constituents of Cimicifuga racemosa. 2. In vitro binding of constituents to estrogen receptors. Planta Med. 1985:316–319. doi: 10.1055/s-2007-969500. [DOI] [PubMed] [Google Scholar]
- 19.Duker EM, Kopanski L, Jarry H, Wuttke W. Effects of extracts from Cimicifuga racemosa on gonadotropin release in menopausal women and ovariectomized rats. Planta Med. 1991;57:420–424. doi: 10.1055/s-2006-960139. [DOI] [PubMed] [Google Scholar]
- 20.Jarry H, Metten M, Spengler B, Christoffel V, Wuttke W. In vitro effects of the Cimicifuga racemosa extract BNO 1055. Maturitas. 2003;44(Suppl 1):S31–38. doi: 10.1016/s0378-5122(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 21.Struck D, Tegtmeier M, Harnischfeger G. Flavones in extracts of Cimicifuga racemosa. Planta Med. 1997;63:289–290. doi: 10.1055/s-2006-957682. [DOI] [PubMed] [Google Scholar]
- 22.Kennelly EJ, Baggett S, Nuntanakorn P, Ososki AL, Mori SA, et al. Analysis of thirteen populations of black cohosh for formononetin. Phytomedicine. 2002;9:461–467. doi: 10.1078/09447110260571733. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 24.Liske E, Hanggi W, Henneicke-von Zepelin HH, Boblitz N, Wustenberg P, et al. Physiological investigation of a unique extract of black cohosh (Cimicifugae racemosae rhizoma): a 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med. 2002;11:163–174. doi: 10.1089/152460902753645308. [DOI] [PubMed] [Google Scholar]
- 25.Borrelli F, Izzo AA, Ernst E. Pharmacological effects of Cimicifuga racemosa. Life Sci. 2003;73:1215–1229. doi: 10.1016/s0024-3205(03)00378-3. [DOI] [PubMed] [Google Scholar]
- 26.Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77:1207–1218. doi: 10.4065/77.11.1207. [DOI] [PubMed] [Google Scholar]
- 27.Stomati M, Rubino S, Spinetti A, Parrini D, Luisi S, et al. Endocrine, neuroendocrine and behavioral effects of oral dehydroepiandrosterone sulfate supplementation in postmenopausal women. Gynecol Endocrinol. 1999;13:15–25. doi: 10.1080/09513599909167527. [DOI] [PubMed] [Google Scholar]
- 28.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 29.Burdette JE, Liu J, Chen SN, Fabricant DS, Piersen CE, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem. 2003;51:5661–5670. doi: 10.1021/jf034264r. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Fabricant DS, Piersen CE, Bolton JL, Pezzuto JM, et al. A preliminary RAPD-PCR analysis of Cimicifuga species and other botanicals used for women’s health. Phytomedicine. 2002;9:757–762. doi: 10.1078/094471102321621403. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Chen SN, Fabricant D, Angerhofer CK, Fong HHS, et al. High-performance liquid chromatographic analysis of Black Cohosh (Cimicifuga racemosa) constituents with in-line evaporative light scattering and photodiode array detection. Anal Chim Acta. 2002;471:61–75. [Google Scholar]
- 32.Chen SN, Fabricant DS, Pauli GR, Fong HH, Farnsworth NR. Synthesis of cimiracemate B, a phenylpropanoid found in Cimicifuga racemosa. Nat Prod Res. 2005;19:287–290. doi: 10.1080/14786410410001714650. [DOI] [PubMed] [Google Scholar]
- 33.Fabricant DS, Nikolic D, Lankin DC, Chen SN, Jaki BU, et al. Cimipronidine, a cyclic guanidine alkaloid from Cimicifuga racemosa. J Nat Prod. 2005;68:1266–1270. doi: 10.1021/np050066d. [DOI] [PubMed] [Google Scholar]
- 34.Chen SN, Fabricant DS, Lu ZZ, Fong HH, Farnsworth NR. Cimiracemosides I-P, new 9,19-cyclolanostane triterpene glycosides from Cimicifuga racemosa. J Nat Prod. 2002;65:1391–1397. doi: 10.1021/np0200818. [DOI] [PubMed] [Google Scholar]
- 35.Chen SN, Fabricant DS, Lu ZZ, Zhang H, Fong HH, et al. Cimiracemates A-D, phenylpropanoid esters from the rhizomes of Cimicifuga racemosa. Phytochemistry. 2002;61:409–413. doi: 10.1016/s0031-9422(02)00209-1. [DOI] [PubMed] [Google Scholar]
- 36.Chen SN, Li W, Fabricant DS, Santarsiero BD, Mesecar A, et al. Isolation, structure elucidation, and absolute configuration of 26-deoxyactein from Cimicifuga racemosa and clarification of nomenclature associated with 27-deoxyactein. J Nat Prod. 2002;65:601–605. doi: 10.1021/np010494t. [DOI] [PubMed] [Google Scholar]
- 37.Fabricant DS. Department of Pharmacognosy. University of Illinois at Chicago; Chicago: 2006. Ph.D. Dissertation: Pharmacognostic investigation of black cohosh (Cimicifuga racemosa (L.) Nutt) p. 210. [Google Scholar]
- 38.Webster DE, Lu J, Chen SN, Farnsworth NR, Wang ZJ. Activation of the mu-opiate receptor by Vitex agnus-castus methanol extracts: implication for its use in PMS. J Ethnopharmacol. 2006;106:216–221. doi: 10.1016/j.jep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, et al. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu J, Jeon E, Lee BS, Onyuksel H, Wang ZJ. Targeted drug delivery crossing cytoplasmic membranes of intended cells via ligand-grafted sterically stabilized liposomes. J Control Release. 2006;110:505–513. doi: 10.1016/j.jconrel.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 42.Grady D, Wenger NK, Herrington D, Khan S, Furberg C, et al. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132:689–696. doi: 10.7326/0003-4819-132-9-200005020-00002. [DOI] [PubMed] [Google Scholar]
- 43.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 44.Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 45.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 46.Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- 47.Delitala G, Trainer PJ, Oliva O, Fanciulli G, Grossman AB. Opioid peptide and alpha-adrenoceptor pathways in the regulation of the pituitary-adrenal axis in man. J Endocrinol. 1994;141:163–168. doi: 10.1677/joe.0.1410163. [DOI] [PubMed] [Google Scholar]
- 48.Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215–2222. doi: 10.1210/jcem.85.6.6615. [DOI] [PubMed] [Google Scholar]
- 49.Genazzani AR, Petraglia F. Opioid control of luteinizing hormone secretion in humans. J Steroid Biochem. 1989;33:751–755. doi: 10.1016/0022-4731(89)90487-1. [DOI] [PubMed] [Google Scholar]
- 50.Acosta-Martinez M, Etgen AM. Estrogen modulation of mu-opioid receptor-stimulated [35S]-GTP-gamma-S binding in female rat brain visualized by in vitro autoradiography. Neuroendocrinology. 2002;76:235–242. doi: 10.1159/000065953. [DOI] [PubMed] [Google Scholar]
- 51.Acosta-Martinez M, Etgen AM. Activation of mu-opioid receptors inhibits lordosis behavior in estrogen and progesterone-primed female rats. Horm Behav. 2002;41:88–100. doi: 10.1006/hbeh.2001.1741. [DOI] [PubMed] [Google Scholar]
- 52.Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:117–125. doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- 53.Kaufman JM, Kesner JS, Wilson RC, Knobil E. Electrophysiological manifestation of luteinizing hormone-releasing hormone pulse generator activity in the rhesus monkey: influence of alpha-adrenergic and dopaminergic blocking agents. Endocrinology. 1985;116:1327–1333. doi: 10.1210/endo-116-4-1327. [DOI] [PubMed] [Google Scholar]
- 54.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 55.Kronenberg F, Downey JA. Thermoregulatory physiology of menopausal hot flashes: a review. Can J Physiol Pharmacol. 1987;65:1312–1324. doi: 10.1139/y87-208. [DOI] [PubMed] [Google Scholar]
- 56.Simpkins JW, Katovich MJ, Song IC. Similarities between morphine withdrawal in the rat and the menopausal hot flush. Life Sci. 1983;32:1957–1966. doi: 10.1016/0024-3205(83)90047-4. [DOI] [PubMed] [Google Scholar]
- 57.Katovich MJ, Simpkins JW, O’Meara J. Effects of acute central LH-RH administration of the skin temperature response in morphine dependent rats. Brain Res. 1989;494:85–94. doi: 10.1016/0006-8993(89)90146-7. [DOI] [PubMed] [Google Scholar]