Abstract
Cancer is associated with a pro-thrombogenic state capable of platelet activation. Platelets on the other hand can support angiogenesis, a process involved in the progression of tumor growth and metastasis. However, it is unclear whether platelet/tumor interactions substantially contribute to tumor physiology. We investigated whether platelets stabilize tumor vessels and studied the underlying mechanisms. We induced severe acute thrombocytopenia in mice bearing subcutaneous Lewis lung carcinoma or B16F10 melanoma. Intravital microscopy revealed that platelet depletion led to a rapid destabilization of tumor vessels with intratumor hemorrhage starting as soon as 30 minutes after induction of thrombocytopenia. Using an inhibitor of GPIbα and genetically engineered mice with platelet adhesion defects, we investigated the role of platelet adhesion receptors in stabilizing tumor vessels. We found that a single defect in either GPIbα, VWF, P-selectin, or platelet integrin activation did not lead to intratumor hemorrhage. We then compared the ability of transfused resting and degranulated platelets to prevent intratumor hemorrhage. While resting platelets prevented thrombocytopenia-induced tumor bleeding, circulating degranulated platelets did not. This suggests that the prevention of intratumor hemorrhage by platelets relies on the secretion of platelet granules’ content. Supporting this hypothesis, we further found that thrombocytopenia dramatically impairs the balance between propermeability and antipermeability factors in tumor-bearing animals, in particular depleting blood of angiopoietin-1 and serotonin. Our results show a crucial contribution of platelets to tumor homeostasis through continuous prevention of severe intratumor hemorrhage and consequent cell death. The study also suggests platelet function as a reasonable target for specific destabilization of tumor vessels.
Keywords: primary tumor, platelet, intratumor hemorrhage, thrombocytopenia, platelet granules
Introduction
By 1865, Armand Trousseau had already reported the association between cancer and thrombosis (1). The thrombogenic properties of tumors have since been widely studied. Tumor cells promote coagulation and inflammation through various mechanisms including overexpression of tissue factor (2), the initiator of the coagulation cascade, secretion of pro-inflammatory cytokines (3, 4) and of metalloproteinases (5) which lead to endothelial activation, an important step in thrombosis. In addition, sluggish blood flow, hyperpermeability and discontinuous endothelial lining are recognized features of tumor microcirculation (6, 7) that may also contribute to the tumor thrombogenic environment. This thrombogenic environment could activate platelets, the major orchestrators of coagulation and thrombosis.
Platelets may influence cancer progression. Both depletion of platelets and anti-platelet treatments have been shown to reduce the number of experimental metastases, indicating that platelets support the metastatic process (8–10). Various mechanisms have been proposed to explain this effect. Coating of circulating cancer cells with platelets may protect cancer cells from the immune response (8) and facilitation of cancer cell adhesion to leukocytes and endothelial cells by platelets may promote the essential step of extravasation in the metastatic process (8, 10, 11). Several studies have shown that platelets enhance the formation of capillary-like structures by endothelial cells in vitro (12) and angiogenesis in vivo (13), a process that is essential to tumor growth and metastasis. Platelets are a rich source of pro- and anti-angiogenic factors (VEGF (14), platelet-derived growth factor (PDGF) (15), basic FGF (bFGF) (16), EGF (17), TGF (18), insulin-like growth factors (19), angiopoietin-1 (20), sphingosine-1-phosphate (21), matrix metalloproteinases (22), thrombospondin I (23), platelet factor 4 (24), plasminogen activator inhibitor I (25) endostatin (26) and angiostatin (27)) that are released upon platelet activation. These pro- and antiangiogenic factors have been shown to be mostly organized into separate platelet α-granules that could be differentially released, suggesting that platelets may actively stimulate or inhibit angiogenesis (28).
Recently, we showed that platelets and their adhesion support angiogenesis in vivo in experimental models of angiogenesis. Platelets prevented excessive hemorrhage from the growing vessels in Matrigel and corneal micropocket assays (29). We now investigated the contribution of platelets to the function of tumor vessels and the mechanisms involved. We found that platelets continuously support tumor vascular homeostasis by regulating the stability of tumor vessels through the secretion of their granules’ content.
Materials and methods
Reagents
Fetal Bovine Serum (FBS) was from the American Type Culture Collection (ATCC, Rockville, MD). Penicillin/streptomycin and high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) were from Gibco/Invitrogen (Carlsbad, CA). Medium titanium skinfold chambers were from APJ Trading Co. (Ventura, CA). Polyclonal anti-GPIbα rat IgG R300 and polyclonal non-immune rat IgG C301 were from emfret Analytics (Eibelstadt, Germany). Hematoxylin, eosin, prostacyclin (PGI2), trypsin-EDTA and Drabkin’s reagent were from Sigma-Aldrich (St. Louis, MO). O-sialoglycoprotein endopeptidase was from Cedarlane Laboratories (Hornby, ON, Canada). 4′, 6-diamino-2-phenylindole (DAPI) was from Molecular Probes Invitrogen (Eugene, OR). GPG-290, an inhibitor of the vWF/GPIbα interaction (30) was prepared by Wyeth Research (Cambridge, MA) and was a kind gift of Dr. Gray D. Shaw (Wyeth Research, Cambridge, MA).
Animals
All animal procedures described in this study were performed using 6 to 8 week old C57Bl/6J female mice (purchased from the Jackson Laboratory, Bar Harbor, ME) except in experiments using dorsal skinfold chamber for which 12-weeks-old C57BL/6J male mice were used. Mice deficient in VWF, P-selectin and CalDAG-GEFI were bred and housed in our animal facility. All experimental procedures involving the use of mice were approved by the Animal Care and Use Committee of the Immune Disease Institute.
Cell culture
Murine B16F10 melanoma cells (CRL-6475) and Lewis lung carcinoma cells (LLC, CRL-1642) were purchased from ATCC. Cells were cultured at 37°C in a humidified atmosphere of 5 % CO2, high glucose (4.5 g/L) DMEM supplemented with 10 % fetal bovine serum and 1 % glutamine and were used by passage 10 for implantation into syngenic C57BL/6J recipient mice.
Tumor cell implantation
100 μL of either LLC or B16F10 melanoma cells at 1.0 × 107/mL in Dulbecco’s phosphate-buffered saline (PBS) were injected subcutaneously into the back of 6 to 8 week old C57BL/6J female mice.
Induction of thrombocytopenia
Thrombocytopenia was induced at indicated time points following tumor cell implantation by an intravenous injection of 2.5 μg/g mouse of the platelet-depleting antibody (8) (polyclonal anti-mouse GPIbα rat IgG, emfret Analytics). Control mice were injected with a non-immune rat polyclonal IgG (emfret Analytics). Thromboctyopenia was evaluated by flow cytometry.
The intravenous injection of the depleting antibody resulted in ≥ 97 % reduction in circulating platelets at 1 hour post-injection in all mice.
Determination of intratumor hemoglobin content
Tumors were excised from the back of the sacrificed animals, weighed, homogenized in Drabkin’s reagent (Sigma), centrifuged (2000 × g; 10 min) and hemoglobin content of supernatants was measured by absorbance reading at 540 nm.
Immunohistology of LLC tumors
Subcutaneous LLC tumors were harvested from sacrificed animals, fixed in zinc-fixative (100 mM Tris-HCl containing 37 mM zinc chloride, 23 mM zinc acetate, 3.2 mM calcium acetate), paraffin-embedded and sectioned. Tumor sections were stained with hematoxylin and eosin (H&E) or with Terminal Deoxynucleotidyltransferase-mediated dUTP Nick End Labeling (TUNEL, Roche Applied Science, Indianapolis, IN) to visualize DNA fragmentation. Tumor cell mitosis was quantified by immunostaining of bromodeoxyuridine (BrdUrd) incorporation using a BrdUrd labeling and detection kit (Roche Applied Science). Tumor sections stained for BrdUrd incorporation or for TUNEL were counterstained with DAPI to visualize all nuclei. After washing, the slides were mounted with Gel/Mount aqueous mounting medium (Biomeda, Foster City, CA) and observed under an epifluorescence microscope. The proliferative and apoptotic indexes were calculated as the percentage of either BrdUrd or TUNEL positive nuclei relative to DAPI-stained nuclei, respectively. For HE staining, slides were mounted with DPX Mountant (Fluka BioChemika, Buchs, Switzerland) and observed in light microscopy.
Metastasis
Subconfluent B16F10 melanoma cells (70–80%) were washed with PBS and detached by brief exposure to 0.25% trypsin and 0.2% EDTA. Cells were washed twice with PBS, resuspended in serum-free medium and kept on ice until injection. 100 μL of a tumor cell (1 × 105 cells) suspension were injected to the lateral tail vein of mice. Ten days later, mice were injected with either the control IgG or the platelet-depleting IgG. The day following the induction of thrombocytopenia, lungs were harvested, perfused and washed with PBS and photographed.
Tail bleeding time
Vehicle (PBS) or GPG-290 (5 mg/kg mouse) was injected intravenously at day 8 following subcutaneous LLC cells implantation and tail bleeding time was assessed the following day, prior to tumor excision. Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) before cutting 3-mm of the distal tip of the tail using a sharp razor blade. The tail was immediately immersed in 37°C PBS and the time required for the bleeding to stop determined. If bleeding did not recur within 15 seconds of cessation, it was considered to have stopped. Experiments were terminated after 15 minutes if no cessation of blood flow occurred.
In vivo imaging of LLC
Dorsal skinfold chambers and surgical preparation were performed as described (31), After 2 days of recovery, 5 × 105 LLC cells were implanted in the conjunctive tissue below the striated skin muscle layer of the remaining skin layer and allowed to grow for 5 days.
Mice were then injected intravenously with 100 μL of 5 % Evans blue and tumors were observed through the dorsal skinfold chamber for 3 hours starting from the injection of either the control or the platelet-depleting antibody. During in vivo microscopy, mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. Light microscopy imaging was performed using an upright microscope (Axioplan; Zeiss, Oberkochen, Germany) with a 2.5 × magnification objective and recorded by a digital camera (AxioCam HSc) attached to it. Data acquisition was done with the time-lapse function in the software from the same manufacturer (Axiovision 4.6.3).
Quantification of VEGF, angiopoietin-1 and serotonin levels
VEGF levels in platelet-poor plasma (PPP), serum and tumor were assayed using an ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Angiopoietin-1 levels were quantified by ELISA using glutaraldehyde-immobilized recombinant mouse Tie2-Fc chimera (R&D Systems) for capture and a goat polyclonal to angiopoietin-1 (Santa Cruz Biotechnology, Santa Cruz, CA) for detection. In addition, PPP and serum samples from control and platelet-depleted mice were analyzed by SDS-PAGE (10 %, reducing conditions) and Western blotting with a goat polyclonal antibody to angiopoietin-1 (Santa Cruz Biotechnology). Serotonin levels were quantified by enzyme immunoassay (EIA) according to the manufacturer’s instructions (Labor Diagnostika Nord GmbH & Co. KG, Nordhorn, Germany).
Platelet preparation
Donor mice were bled from the retro-orbital venous plexus under anesthesia. Blood was collected into polypropylene tubes containing 7.5 U/mL heparin. Platelet-rich plasma (PRP) was obtained by centrifugation at 200 × g for 5 minutes at room temperature. The PRP was incubated for 2 minutes with PGI2 (0.1 μg/mL) and platelets were isolated by centrifugation at 850 × g for 5 minutes. The resulting pellet was washed and resuspended in Tyrode’s buffer (137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 5.5 mM glucose, 5 mM Hepes, pH 7.3) containing 0.35 % bovine serum albumin.
Treatment of platelets with O-Sialoglycoprotein endopetidase
In order to prevent clearance of transfused platelets by the anti-GPIbα antibody, the external part of GPIbα was removed prior to transfusion by treating the platelets with O-sialoglycoprotein endopeptidase as previously described (32). Briefly, washed platelets were resuspended in Tyrode’s buffer containing 1mM CaCl2 and incubated at 37°C for 30 minutes with 250 μg/ml O-sialoglycoprotein endopeptidase. Aliquots of the platelet suspensions were analyzed by flow cytometry to assess the removal of the N-terminal domain of GPIbα alpha using a FITC-conjugated antibody directed against the extracellular domain of GPIbα (emfret Analytics).
Activation of platelets
For activation, platelets pre-treated with O-Sialoglycoprotein endopetidase were stimulated with human thrombin (1 U/mL) for 10 minutes at 37°C in the presence of 2 mM EDTA to avoid aggregation. Hirudin (2 U/mL) was added to stop the reaction. Platelet degranulation was confirmed by expression of P-selectin using FITC-conjugated anti–P-selectin antibody (BD Pharmingen). EDTA and hirudin were also added to resting platelet preparations. Aliquots of resting and activated platelets supernatant were analyzed for angiopoietin-1 content by ELISA.
Statistical Analysis
Data are presented as mean ± SEM and were analyzed by analysis of variance (ANOVA) and by unpaired, two-tailed Student’s t test. P values <0.05 were regarded as statistically significant.
Results
Acute thrombocytopenia induces severe tumor hemorrhage independent of the tumor type, age, and location
Platelet depletion was induced in mice bearing subcutaneous tumors at days 4, 8, and 12 following LLC tumor cell implantation. Eighteen hours after the induction of platelet depletion, the mice treated with the platelet-depleting antibody had less than 2.5 ± 0.9 % of normal platelet count whereas platelet number was unaffected in mice treated with the control IgG (94 ± 7 % of normal platelet count) as compared to non tumor-bearing untreated control mice. Morphological examination and H&E staining of the subcutaneous tumors revealed extensive hemorrhage in and around all tumors of platelet-depleted mice but not in mice treated with control IgG (Fig. 1A and B). As illustrated in Fig. 1B, massive accumulation of red blood cells was observed at the interface of the tumor and the adjacent connective tissue in the platelet-depleted mice. Hemorrhage was not seen anywhere in areas distant from the tumor in the thrombocytopenic mice.
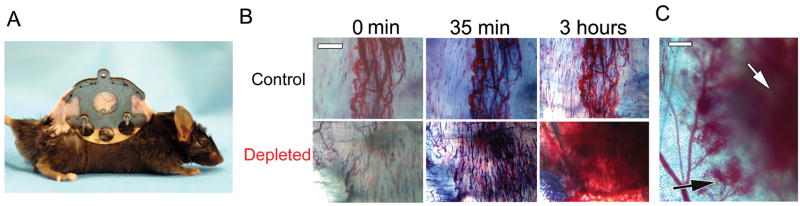
Figure 1. Acute thrombocytopenia induces tumor bleeding independently of the tumor type, age, and location.
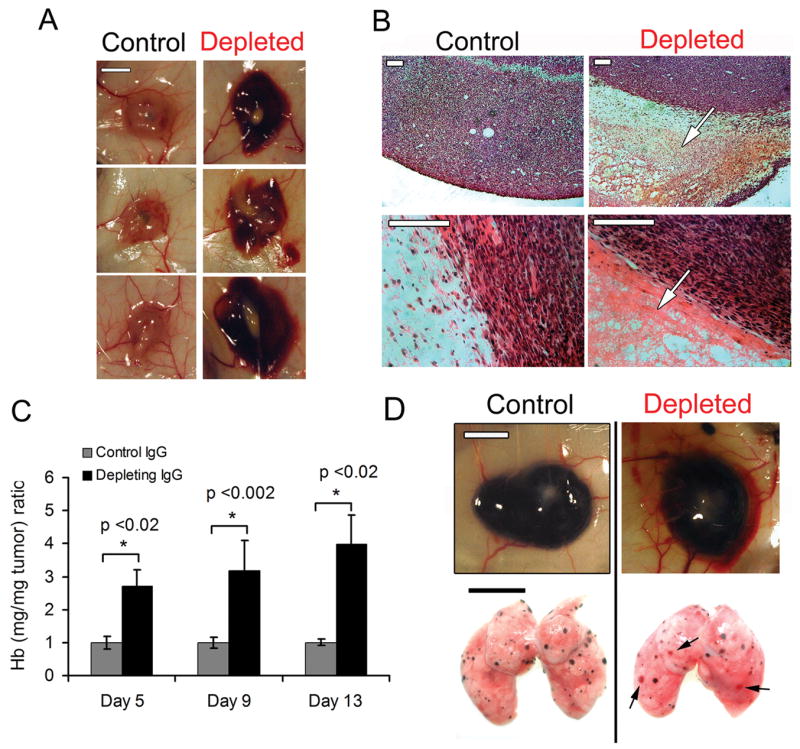
A. At day 8 after subcutaneous implantation of LLC tumor cells, mice were injected with either the control IgG (control) or the platelet-depleting IgG (depleted) and tumors were photographed 18 hours later. Bar = 5 mm. B. H&E staining of the LLC tumors showed massive accumulation of red blood cells in the tumor stroma only in platelet-depleted mice (arrows). Bars = 100 μm. C. Thrombocytopenia was induced at day 4, 8, or 12 following subcutaneous LLC implantation. 18 hours later, intratumor hemoglobin content was determined and compared to control IgG-treated tumors (n = 4). D. 10 days following either subcutaneous (upper panel) or intravenous (lower panel) injection of B16F10 melanoma cells, mice were injected with either the control IgG (control) or the platelet-depleting IgG (depleted). 18 hours later, photographs of skin and lungs were taken. Hemorrhage was observed only in tumors from platelet-depleted mice (arrows). Bars = 5 mm.
Thrombocytopenia-induced tumor bleeding was independent of the age of the tumor. Induction of thrombocytopenia at either day 4, 8 or 12 after tumor cell implantation invariably resulted 18 hours later in a 2 to 3 fold increase in intratumor hemoglobin content as compared to tumors from control mice with normal platelet counts (Fig. 1C). This indicates that platelets are required continuously to prevent hemorrhage from primary tumor vessels. Interestingly, induction of acute severe thrombocytopenia also resulted in hemorrhage in subcutaneously implanted B16F10 melanoma and in established B16F10 melanoma lung metastasis (Fig. 1D), indicating that the requirement of platelets for the prevention of intratumor hemorrhage was likely independent of the tumor type, age, and location.
Intravital observation of subcutaneous LLC tumors through a dorsal skinfold chamber (Fig. 2A) revealed that plasma protein leakage, detected by extravasation of Evans blue, occurs continuously and excessively in both control and thrombocytopenic mice (Fig. 2B and Suppl. Movies). In contrast, tumor hemorrhage was observed only in the thrombocytopenic mice. First signs of tumor hemorrhage occurred as soon as 35 minutes following the injection of the depleting antibody (Fig. 2B and Suppl. Movie 2). In depleted animals, plasma protein leakage and hemorrhage were observed both from intratumor vessels and from post-capillary venules directly surrounding the tumor (Fig. 2B and C). Altogether, these results show that platelets continuously help prevent excessive tumor vessel fragility.
Figure 2. Kinetics and localization of tumor bleeding in platelet-depleted mice.
A. Mouse carrying a dorsal skinfold chamber. B. Mice bearing 5 day old LLC tumors were injected with Evans blue and either the control (upper panel) or the platelet-depleting antibody (lower panel) at time 0. Tumors were observed through the dorsal skinfold window for 3 hours. Times post-infusion are indicated. Bar = 500 μM. C. LLC tumor viewed 3 hours after induction of thrombocytopenia. White arrow: intratumor hemorrhage, black arrow: hemorrhage occurring from vessels surrounding the tumor. Bar = 500 μM.
Acute thrombocytopenia reduces tumor cell proliferation and increases tumor necrosis
Induction of acute thrombocytopenia leads to severe tumor hemorrhage, an event that is likely to affect tumor cell viability. We therefore studied the effect of acute thrombocytopenia on tumor growth and survival. Mice bearing 8 day old LLC tumors were injected with either the control or the platelet-depleting antibody and tumors were allowed to grow for two more days. No significant differences in wet and dry weight could be found between the excised tumors of thrombocytopenic and control mice (not shown). Interestingly however, quantitation of tumor cell mitosis by BrdUrd incorporation and in situ immunostaining revealed a decrease in the proliferative index of tumors from thrombocytopenic mice as compared to tumors from control mice (0.9 % ± 0.14 vs 2.3 % ± 0.47, p<0.004). Less proliferation was observed in areas both distant and proximal to the hemorrhage (Fig. 3A).
Figure 3. Thrombocytopenia reduces cancer cell proliferation and locally affects tumor viability.
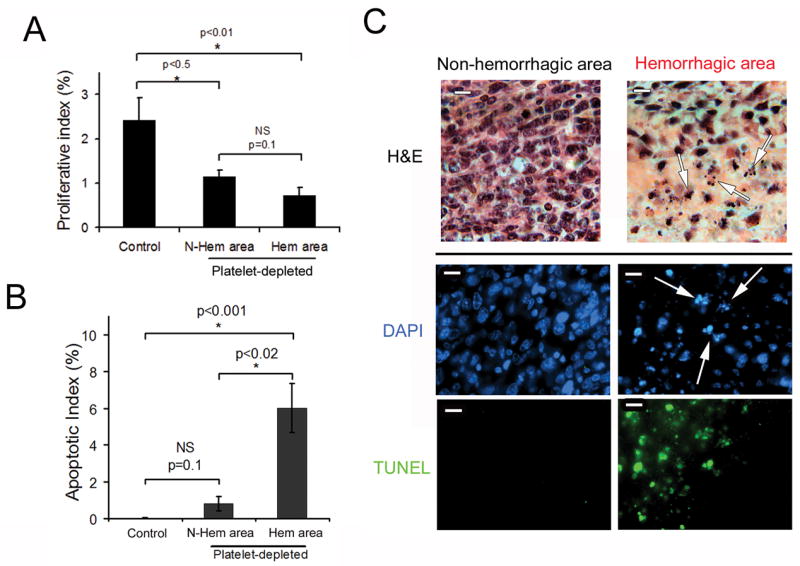
At day 8 after subcutaneous implantation of LLC tumors, thrombocytopenia was induced and 48 hours later, tumors were harvested and sectioned. A. Mice were injected with BrdUrd 3 hours before sacrifice and the proliferative index was calculated as the percentage of BrdUrd positive nuclei relative to DAPI-stained nuclei (n = 10 microscopic fields out of 4 tumors for each). B. The apoptotic index was calculated as the percentage of TUNEL positive nuclei relative to DAPI-stained nuclei (n = 10 microscopic fields out of 5 tumors for each). C. H&E, DAPI and TUNEL staining of non-hemorrhagic and hemorrhagic areas of the LLC tumors. Arrows indicate fragmented and condensed nuclei. Bars = 20 μm.
H&E staining of LLC tumors from thrombocytopenic mice revealed that tumor necrosis could be observed in the area next to the hemorrhage as indicated by morphological changes such as nuclear condensation and fragmentation (Fig. 3C, upper panel). This observation was further confirmed by quantitation of apoptotic cells by TUNEL staining (Fig. 3B and C). The TUNEL apoptotic index in the non-hemorrhagic areas of tumors from platelet-depleted mice (0.8 ± 0.9 %) was not significantly different (p=0.1) from that of tumors from control mice (0.1 ± 0.05 %). In contrast, the TUNEL apoptotic index in the hemorrhagic areas of tumors from platelet-depleted mice was significantly increased as compared to non-hemorrhagic regions (6.01 ± 4.03 %, p<0.001, Fig. 3B). This indicates that thrombocytopenia-induced tumor bleeding is injurious to the cancer cells.
Prevention of tumor-associated hemorrhage by platelets does not rely on their ability to form thrombi
We asked whether the continuous requirement of platelets to prevent tumor hemorrhage was dependent on their capacity to adhere to the vessel wall and to form thrombi. To determine this, we first studied the effect of GPG-290, a soluble competitive inhibitor of the platelet GPIbα/VWF interaction (30), on intratumor hemoglobin content. GPIbα-mediated platelet adhesion was previously found by our group to play a role in preventing hemorrhage during experimental angiogenesis not associated with tumors (29). However, although the GPG-290-treated mice could not arrest their bleeding as indicated by their increased tail bleeding time (Fig. 4A), no increase in their tumor hemoglobin content was found as compared to control mice (Fig. 4B). This indicates that prevention of tumor-associated hemorrhage by platelets is independent of platelet GPIbα/VWF interaction. This was further confirmed by the absence of severe hemorrhage in LLC tumors implanted in VWF−/− mice (0 hemorrhage in 5 tumors examined) that also have prolonged tail bleeding time (33). Besides VWF, P-selectin mediates platelet rolling on the activated vessel wall (34). As for VWF−/− mice, no severe hemorrhage was found in LLC tumors grown in P-selectin−/− mice (0 hemorrhage in 6 tumors examined), thus indicating that P-selectin was also not crucial for the prevention of intratumor hemorrhage by platelets.
Figure 4. Prevention of tumor hemorrhage by platelets is independent of platelet GPIbα.
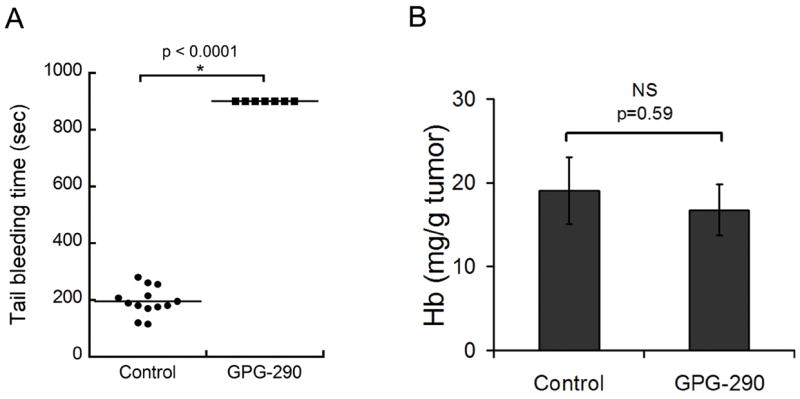
PBS or the GPIbα chimera (GPG-290) was injected intravenously at day 8 following subcutaneous LLC tumors implantation. A. Tail bleeding time was assessed 18 hours after GPG-290 injection. B. Comparison of hemoglobin content of control and GPG-290-treated tumors (n = 5). No difference in hemoglobin content was found between the two groups.
Mouse platelets lacking CalDAG-GEFI are severely compromised in integrin-dependent platelet aggregation since CalDAG-GEFI is a key signal integrator in the cascade leading to the activation of the integrin αIIbβ3 (35). LLC tumors grown in CalDAG-GEFI−/− mice did not show hemorrhage (0 hemorrhage in 7 tumors examined) as compared to LLC tumors grown in wild-type mice. This suggests that platelet integrin activation is also not required to prevent hemorrhage from angiogenic tumor vessels. Moreover, immunofluorescent staining using anti-GPIbα and anti-αIIbβ3 antibodies as well as H&E staining of Lewis lung carcinoma sections did not reveal any platelet aggregates or significant numbers of platelets adhering to the lumens of tumor vessels (not shown). Altogether, these results suggest that the classical mechanisms of platelet adhesion and aggregation involved in primary hemostasis/thrombus formation might not be required for the prevention of intratumor hemorrhage by platelets.
Thrombocytopenia leads to an altered balance between pro- and anti-permeability factors
Platelets are an important source of angiogenic factors and of regulators of vascular permeability such as VEGF, angiopoietin-1 and serotonin (14, 20, 36). Both VEGF and angiopoietin-1 have been previously reported to affect blood vessel maturation and stability as well as vascular permeability in adult mice (37). We therefore investigated whether the absence of platelets could substantially affect the concentrations of these factors in platelet-poor plasma, serum and tumor, thus resulting in a modification of the balance between pro- and anti-permeability factors. VEGF was found most abundantly in tumors and its levels in platelet-poor plasma, serum and tumor were not significantly affected by thrombocytopenia (Fig. 5A). Angiopoietin-1 and serotonin were mostly found in serum in tumor-bearing mice (Fig. 5B and C) and were found in similar levels in serum of non-tumor-bearing animals (not shown). In contrast to VEGF (Fig. 5A), angiopoietin-1 and serotonin levels in serum were dramatically decreased in tumor-bearing thrombocytopenic mice as compared to tumor-bearing control mice (Fig. 5B and C). Similarly, thrombocytopenia led to the disappearance of serum angiopoietin-1 and serotonin in mice without tumors (not shown). This indicates that the serotonin- and angiopoietin-1-load in platelets accounts for the majority of the circulating levels of these two key regulators of vascular permeability. Thus, whereas the potent pro-permeability factor VEGF is consistently produced by the LLC tumor and relatively little is found in plasma or serum, the two anti-permeability factors angiopoietin-1 and serotonin are primarily found in platelets and not in tumors. Our results document that severe acute thrombocytopenia leads to an altered balance between the platelet-derived pool of anti-permeability factors and the tumor-derived pro-permeability factor VEGF, also known as vascular permeability factor.
Figure 5. Platelet depletion effects on serum concentrations of VEGF, angiopoietin-1 and serotonin.
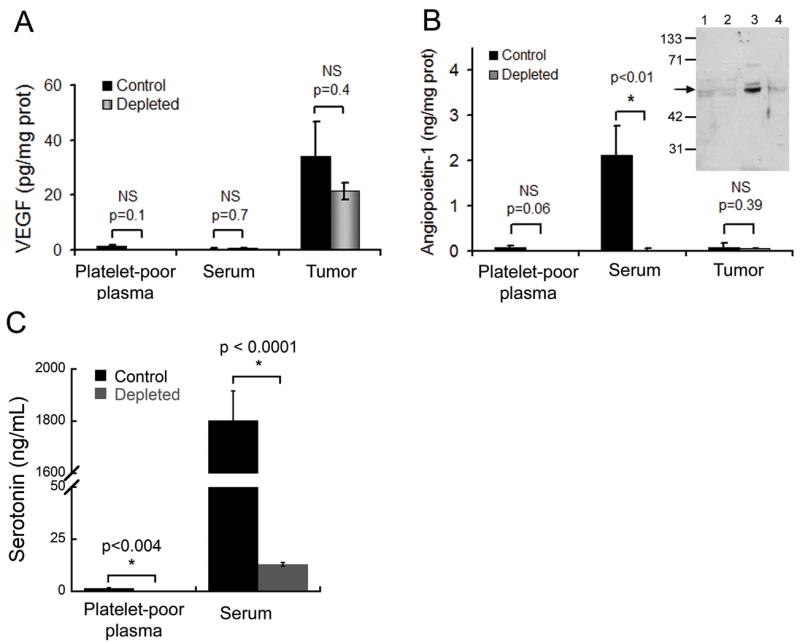
A. Comparison of VEGF levels in LLC tumor-bearing control and platelet-depleted mice (n = 5). B. Comparison of angiopoietin-1 levels between control and platelet-depleted mice (n = 5). Inset. Western blot detection of angiopoietin-1. Lane 1: platelet poor plasma from control mouse, lane 2: plasma from platelet-depleted mouse, lane 3: serum from control mouse, lane 4: serum from platelet-depleted mouse. Arrow indicates angiopoietin-1. C. Comparison of serotonin levels between control and platelet-depleted mice (n = 4–6). Platelet depletion led to disappearance of serotonin and angiopietin-1 from serum without affecting VEGF levels.
Degranulated platelets are unable to prevent thrombocytopenia-induced tumor bleeding
In order to investigate the role of platelet granules’ content in preventing tumor hemorrhage, we compared the ability of transfused resting platelets and thrombin-stimulated platelets (with released granules) to rescue tumor bleeding in thrombocytopenic tumors. Thrombin-activated platelets have been previously shown to rapidly lose surface P-selectin when transfused but to continue to circulate and function (38, 39). To avoid clearance of the transfused platelets by the depleting anti-GPIbα antibody, platelets lacking the extracellular domain of GPIbα were used. The removal of the extracellular domain of GPIbα was performed with O-sialoglycoprotein endopeptidase prior to transfusion (32). Degranulation of thrombin-stimulated platelets was verified by FACS analysis of P-selectin surface expression and by the presence of angiopoietin-1 in platelet supernatants (Fig. 6A and B). While transfusion of resting platelets could prevent tumor bleeding in thrombocytopenic mice, transfusion of degranulated (P-selectin-positive) platelets could not (Fig. 6C and D), indicating that prevention of tumor vessel bleeding by platelets likely relies on the local release of a soluble factor from platelet granules rather than on the formation of platelet plugs.
Figure 6. Degranulated platelets are unable to prevent thrombocytopenia-induced tumor bleeding.
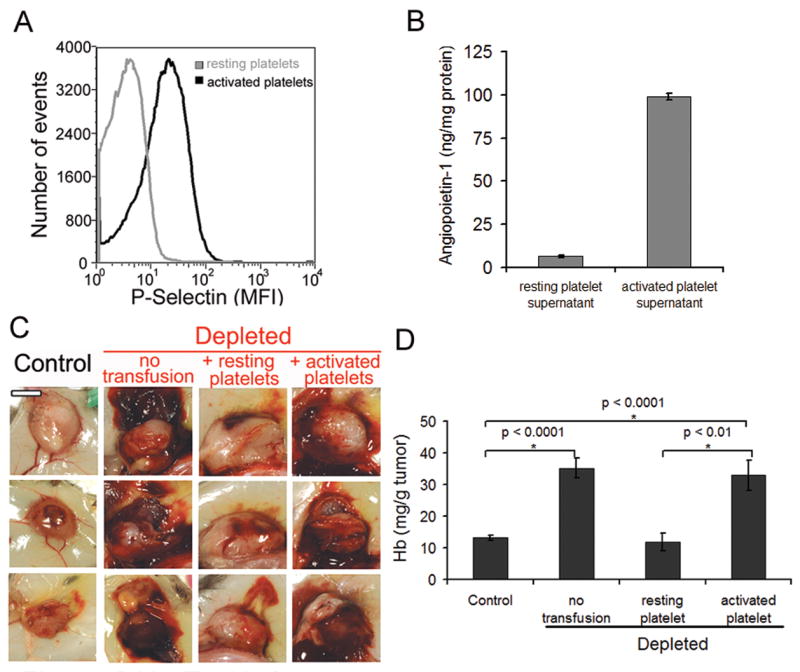
A. Degranulation of thrombin-stimulated platelets was assessed by FACS analysis of P-selectin surface expression and by B. Quantitation of angiopoietin-1 in platelet supernatants by ELISA. C. At day 8 after tumor cells implantation, mice were injected with either the control IgG (control) or the platelet-depleting IgG (depleted). A subset of mice was transfused 30 minutes prior to the induction of thrombocytopenia with tyrode buffer (no transfusion) or with 7 × 108 of either resting (resting platelets) or activated platelets (activated platelets) and subcutaneous LLC were photographed 18 hours later. Bar = 5 mm. D. Comparison of the hemoglobin content of control tumors and platelet-depleted tumors from mice transfused with either tyrode buffer, resting platelets, or activated platelets (n = 17–20).
Discussion
In the present report, we addressed the contribution of platelets to tumor vascular homeostasis. We show that platelets are crucial regulators of tumor vessel stability that are continuously needed to prevent severe tumor hemorrhage. In fact, absence of platelets leads to an immediate destabilization of tumor vessels with intratumor hemorrhage starting within the first hour following the induction of thrombocytopenia. This thrombocytopenia-induced tumor bleeding appears to be independent of the tumor type and location since it could be seen in subcutaneous LLC tumors, B16F10 melanoma, and in lung metastasis (Fig. 1). We recently reported that absence of platelets leads to high susceptibility to hemorrhage in blood vessels during inflammation (40). In this previous study, we showed that in thrombocytopenic mice inflammation induces hemorrhage at the inflamed site indicating that platelets prevent tissue-damaging hemorrhage in inflamed organs (40). Solid tumors often show signs of chronic inflammation. These include the presence of leukocyte infiltration, the expression of cytokines such as tumor necrosis factor-α or interleukin-1, chemokines and active tissue remodeling (41, 42). We hypothesize that the requirement of platelets for the prevention of tumor hemorrhage might not only include their supportive role in angiogenesis (29) but also their ability to prevent inflammation-induced vascular injury (40).
Platelets are known to carry biologically active agents, such as, angiopoietin-1 and serotonin, that have been shown to promote endothelial integrity and barrier function in vitro and in vivo (36, 37, 43–46). Angiopoietin-1 is known to stabilize blood vessels, inhibit vascular permeability and to have anti-inflammatory properties (47–49). Serotonin was shown to prevent red blood cell extravasation in thrombocytopenic hamsters (46). In contrast, tumors release destabilizing factors, the most prominent being VEGF, and activated endothelium, as would be found in tumors, secretes angiopoietin-2 another potent destabilizer of vasculature (50). Angiopoeitin-2 action is through competitive inhibition of angiopoietin 1 (50). We hypothesize that the balance between the tumor and platelet-derived agents is regulating tumor vessel stability. Indeed, our study reveals the crucial contribution of the platelet-derived products in the prevention of tumor hemorrhage. We show that platelet depletion leads to a dramatic decrease in both angiopoietin-1 and serotonin levels in serum, while VEGF levels remain unaffected. This illustrates that severe acute thrombocytopenia leads to an impaired balance between available pro- and anti-permeability factors that may contribute to tumor vessel destabilization. It is of note, however, that systemic intravenous infusion of platelet releasate did not prevent tumor bleeding in thrombocytopenic mice (not shown). We speculate that the platelet-derived factors responsible for tumor vessel stabilization have to be delivered by platelets to the tumor site for optimal activity.
Surprisingly, prevention of severe intratumor hemorrhage by platelets does not seem to require platelet plug formation, which relies on platelet adhesion receptors GPIbα/VWF and activation of the integrin αIIbβ3. Although CalDAG-GEFI−/−, VWF−/− or GPIbα inhibitor-treated mice all have a severe bleeding phenotype, manifesting a highly prolonged bleeding time upon injury, severe intratumor hemorrhage did not occur in any of these animals. Thus, similar to the prevention of hemorrhage in inflamed tissues (40), tumor vessel stabilization by platelets might require neither platelet adhesion to the vessel wall nor platelet aggregation. These observations are in agreement with those of Manegold and colleagues who could not detect an increase in platelet adhesion in tumor vessels (51). The fact that genetic inhibition of platelet adhesion and aggregation did not affect their capacity to stabilize tumor vessels raises questions about how platelets deliver vasoactive compounds to tumor vessels. As previously hypothesized by Folkman and colleagues (52), platelet/tumor interactions might be facilitated by the impaired blood flow (51, 53–55) and the localized granular release by the procoagulant environment of the tumor (56–58).
The identification of the tumor vessel stabilizing factor(s) delivered by platelets to solid tumors could lead to new therapeutic strategies. Inhibition of these platelet-derived factors may allow selective induction of tumor bleeding and thus decrease tumor viability and/or growth (Fig. 3) without affecting the immediate function of blood vessels in other tissues. Induction of tumor hemorrhage may also facilitate the selective delivery of chemotherapeutic agents to tumors and enhance antitumor immunity through better exposure of tumor antigens to circulating immune cells. Alternatively, mimicking the stabilizing effect of platelets on tumor vessels might help to normalize the tumor vasculature and its function, a strategy that was shown to have a synergistic effect when combined with cytotoxic therapy (55).
Supplementary Material
Mice were injected with Evan’s blue and LLC tumors were observed through a dorsal skinfold chamber for 3 hours starting from the injection of either the control (Movie 1) or the platelet-depleting (Movie 2) antibody.
Acknowledgments
We thank Dr. Rakesh K. Jain and Julia Kahn for teaching us the dorsal skinfold chamber method, and Dr. Robert Schaub for helpful discussion. The assistance of Lesley Cowan in the preparation of the manuscript is gratefully acknowledged.
Financial support: This work was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R37 HL041002 and P01 HL066105 to D.D.W.), La Fondation pour la Recherche Médicale (to B.H.), and from the Deutsche Forschungsgemeinschaft (GO1360 to T.G.).
References
- 1.Trousseau A. Phlegmatia alba dolens. Clinique Medicale de l’Hotel-Dieu de Paris. Vol. 3. Paris: JB Baillere et Fils; 1865. pp. 654–712. [Google Scholar]
- 2.Andoh K, Kubota T, Takada M, Tanaka H, Kobayashi N, Maekawa T. Tissue factor activity in leukemia cells. Special reference to disseminated intravascular coagulation. Cancer. 1987;59:748–54. doi: 10.1002/1097-0142(19870215)59:4<748::aid-cncr2820590414>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Falanga A, Marchetti M, Giovanelli S, Barbui T. All-trans-retinoic acid counteracts endothelial cell procoagulant activity induced by a human promyelocytic leukemia-derived cell line (NB4) Blood. 1996;87:613–7. [PubMed] [Google Scholar]
- 4.Gianni M, Norio P, Terao M, et al. Effects of dexamethasone on pro-inflammatory cytokine expression, cell growth and maturation during granulocytic differentiation of acute promyelocytic leukemia cells. Eur Cytokine Netw. 1995;6:157–65. [PubMed] [Google Scholar]
- 5.Goerge T, Barg A, Schnaeker EM, et al. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006;66:7766–74. doi: 10.1158/0008-5472.CAN-05-3897. [DOI] [PubMed] [Google Scholar]
- 6.Sun C, Jain RK, Munn LL. Non-uniform plasma leakage affects local hematocrit and blood flow: implications for inflammation and tumor perfusion. Ann Biomed Eng. 2007;35:2121–9. doi: 10.1007/s10439-007-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvorak HF, Nagy JA, Berse B, et al. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann N Y Acad Sci. 1992;667:101–11. doi: 10.1111/j.1749-6632.1992.tb51603.x. [DOI] [PubMed] [Google Scholar]
- 8.Nieswandt B, Bergmeier W, Rackebrandt K, Gessner JE, Zirngibl H. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood. 2000;96:2520–7. [PubMed] [Google Scholar]
- 9.Skolnik G, Ericson LE, Bagge U. The effect of thrombocytopenia and antiserotonin treatment on the lodgement of circulating tumor cells. A vital fluorescence microscopic, electron microscopic and isotope study in the rat. J Cancer Res Clin Oncol. 1983;105:30–7. doi: 10.1007/BF00391829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nierodzik ML, Klepfish A, Karpatkin S. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb Haemost. 1995;74:282–90. [PubMed] [Google Scholar]
- 11.Jain S, Zuka M, Liu J, et al. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A. 2007;104:9024–8. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pipili-Synetos E, Papadimitriou E, Maragoudakis ME. Evidence that platelets promote tube formation by endothelial cells on matrigel. Br J Pharmacol. 1998;125:1252–7. doi: 10.1038/sj.bjp.0702191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iba O, Matsubara H, Nozawa Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–25. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 14.Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94:663–8. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldin CH, Westermark B, Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981;193:907–13. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan DR, Chao FC, Stiles CD, Antoniades HN, Scher CD. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979;53:1043–52. [PubMed] [Google Scholar]
- 17.Ben-Ezra J, Sheibani K, Hwang DL, Lev-Ran A. Megakaryocyte synthesis is the source of epidermal growth factor in human platelets. Am J Pathol. 1990;137:755–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T, Tomita Y, Hirai R, Yamaoka K, Kaji K, Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985;133:1042–50. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- 19.Karey KP, Marquardt H, Sirbasku DA. Human platelet-derived mitogens. I Identification of insulinlike growth factors I and II by purification and N alpha amino acid sequence analysis. Blood. 1989;74:1084–92. [PubMed] [Google Scholar]
- 20.Li JJ, Huang YQ, Basch R, Karpatkin S. Thrombin induces the release of angiopoietin-1 from platelets. Thromb Haemost. 2001;85:204–6. [PubMed] [Google Scholar]
- 21.English D, Welch Z, Kovala AT, et al. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. Faseb J. 2000;14:2255–65. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 22.Galt SW, Lindemann S, Allen L, et al. Outside-in signals delivered by matrix metalloproteinase-1 regulate platelet function. Circ Res. 2002;90:1093–9. doi: 10.1161/01.res.0000019241.12929.eb. [DOI] [PubMed] [Google Scholar]
- 23.McLaren KM. Immunohistochemical localisation of thrombospondin in human megakaryocytes and platelets. J Clin Pathol. 1983;36:197–9. doi: 10.1136/jcp.36.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maione TE, Gray GS, Petro J, et al. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990;247:77–9. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- 25.Booth NA, Simpson AJ, Croll A, Bennett B, MacGregor IR. Plasminogen activator inhibitor (PAI-1) in plasma and platelets. Br J Haematol. 1988;70:327–33. doi: 10.1111/j.1365-2141.1988.tb02490.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma L, Elliott SN, Cirino G, Buret A, Ignarro LJ, Wallace JL. Platelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor release. Proc Natl Acad Sci U S A. 2001;98:6470–5. doi: 10.1073/pnas.111150798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurasz P, Alonso D, Castro-Blanco S, Murad F, Radomski MW. Generation and role of angiostatin in human platelets. Blood. 2003;102:3217–23. doi: 10.1182/blood-2003-02-0378. [DOI] [PubMed] [Google Scholar]
- 28.Italiano JE, Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet {alpha} granules and differentially released. Blood. 2008;111:1227–33. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kisucka J, Butterfield CE, Duda DG, et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci U S A. 2006;103:855–60. doi: 10.1073/pnas.0510412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennan JK, Swillo RE, Morgan GA, et al. Pharmacologic inhibition of platelet vWF-GPIb alpha interaction prevents coronary artery thrombosis. Thromb Haemost. 2006;95:469–75. doi: 10.1160/TH05-09-0640. [DOI] [PubMed] [Google Scholar]
- 31.Leunig M, Yuan F, Menger MD, et al. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992;52:6553–60. [PubMed] [Google Scholar]
- 32.Bergmeier W, Bouvard D, Eble JA, et al. Rhodocytin (aggretin) activates platelets lacking alpha(2)beta(1) integrin, glycoprotein VI, and the ligand-binding domain of glycoprotein Ibalpha. J Biol Chem. 2001;276:25121–6. doi: 10.1074/jbc.M103892200. [DOI] [PubMed] [Google Scholar]
- 33.Denis C, Methia N, Frenette PS, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95:9524–9. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A. 1995;92:7450–4. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crittenden JR, Bergmeier W, Zhang Y, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–6. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 36.D’Amore P. First annual Lamport award manuscript. Platelet--endothelial interaction and the maintenance of the microvasculature. Microvasc Res. 1978;15:137–45. doi: 10.1016/0026-2862(78)90014-6. [DOI] [PubMed] [Google Scholar]
- 37.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 38.Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood. 2005;106:2334–9. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michelson AD, Barnard MR, Hechtman HB, et al. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A. 1996;93:11877–82. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goerge T, Ho-Tin-Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008 doi: 10.1182/blood-2007-11-123620. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 42.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92:1075–85. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 44.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 45.Schaphorst KL, Chiang E, Jacobs KN, et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–67. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 46.Shepro D, Welles SL, Hechtman HB. Vasoactive agonists prevent erythrocyte extravasation in thrombocytopenic hamsters. Thromb Res. 1984;35:421–30. doi: 10.1016/0049-3848(84)90234-2. [DOI] [PubMed] [Google Scholar]
- 47.Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139:329–36. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarter SD, Lai PF, Suen RS, Stewart DJ. Regulation of endothelin-1 by angiopoietin-1: implications for inflammation. Exp Biol Med (Maywood) 2006;231:985–91. [PubMed] [Google Scholar]
- 49.McCarter SD, Mei SH, Lai PF, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007;175:1014–26. doi: 10.1164/rccm.200609-1370OC. [DOI] [PubMed] [Google Scholar]
- 50.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–8. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Manegold PC, Hutter J, Pahernik SA, Messmer K, Dellian M. Platelet-endothelial interaction in tumor angiogenesis and microcirculation. Blood. 2003;101:1970–6. doi: 10.1182/blood.V101.5.1970. [DOI] [PubMed] [Google Scholar]
- 52.Pinedo HM, Verheul HM, D’Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352:1775–7. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- 53.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen consumption and tissue oxygenation of human tumors. Adv Exp Med Biol. 1990;277:895–905. doi: 10.1007/978-1-4684-8181-5_103. [DOI] [PubMed] [Google Scholar]
- 54.Fukumura Dh, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–49. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 55.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvasc Res. 2007 doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green KB, Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10:499–530. doi: 10.1016/s0889-8588(05)70349-x. [DOI] [PubMed] [Google Scholar]
- 57.Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patient. Semin Thromb Hemost. 1999;25:173–82. doi: 10.1055/s-2007-994919. [DOI] [PubMed] [Google Scholar]
- 58.Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–24. doi: 10.1016/s0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mice were injected with Evan’s blue and LLC tumors were observed through a dorsal skinfold chamber for 3 hours starting from the injection of either the control (Movie 1) or the platelet-depleting (Movie 2) antibody.