Abstract
The amount of mucin on the ocular surface is regulated by the rate of mucin synthesis, mucin secretion, and the number of goblet cells. We have previously shown that cholinergic agonists are potent stimuli of mucin secretion. In contrast, there have been no studies on the control of goblet cell proliferation. In this study we investigate the presence of the EGF family of growth factors and their receptors in rat conjunctiva and cultured rat conjunctival goblet cells as well as their effects on activation of signaling intermediates and goblet cell proliferation. Rat conjunctival goblet cells were grown in organ culture and identified as goblet cells by their morphology and positive staining for the lectin UEA-1 and cytokeratin 7. In the rat conjunctiva, the presence of the EGF family members epidermal growth factor (EGF), transforming growth factor α (TGF-α), heparin binding EGF (HB-EGF), and heregulin was determined by RT-PCR. The receptors for these ligands, EGF receptor (EGFR), erbB2, erbB3, and erbB4 were detected in both rat conjunctiva and goblet cells by Western blot analysis. Immunofluorescence microscopy of conjunctival tissue determined that EGFR was present as punctate staining in the cytoplasm of conjunctival goblet cells while ErbB2 was present in the basolateral and lateral membranes of goblet cells. ErbB3 was localized to the cytosol of rat conjunctival goblet cells. In cultured goblet cells, EGFR and ErbB2 were present in the perinuclear area of the cells. ErbB3 was widely distributed throughout the cytoplasm of the cells. ErbB4 was not detected in either the conjunctiva or goblet cells by immunofluorescence microscopy. Using a multiplex assay system we measured phosphorylation (activation) of p44/p42 mitogen-activated protein kinase (MAPK), also known as ERK, Jun N-terminal kinase (JNK), p38 MAPK and AKT (also known as protein kinase B), molecules known to be activated by EGF receptor members. EGF, TGF-α and HB-EGF activated the signaling intermediate proteins whereas heregulin did not. No EGF family member significantly activated AKT. Consistent with these findings, EGF, TGF-α and HB-EGF each stimulated goblet cell proliferation as measured by WST-1 assay or immunofluorescence microscopy using an antibody against Ki-67, a protein expressed in dividing cells. Heregulin did not cause goblet cell proliferation. We conclude that multiple members of the EGF family, EGF, TGF-α and HB-EGF, and heregulin are present with three of the four erbB receptor subtypes. EGF, TGF-α and HB-EGF all stimulated the activation of signaling intermediates and caused goblet cell proliferation.
Keywords: goblet cell, proliferation, secretion, conjunctiva
1. Introduction
The EGF family of ligands interacts with a family of receptor tyrosine kinases known as ErbB receptors. There are four ErbB receptors, the EGF receptor (EGFR, HER1, or ErbB1), ErbB2 (HER2/neu), ErbB3, and ErbB4. The ligands for ErbB receptors can be divided into three groups. The first group is comprised of epidermal growth factor (EGF), transforming growth factor alpha (TGF-α), betacellulin, and amphiregulin, which bind to EGFR. Heparin-binding EGF (HB-EGF) and epiregulin, which bind to EGFR and ErbB4, represent the second group. The third group consists of heregulin (HG) isoforms and neu differentiation factor, which bind to ErbB3 and ErbB4. Ligands bind to the ErbB receptors resulting in the formation of homo- and heterodimers and autophosphorylation of specific tyrosine residues in the cytoplasmic domain of the receptor (Iwamoto and Mekada, 2006). Tyrosine phosphorylated ErbB receptors bind to adaptor proteins, which mediate a variety of signal transduction pathways that elicit downstream functional responses. Downstream adaptors include Shc/Grb2, phosphoinositide-3 kinase (PI3K), phospholipase C γ (PLCγ), Src, Vav, Nck, Grb7, and Crk (Yarden and Sliwkowski, 2001). Downstream adaptors activate cascades of enzymes such as protein kinase C (PKC), AKT (also known as protein kinase B), extracellular regulated kinase (ERK 1/2, also known as p44/p42 mitogen-activated protein kinase (MAPK)), Jun N-terminal kinase (JNK), and Abelson tyrosine kinase (Abl) that stimulate nuclear transcription factors to activate functions such as proliferation, apoptosis, migration, adhesion and differentiation. In this study, we explored the role of members of each group of the EGF family of growth factors, EGF, TGF-α, HB-EGF, and heregulin in stimulating conjunctival goblet cell signaling pathways leading to proliferation.
In most vertebrate tissues, the different EGF ligands bind to the appropriate ErbB receptors with varying degrees of preference causing formation and activation of distinct homo- and heterodimers. Each type of ErbB receptor dimer has different sets of phosphorylated tyrosines attracting distinct sets of adaptor proteins causing ligand specific responses. The existence of an ErbB receptor, ErbB2, for which no ligand has yet been identified and an ErbB receptor, ErbB3, with no kinase activity adds an additional level of complexity. Furthermore, certain types of heterodimers are preferentially formed, especially those with ErbB2 as a partner, and certain heterodimers (Erb2/Erb3) have enhanced activity. Thus, the EGF family of ligands can differentially stimulate a given function or stimulate diverse functions. For examples, TGF-α and EGFR are expressed in lung, ovary, and colon tumors (Yarden and Sliwkowski, 2001). ErbB2 is associated with breast cancer (Meric-Bernstam and Hung, 2006). ErbB2/ErbB3 heterodimers are found in prostate cancer (Li et al., 2006). Some epithelial tumors express ErbB4/ErbB2 (Normanno et al., 2005). In addition, HB-EGF's activation of ErbB2 is needed for the maintenance of homeostasis in the heart, whereas its activation of EGFR is required for cardiac development (Iwamoto and Mekada, 2006). In this study we investigated if representatives of the EGF ligands that bind to and activate the four ErbB receptors cause differential effects on goblet cell proliferation.
Goblet cells are present in all wet-surfaced epithelia and synthesize, store, and secrete high molecular weight glycoproteins, including mucins that function to protect these epithelia from changes in the external environment (Perez-Vilar and Mabolo, 2007). The conjunctiva is no exception. This epithelium, with the cornea, forms the ocular surface. Goblet cells in the conjunctiva secrete the mucin MUC5AC into the inner mucous layer of the tear film providing a physical and chemical barrier to maintain a healthy ocular surface (Dartt, 2002). A mucus layer that is optimum in amount and composition is critical to the maintenance of the ocular surface, as either an increase or a decrease in the amount of mucin induces disease. Mucin deficiency is a consequence of diseases such as dry eye syndromes, ocular cicatricial pemphigoid, Stevens-Johnson syndrome, alkali burns, herpes simplex keratitis, and neurotrophic keratitis (Tseng et al., 1984). In contrast, overproduction of mucin leads to the ocular surface disease that occurs in atopy, seasonal allergic conjunctivitis, and mucus fishing syndromes (McCulley et al., 1985; Roat et al., 1993; Dogru et al., 2005). Both an increase and a decrease in mucin production cause disease suggesting that mucin production is tightly regulated.
The amount of mucin on the ocular surface is regulated by controlling the rate of mucin synthesis, the rate of mucin secretion, and the number of goblet cells. We previously found that cholinergic agonists are potent and effective stimuli of goblet cell mucin secretion and work by increasing the intracellular Ca2+ concentration and activating PKC isoforms that stimulate the non-receptor tyrosine kinases Pyk2 and Src (Dartt et al., 2000; Kanno et al., 2003). These kinases in turn transactivate the EGFR receptor thereby inducing the ERK pathway. In contrast to secretion there are no studies on the control of conjunctival goblet cell number i.e. goblet cell proliferation. It has been demonstrated that the number of conjunctival goblet cells changes in diseases of mucus over- and underproduction (Lemp, 1992). Conjunctival goblet cell proliferation has been indirectly linked to EGF as Pflugfelder et al. (1999) found that the tear EGF concentration was decreased in patients with Sjogren's syndrome compared to normal controls and that EGF concentration was correlated with conjunctival goblet cell number. There are no studies on the role of other members of the EGF family of growth factors on conjunctival goblet cell function. Further study of goblet cell proliferation and the role of EGF and its family members has been hampered by the lack of an appropriate model that allows easy, accurate study of the proliferation of goblet cells uncontaminated by other conjunctival cell types. Our laboratory has developed a method to culture both rat and human goblet cells in primary culture (Shatos et al., 2001, 2003). We extensively characterized these cells using multiple markers of goblet cell secretory products and cell bodies. In the present study we investigated the regulation of the number of conjunctival goblet cells by studying goblet cell proliferation in culture. We found that EGF, TGF-α, and HB-EGF are: (1) present in conjunctival goblet cells; (2) interact with their appropriate receptors that are present; (3) differentially activate p44/p42 MAPK, p38 MAPK, and JNK, and possibly AKT, signaling pathways; and (4) stimulate goblet cell proliferation. Although heregulin and the ErbB receptor subtypes to which it binds are present in the conjunctiva and in goblet cells, heregulin does not activate any of the above signaling pathways, nor does it stimulate goblet cell proliferation.
2. Materials and methods
2.1. Materials
EGF and TGF-α were from PeproTech, Inc. (Rocky Hill, NJ); HB-EGF and Heregulin were from Sigma-Aldrich (St. Louis, MO). Cell Proliferation Reagent WST-1 came from Roche Molecular Biochemicals (Mannheim, Germany). RNA extraction reagent (TriZol) was from GibcoBRL (Grand Island, NY). Reverse Transcription and PCR Systems were purchased from Promega (Madison, WI). All other reagents were obtained from Sigma-Aldrich.
Antibodies: A rabbit polyclonal antibody to human EGFR receptor was used in Western blotting experiments (Santa Cruz Biotechnology, Santa Cruz, CA), whereas a rabbit polyclonal antibody from Cell Signaling (Beverly, MA) and the human EGFR antibody was used in immunofluorescence microscopy experiments. Competing peptide was from Santa Cruz Biotechnology. To detect the ErbB2 receptors, two mouse monoclonal antibodies to human ErbB2 were used (Ab17 and Ab18, NeoMarkers, Fremont, CA) for immunofluorescence microscopy experiments while Ab18 antibody was used for Western blotting analysis. To detect the ErbB3 receptors, two mouse monoclonal antibodies to human ErbB3 were used for Western blotting analysis and immunofluorescence microscopy experiments (Santa Cruz Biotechnology, Santa Cruz, CA). The competing peptide was also from Santa Cruz Biotechnology. We detected the ErbB4 receptors using a rabbit polyclonal antibody to human ErbB4 in Western blotting experiments and immunofluorescence microscopy experiments (Santa Cruz Biotechnology, Santa Cruz, CA). We also tried a mouse monoclonal antibody to human ErbB4 for immunofluorescence microscopy experiments (Santa Cruz Biotechnology, Santa Cruz, CA). The anti-human ErbB receptor antibodies cross-reacted with rat ErbB receptors. The antibody to EGFR phosphorylated at Tyr1068 was purchased from Sigma Chemical Company (St. Louis, MO). The secondary antibodies used for immunofluorescence microscopy were fluorescein isothiocyanate (FITC) conjugated goat anti-rabbit IgG and tetramethyl rhodamine isothiocyanate (TRITC) conjugated donkey anti-mouse IgG from Jackson Immunoresearch Laboratories, Inc. (West Grove, PA). Horseradish peroxidase (HRP)-conjugated secondary antibodies for Western blotting analysis were from Santa Cruz Biotechnology.
2.2. Animals
Male Sprague–Dawley rats weighing between 125 and 150 g were obtained from Taconic Farms (Germantown, NY). Rats were anaesthetized with CO2 for 1 min, decapitated, and the nictitating membranes and conjunctival fornix removed from both eyes and minced. The procedure for removal of the conjunctiva was in accordance with guidelines established by the US Department of Agriculture Animal Welfare Act and approved by the Schepens Eye Research Institute Animal Care and Use Committee. Goblet cells were grown in organ culture as described previously (Shatos et al., 2001). In brief minced pieces of conjunctiva were placed in culture with RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 μg/ml penicillinstreptomycin. After nodules of cells were observed, the tissue plug was removed and goblet cells were allowed to grow from the nodules. As described previously (Shatos et al., 2001), cells were identified as goblet cells by: (1) their morphology as visualized by light microscopy; (2) their positive staining with the lectin Ulex Europaeus Agglutinin 1 (UEA-1) and antibody to cytokeratin 7 viewed by immunofluorescence microscopy; and (3) their negative staining for the stratified squamous cell markers the lectin Bandeira and antibody to cytokeratin 4 viewed by immunofluorescence microscopy. First passage goblet cells were used in all experiments.
2.3. RT-PCR analysis of expression of EGF family ligands
Conjunctiva removed from Sprague–Dawley rats and goblet cells in primary culture were homogenized in TRIzol, and total RNA isolated according to the manufacturer's protocol. Purified total RNA (1 μg) was used for complementary DNA (cDNA) synthesis using the Reverse Transcription System as described in the manufacturer's instructions. The cDNA was amplified by the polymerase chain reaction (PCR) using primers unique for EGF, TGF-α, HB-EGF, and heregulin in a thermal cycler (PCR Sprint; Thermo Hybaid, Ashton, UK). The sense and antisense oligonucleotide primers were derived from previously published sequences (Dvorak et al., 1998; Park and Borer, 1998; Sundaresan et al., 1998; Zieske et al., 2000) and are described in Table 1 (Chen et al., 2005). The primer for heregulin was common to all heregulin and neuregulin isoforms.
Table 1.
RT-PCR primers
| Oligonucleotide sequence (position) | Annealing temp |
Fragment size | Accession no. | |
|---|---|---|---|---|
| EGF | 5′-GACAACTCCCCTAAGGCTTA-3′ (2804–2823) | 55 °C | 567 bp | U04842 |
| 5′-CATGCACACGCCACCATTGA-3′ (3351–3370) | ||||
| TGF-α | 5′-ATGGTCCCCGCGGCCGGACAG-3′ (145–165) | 64 °C | 294 bp | M31076 |
| 5′-GGCCTGCTTCTTCTGGCTGGCA-3′ (417–438) | ||||
| HB-EGF | 5′-TCCCACTGGAACCACAAACCAG-3′ (157–178) | 61 °C | 414 bp | NM_012945 |
| 5′-CCCACGATGACAAGAAGACAGAC-3′ (548–570) | ||||
| Heregulin | 5′-TGTGCGGAGAAGGAGAAAA-3′ (888–906) | 53 °C | 233 bp | U02323 |
| 5′-ACCACACACATGATGCCGA-3′ (1102–1120) |
Each PCR reaction consisted of 0.5 μM each of sense and antisense primers, 200 mM each of dNTP, 1.5 μM MgCl2, 1.25 units of Taq polymerase, and 1 ml of cDNA. The cycling conditions were: 5 min hot start at 94 °C, followed by 35 cycles of denaturation for 1 min at 94 °C, annealing for 1 min at the indicated temperature, and extension for 1.5 min at 72 °C for 7 min. Total RNA isolated from brain and lacrimal gland which are known to express the EGF family ligands (Kaser et al., 1992; Chen et al., 1994; Hayase et al., 1998; Chen et al., 2005), served as the positive control. Samples with no cDNA were also amplified as the negative controls. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) primers (Clonetech Laboratories, Palo Alto, CA) were used to monitor the quality of the cDNA. After amplification, 10 μl of products were separated by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining under UV light.
2.4. Western blotting analysis
Conjunctiva removed from Sprague–Dawley rats and goblet cells from primary culture were homogenized in homogenization buffer (30 mM Tris–HCl pH 7.5, 10 mM EGTA, 5 mM EDTA, 1 mM dithiothreitol, and 250 mM sucrose) containing proteinase inhibitors (phenylmethysulfonyl fluoride 100 μg/ml, aprotinin 30 μl/ml, and sodium orthovanadate 100 nM). After homogenization, the samples were sonicated and centrifuged at 2000 × g for 15 min at 4 °C. The supernatant was resuspended in homogenization buffer and centrifuged at 100,000 × g for 1 h at 4 °C. Proteins in the pellet were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel. Separated proteins were transferred onto nitrocellulose membranes, blocked overnight at 4 °C in 5% non-fat dried milk in buffer containing 10 mM Tris–HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween-20 (TBST), and then incubated with the primary antibody (1 μg/ml) for 1 h at room temperature or overnight at 4 °C. After washing the membranes three times in TBST, the membranes were incubated with HRP-conjugated secondary antibody. Immunoreactive bands were detected by the enhanced chemiluminescence method.
2.5. Phosphorylation of EGFR
Primary cultures of rat conjunctival goblet cells were trypsinized and seeded into 6 well plates. Cells were grown to approximately 80% confluency, serum starved for 24 h. Cells were then stimulated for 5 min either with no addition or EGF, TGF-α or HB-EGF (each at 10−7 M). The reaction was stopped by the addition of ice-cold PBS. Cells were homogenized in RIPA buffer (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 100 mg/ml PMSF, 30 ml/ml aprotinin, and 1 mM Na3VO3). Proteins were separated by SDS-PAGE and Western blot analysis as described previously using antibodies that recognized phosphorylated Try1068 in EGFR and total EGFR.
2.6. Immunohistochemical analysis
Rat conjunctiva was removed, fixed in 4% formaldehyde in PBS for 4 h and preserved in 30% sucrose in PBS overnight at 4 °C. The conjunctiva was then frozen in optimal-cutting embedding compound and sections (6 μm) were cut and placed on gelatin-coated slides.
Primary cultures of rat conjunctival goblet cells were trypsinized, seeded onto glass coverslips, and grown to 75% confluence in RPMI medium containing 10% FBS. Cells were fixed with 4% paraformaldehyde and subjected to analysis by immunofluorescence microscopy for the presence of EGFR, ErbB2, ErbB3 and ErbB4. Cells fixed on coverslips were rinsed for 5 min in TBS (10 mM Tris–HCl, pH 7.4 and 150 mM NaCl), followed by blocking of non-specific sites in phosphate buffered saline (PBS, 145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 mM NaH2PO4 pH 7.2) containing 1% bovine serum albumin (BSA). Primary antibodies were diluted in TBS containing 1% BSA and applied for 2 h at room temperature or overnight at 4 °C. Antibodies to EGFR and ErbB4 were diluted 1:400 and antibodies to ErbB2 and ErbB3 diluted 1:40 and 1:50, respectively. The secondary antibodies, conjugated to either Cy2 or Cy3, were diluted 1:200 in TBS containing 1% BSA and applied to the cells for 1 h at room temperature. Coverslips were mounted on glass slides. The negative control was omission of the primary antibody. The specificity of the antibodies was confirmed by incubating slides with antibody that had been preincubated overnight with 10 fold excess of blocking peptide. Cells were viewed with a Nikon Eclipse E80i microscope with SPOT Diagnostic Instruments Inc camera.
2.7. Measurement of goblet cell entry into the cell cycle using Ki67-immunostaining
Goblet cells grown in primary culture were trypsinized, seeded onto chamber slides, and grown to 75% confluence in RPMI medium supplemented with 10% FBS. Cells were then serum starved in RPMI-1640 medium supplemented with 0.5% BSA for 24 h. RPMI medium with 0.5% BSA (basal) or 10% FBS (the positive control) were added for 24 h. EGF, TGF-α, HB-EGF, or heregulin all at 10−7 M were added for 1–24 h. Incubation was terminated and cells fixed by addition of ice-cold methanol (100%). Fixed cells were incubated for either 1 h at room temperature of overnight at 4 °C with anti-Ki67 antibody (1:100). The lectin UEA-1 directly conjugated to FITC was added with secondary antibody for 1 h at room temperature. Slides were mounted in OCT containing DAPI, which stains all cell nuclei independent of cell cycle stage. Ki-67-labeling of nuclei indicates cells that have entered into the cell cycle. UEA-1-labeling of cells identifies goblet cells. DAPI stained nuclei were counted to determine the total number of cells. The number of Ki67-labeled, UEA-1 positive goblet cells and the total number of cells were counted in nine fields per chamber. The number of goblet cells having entered the cell cycle was expressed as a percentage of the total number of cells.
2.8. Measurement of goblet cell proliferation using WST-1 assay
Rat conjunctival goblet cells in primary culture were trypsinized and seeded on 96-well culture plates at a density of 200 cells per well. Cells were grown for 24 h to subconfluency. After 24 h of serum starvation RPMI with 0.5% BSA (basal), 10% FBS (the positive control), and EGF, TGF-α, HB-EGF, or heregulin (each at 10−7 M) were added for 24 h. Cell proliferation was determined by the WST-1 assay, a colorimetric assay for the quantification of cell proliferation and cell viability, based on the cleavage of the tetrazolium salt WST-1 by mitochondrial dehydrogenases in viable cells. WST-1 reagent (1:10) was added after a 24-h stimulation. The absorbance was then read at 465 nm after 60 min incubation at 37 °C.
2.9. Measurement of phosphorylated signaling intermediates with multiplex assay
Goblet cells from primary culture were trypsinized, seeded on 24 well plates, and grown to confluency in RPMI containing 10% FBS. Medium was removed and replaced with RPMI containing 0.5% BSA (basal medium) for 24 h prior to addition of EGF, TGF-α, HB-EGF, or heregulin each at 10−7 M for 5 min. The reaction was stopped by addition of 1 ml of ice-cold homogenization buffer. The cells were scraped and protein measured by the Bradford assay. Multiplex assays were performed on equivalent protein from each sample.
Multiplex phosphoprotein assays are bead-based assays that can directly detect multiple phosphoproteins in a single sample of cell or tissue lysates using a 96-well cell culture plate format. Bead based assays contain fluorescently dyed beads covalently coupled to the antibody directed against the desired target protein or phosphoprotein. A four-plex phosphoprotein assay kit was obtained from Bio-Rad (Bio-Rad Laboratories Inc., Hercules, CA) to detect the phosphorylated form of four different proteins in conjunctival lysates: ERK, JNK, p38 mitogen-activated protein kinase (MAPK), and AKT. Total ERK2 assay kit was used to determine the total target protein in the sample lysates.
The sample lysates were diluted 1:1 with the assay buffer according to the manufacturer's protocol. The diluted sample lysates were incubated with antibody coated capture beads for 15 h at room temperature. After incubation, the sample was washed three times to remove the unbound protein; 100 μl of wash buffer was added to the wells, the filter plate was placed on the calibrated vacuum apparatus and the buffer removed by vacuum filtration. Washed beads were incubated with biotin labeled detection antibodies for 30 min. After a series of wash steps to remove the unbound detection antibodies, streptavidin-phycoerythrin was added (final volume of 50 μl). The reaction proceeded for 10 min, the wells were rinsed, and the data were acquired using the Bio-Plex suspension array system with Bio-Rad manager software.
2.10. Data analysis
The amount of phosphorylated proteins were divided by the amount of total ERK2 and expressed as a ratio. The values obtained under basal conditions were set at 1. Data were expressed as mean ± SEM and analyzed by the Student's t-test. Values were considered significant if p < 0.05.
3. Results
3.1. mRNA expression of the EGF family of ligands in conjunctival tissue and cultured goblet cells
To examine the expression of the representative ligands for ErbB receptors in conjunctival goblet cells, RT-PCR was performed using specific primers (Table 1) for the EGF family of growth factors. Four selected ligands of this family, EGF, TGF-α, HB-EGF, and heregulin that activate different ErbB receptors were studied. A single band at 567, 294, 414, and 233 base pairs was generated using primers specific to EGF, TGF-α, HB-EGF, and heregulin, respectively in all tissues used (Fig. 1). The primer to heregulin measured all forms of heregulin and neuregulins. mRNA expression was detected in conjunctival tissue that includes goblet cells as well as stratified squamous cells, fibroblasts, immune cells, nerves, and conjunctival stromal cells. The same size band was also detected in mRNA isolated from goblet cells propagated from three different rats. The appropriate size bands were also detected in lacrimal gland and brain, the positive controls. No amplification products were detected when RNA sample was omitted, the negative control. Thus EGF, TGF-α, HB-EGF, and the heregulin/neuregulin family mRNA are expressed in conjunctival tissue that includes goblet cells, as well as in cultured goblet cells.
Fig. 1.
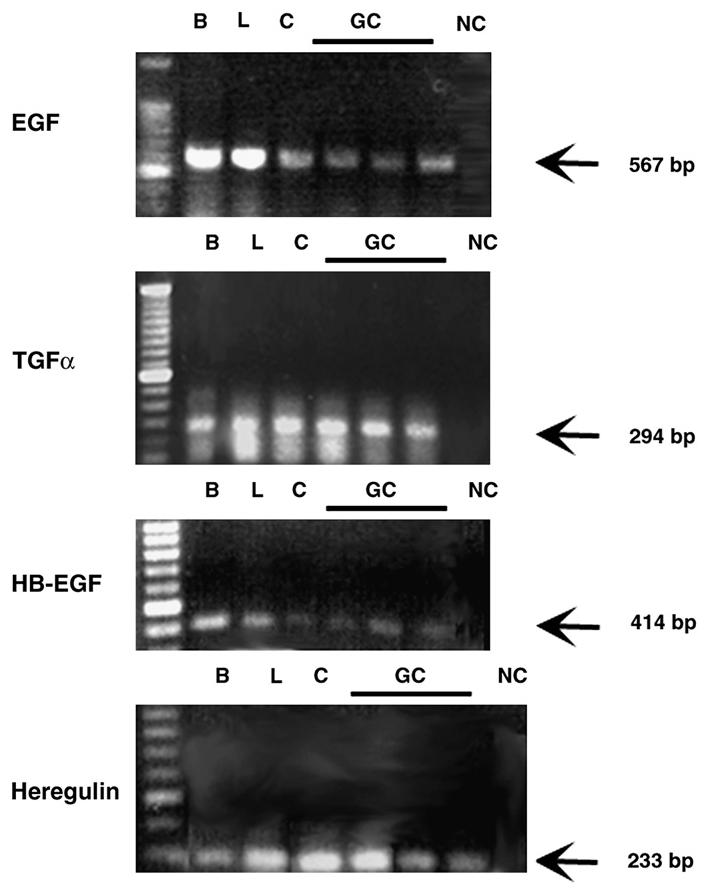
Identification of the EGF family of growth factors in the conjunctiva and cultured goblet cells by RT-PCR. Rat conjunctiva (C) and cultured goblet cells (GC) from primary culture were homogenized and the RNA isolated. RT-PCR was performed using primers specific for EGF, TGF-α, HB-EGF, and heregulin. RNA from rat brain (B) and lacrimal gland (L) was also isolated and serve as positive controls. The negative control (NC) indicates RT-PCR performed in the absence of cDNA. Each GC lane is the RT-PCR reaction from a single animal.
3.2. Identification of ErbB receptors in conjunctival tissue and cultured goblet cells
To determine which ErbB receptors were present in conjunctival tissue and cultured goblet cells, membrane preparations from these tissues were incubated with antibodies specific to each receptor subtype. The EGFR was present as a major band of 170 kDa in membranes isolated from conjunctival tissue (Fig. 2). A band of similar molecular weight was detected in membranes from cultured goblet cells and of a slightly lower molecular weight in lacrimal gland membranes. Minor bands of lower molecular weight were also detected in the lacrimal gland. The slightly different molecular weights and the multiple bands can result from differential glycosylation of the receptor and occurred with all four receptors.
Fig. 2.
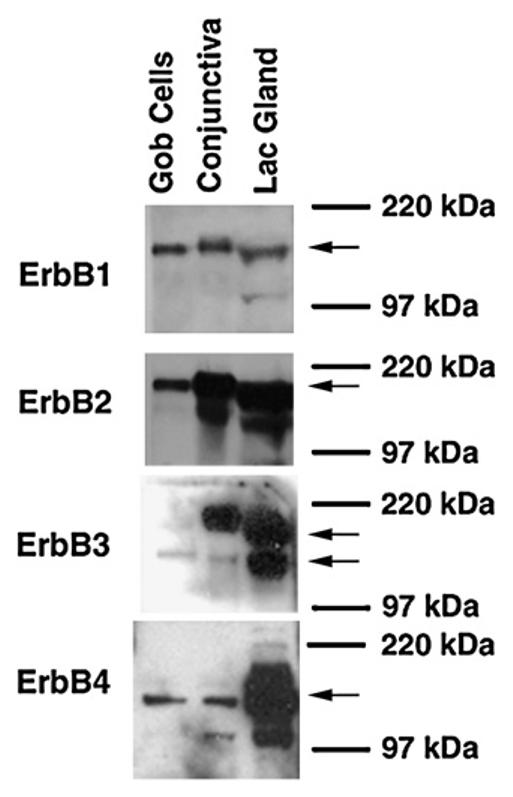
Identification of the ErbB receptors in the conjunctiva and cultured goblet cells by Western blot analysis. Proteins from rat conjunctiva and cultured goblet cells from primary culture were analyzed with antibodies specific to individual ErbB receptors. Protein from rat lacrimal gland is the positive control. Blots are representative of 3 independent experiments. Arrows indicate the major band detected.
A major band of 185 kDa indicated that the ErbB2 receptor was present in the membranes from conjunctival tissue, as well as from the cultured goblet cells. A band of slightly lower molecular weight was detected in the lacrimal gland. Multiple bands were detected in both lacrimal gland and conjunctiva.
For the ErbB3 receptor, two bands were identified. The upper band was present only in the conjunctival tissue and the lacrimal gland. A second, lower band of approximately 180 kDa was identified in all three tissues tested. This band was the only band present in conjunctival goblet cells. ErbB3, similar to the other ErbB receptors, is known to be glycosyated and this glycosylation may play a role in its actions (Hellyer et al., 1995; Yokoe et al., 2007). Thus the upper band could be a glycosyated form of the receptor.
Indicating the presence of ErbB4 was a band at 180 kDa detected in conjunctival, goblet cell, and lacrimal gland membranes along with multiple bands in the lacrimal gland and conjunctiva. For each ErbB receptor similar results were obtained in samples from three animals. Thus protein for all four ErbB receptors was detected in conjunctival tissue that includes goblet cells and cultured conjunctival goblet cells.
3.3. Cellular localization of ErbB receptors in conjunctival cells and cultured goblet cells
Using immunofluorescence microscopy the presence and localization of all four ErbB receptors was investigated in the fixed conjunctival epithelium with its underlying stroma. Goblet cells are identified using the lectin UEA-1 conjugated to FITC. UEA-1 binds to the goblet cell secretory product MUC5AC (Fig. 3). The EGFR (shown in red) was localized subjacent to the goblet cell secretory product (shown in green) as bright punctate staining in selected goblet cell clusters and in the cytoplasm of both goblet and stratified squamous cells. Limited immunoreactivity was detected in the stroma (Fig. 3A). Inhibition of the EGFR antibody with blocking peptide eliminated this staining (data not shown).
Fig. 3.
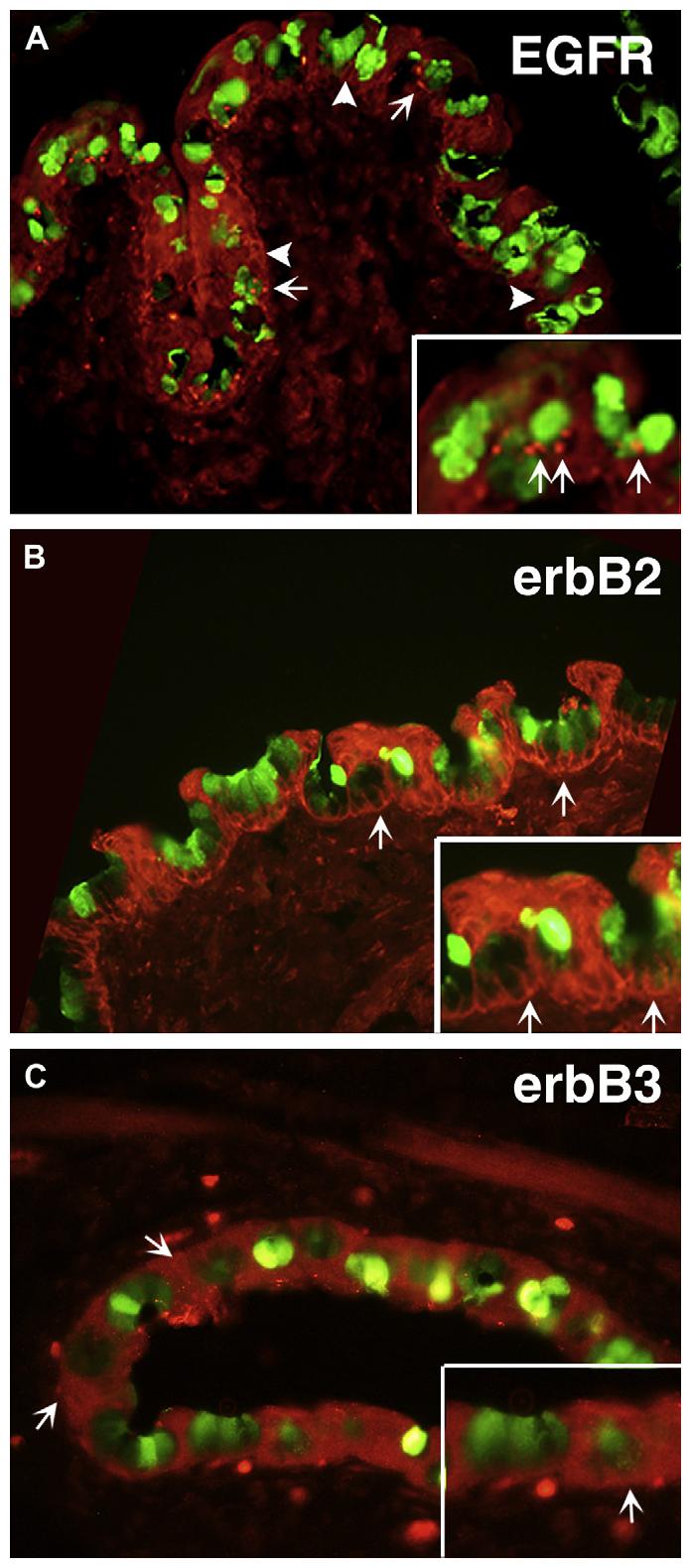
Localization of the ErbB receptors in the conjunctiva. Localization of the EGFR is shown in red (A). Arrows indicate punctate staining in goblet cell clusters; arrowheads indicate cytoplasmic staining of goblet and stratified squamous cells. The goblet cell secretory product is indicated by staining of UEA-1 (green). Localization of erbB2 is shown in red (B). Arrows indicate staining in basolateral membranes of goblet and stratified squamous cells. Localization of erbB3 is shown in red (C). Arrows indicated cytoplasmic staining in goblet and stratified squamous cells. In all micrographs, goblet cells are identified using UEA-1 conjugated to FITC. UEA-1 binds to the goblet cell secretory product MUC5AC and is shown in green. Micrographs are representative of 3 individual animals. Magnification ×400, inset ×800.
Intense ErbB2 immunoreactivity (shown in red) was found in the basolateral and lateral membranes of almost all goblet cells and the cell membranes of stratified squamous cells (Fig. 3B). Limited immunoreactivity was detected in the stroma. A second ErbB2 antibody was used to confirm the location of this receptor with similar results (data not shown).
Using an antibody to ErbB3, ErbB3 (shown in red) was localized to the cytoplasm of both goblet and stratified squamous cells (Fig. 3C). Inhibition of the ErbB3 antibody with blocking peptide eliminated this staining (data not shown).
ErbB4 was not detected in the conjunctival epithelium or stroma, even though ErbB4 receptor protein was found in the conjunctiva when Western blotting analysis was used. Similar results were obtained in conjunctiva from three animals.
Immunoreactivity to the EGFR, ErbB2, and ErbB3, but not ErbB4, was detected in cultured conjunctival goblet cells. The nuclei were counterstained with DAPI and are shown in blue. The EGFR (shown in green) was localized to the cytoplasm of goblet cells, most often in the perinuclear area. In select cells, immunoreactivity to this receptor was detected throughout much of the cytoplasm (Fig. 4A). Immunoreactivity to ErbB2 (shown in red) was similar to that of the EGFR and was often concentrated in the cytoplasm close to the nucleus (Fig. 4B). ErbB3 immunoreactivity (shown in red) was more widely distributed throughout the cytoplasm of goblet cells in culture than the EGFR and ErbB2. Selected cells had a concentrated pocket of reactivity in their cytoplasm (Fig. 4C). ErbB4 immunoreactivity was not detected in goblet cells, as in the conjunctiva, although this receptor was present by Western blotting analysis. Similar results were obtained in goblet cells cultured from three different animals.
Fig. 4.
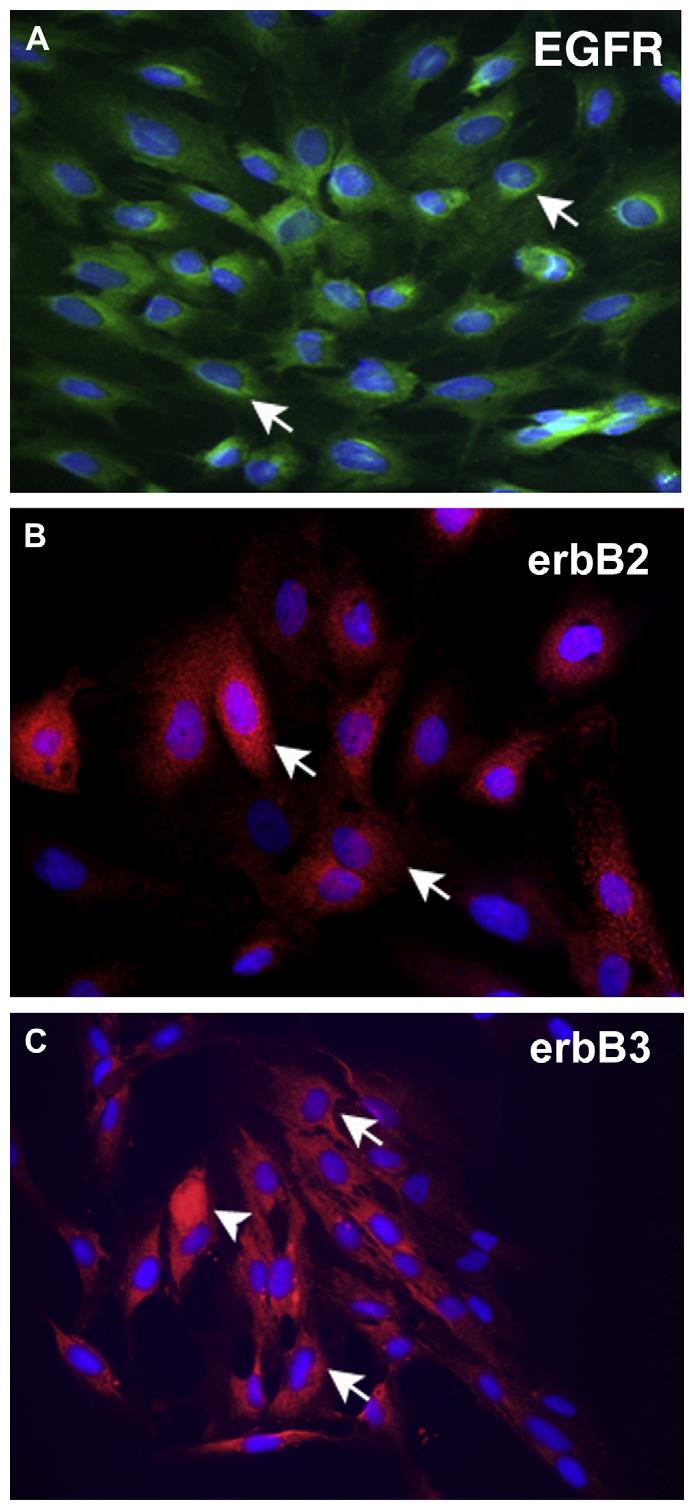
Localization of the ErbB receptors in cultured goblet cells. Localization of the EGFR (green) is shown in (A). Arrows indicate perinuclear staining of cultured goblet cells. Localization of erbB2 (red) is shown in (B). Arrows indicate cytoplasmic staining in cultured goblet cells. Localization of erbB3 (red) is shown in (C). Arrows indicated cytoplasmic staining in cultured goblet cells. Arrowheads indicate cells in which staining is concentrated throughout the cytoplasm. In all micrographs, the nuclei were counterstained with DAPI and are shown in blue. Micrographs are representative of 3 individual animals. Magnification ×400.
Thus the EGFR, ErbB2, and ErbB3, but not ErbB4, were localized to goblet cells both in the conjunctival epithelium and in culture. These receptors were also localized in the stratified squamous cells of the conjunctiva.
3.4. Activation of the EGFR in cultured goblet cells
To ensure that the EGF family of growth factors caused phosphorylation of the EGFR in the cultured goblet cells, cells from four different animals were incubated for 5 min in the presence of EGF, TGF-α, or HB-EGF, each at 10−7 M. We did not use heregulin as it binds only to the erbB3 and erbB4 receptors. The amount of phosphorylated EGFR was determined via Western blot analysis using antibodies against phosphorylated EGFR (Tyr1068) and total EGFR (Fig. 5A). EGF, TGF-α, and HB-EGF significantly stimulated the phosphorylation of EGFR by 21.1 ± 2.5, 22.2 ± 6.7, and 19.9 ± 6.0 fold above basal, respectively (Fig. 5B).
Fig. 5.
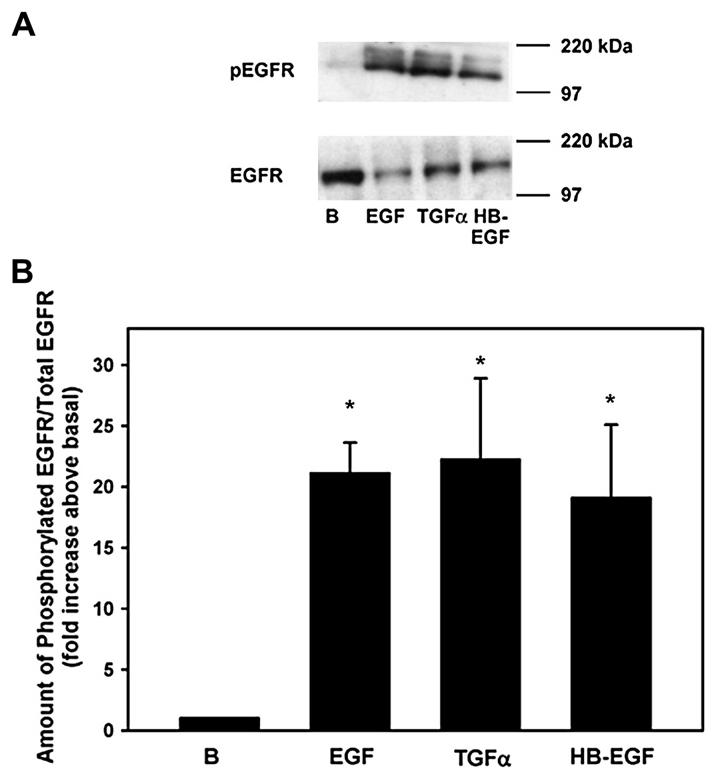
Effect of EGF family of growth factors on phosphorylation of the EGFR. Goblet cells from primary culture were stimulated with EGF (10−7 M), TGF-α (10−7 M) or HB-EGF (10−7 M) for 5 min. Cells were scraped and analyzed via Western blot with antibodies against phosphorylated EGFR (Tyr1068) or total EGFR. A representative blot is shown in A. Data shown in B are mean ± SEM from 4 independent experiments. * indicates p < 0.05.
3.5. Activation of phosphorylated signaling intermediates in cultured conjunctival goblet cells
EGF and its family of ligands activate multiple signaling pathways to stimulate cell proliferation, apoptosis, migration, adhesion and differentiation. These pathways include stimulation of the following adaptor proteins: (1) phospholipase Cγ to increase intracellular Ca2+ and activate protein kinase C; (2) phosphatidylinositol-3 kinase (PI3K) to phosphorylate AKT and JAK/STAT; and (3) Shc and Grb2 to activate Ras that phosphorylates ERK that translocates to the nucleus and phosphorylates JNK. EGF family ligands also phosphorylate mitogen-activated protein kinase kinase (MKK) 4 and 6 to phosphorylate p38MAPK. To determine which of these signaling pathways were activated by the EGF family ligands, serum-starved goblet cells cultured from 5 different animals were stimulated for 5 min with media alone, EGF, TGF-α, HB-EGF, or heregulin each at 10−7 M. ERK was significantly activated 2.6 ± 0.6, 2.7 ± 0.7 and 2.9 ± 0.8 fold compared to the basal level by EGF, TGF-α, and HB-EGF respectively, but was not significantly activated by heregulin (Fig. 6). JNK phosphorylation was significantly increased 1.4 ± 0.1, 1.4 ± 0.2, and 1.8 ± 0.3 fold compared to the basal level by EGF and TGF-α and HB-EGF, but was not significantly increased by heregulin. Similarly to ERK, the activity of p38MAPK was significantly increased 1.8 ± 0.2, 1.8 ± 0.2, and 2.1 ± 0.2 fold compared to the basal level by EGF, TGF-α, and HB-EGF, but was not significantly altered by heregulin. No EGF family of growth factors significantly activated AKT even though the values were increased. Thus the EGF family of growth factors differentially activated the phosphorylated signaling intermediates. Heregulin did not activate any of the four phosphoproteins measured.
Fig. 6.
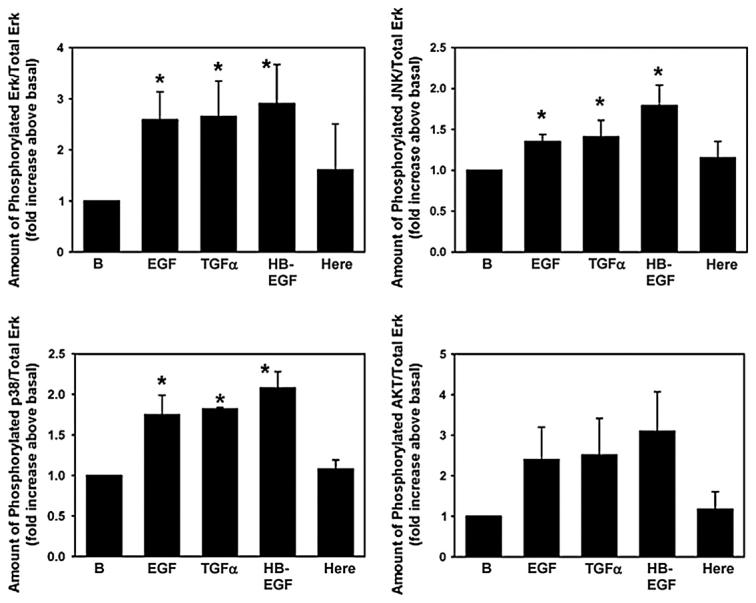
Effect of EGF family of growth factors on signaling intermediates. Goblet cells from primary culture were stimulated with EGF (10−7 M), TGF-α (10−7 M), HB-EGF (10−7 M), and heregulin (10−7 M) for 5 min. Cells were scraped and multiplex analysis was performed for activation of Erk, JNK, p38 MAPK, and AKT. Data are mean ± SEM from 5 independent experiments. * indicates p < 0.05. Here – heregulin.
3.6. Effect of EGF family ligands on goblet cell proliferation
We used two methods to measure goblet cell proliferation. First, the WST-1 assay system was used that measures number of cells. Second, an immunofluorescence method employing an antibody to Ki-67 was used that measures entry into the cell cycle. Cells were incubated for 24 h in the presence of medium alone (basal), EGF, TGF-α, HB-EGF, and heregulin each at 10−7 M, and 10% FBS and the number of cells measured by WST-1 assay. Compared to basal, EGF stimulated proliferation 1.3 ± 0.1 fold, TGF-α increased proliferation 1.2 ± 0.02 fold, and HB-EGF stimulated proliferation 1.1 ± 0.04 fold (Fig. 7). In contrast, heregulin did not stimulate proliferation. The positive control 10% FBS stimulated proliferation 1.6 ± 0.1 fold compared to basal.
Fig. 7.
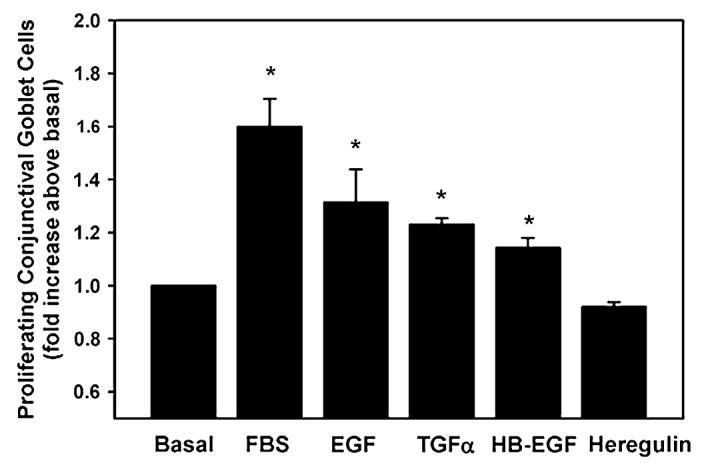
Effect of EGF family of growth factors on goblet cell proliferation. Goblet cells from primary culture were stimulated with EGF (10−7 M), TGF-α (10−7 M), HB-EGF (10−7 M), and heregulin (10−7 M) for 24 h. Cell proliferation was measured using WST-1. Data are mean ± SEM from 5 independent experiments. * indicates p < 0.05.
When entry into the cell cycle was visualized using an antibody to Ki-67 (shown in red), no labeled cells were visible under basal conditions (Fig. 8A). Multiple labeled cells were detected after a 24-h incubation with EGF, TGF-α, and HB-EGF, but few with heregulin, all at 10−7 M. After incubation in 10% FBS most cells had entered the cell cycle. Proliferating cells were goblet cells as they contained reactivity to the lectin UEA-1 (shown in green). When the cells expressing Ki-67 were counted, 0%, 61.4%, 38.1%, 27.8%, 19.5%, and 0% of the total number of cells (as counted using the nuclear stain DAPI, shown in blue) expressed KI-67 after treatment with media alone, FBS, EGF, TGF-α, HB-EGF, and heregulin, respectively (Fig. 8B). Thus, two different methods demonstrate that EGF, TGF-α, and HB-EGF stimulated the cultured goblet cells to enter the cell cycle and to divide. These EGF family members that bind to the EGFR were equally effective in stimulating proliferation. Heregulin that binds to ErbB3 and ErbB4 did not stimulate goblet cell proliferation.
Fig. 8.
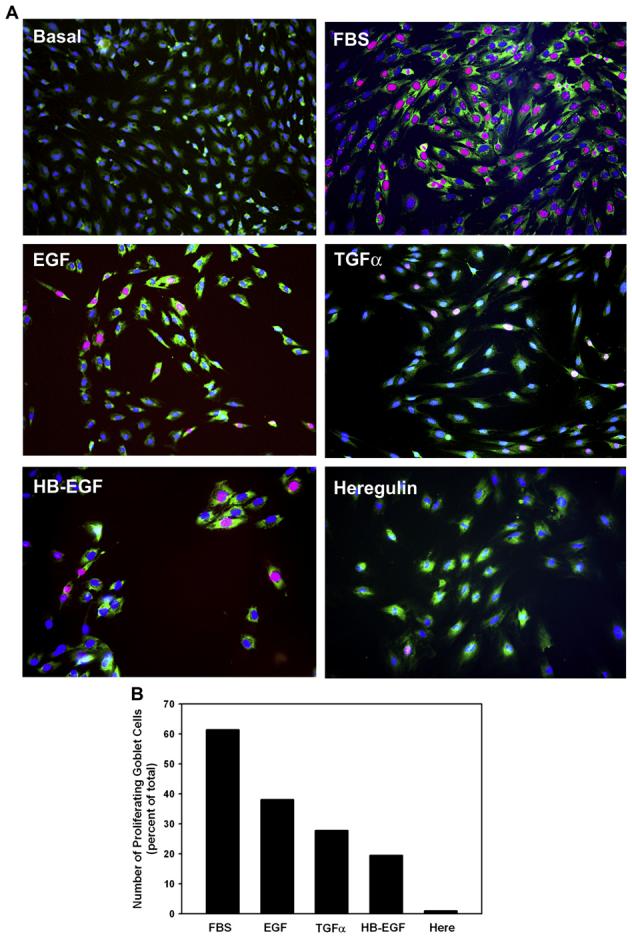
Effect of EGF family of growth factors on goblet cell proliferation. Goblet cells from primary culture were stimulated with either no addition, the positive control FBS (10%), EGF (10−7 M), TGF-α (10−7 M), HB-EGF (10−7 M), or heregulin (10−7 M) for 24 h. Cell proliferation was measured using an antibody directed against Ki-67 (red). MUC5AC, identifying goblet cells, is shown in green and nuclear-staining with DAPI is shown in blue. Micrographs for each condition are shown in A. Magnification ×200. Cells staining positive for Ki-67 were counted and shown in B. Data is the mean from 2 independent experiments. Please note that there are no proliferating cells under basal conditions.
3.7. Time-dependency of cell proliferation stimulated by EGF family ligands
The time course of entry of the cells into the cell cycle was measured at increasing times after the addition of EGF, TGF-α, HB-EGF, and heregulin at 10−7 M. We chose 14–24 h to study as preliminary data measuring cell proliferation using Ki-67 every 2 h, showed that proliferation did not occur before 14 h (data not shown). Entry into the cell cycle induced by all ligands increased slowly from 14 to 24 h, and peaked at 18 h (Fig. 9) as measured by WST-1. Interestingly, TGF-α caused more cells to enter the cell cycle earlier and longer than either EGF or HB-EGF. In these experiments heregulin did not stimulate cell cycle entry (data not shown). These data suggest that ligands from the EGF family each stimulate proliferation with similar time-dependencies.
Fig. 9.
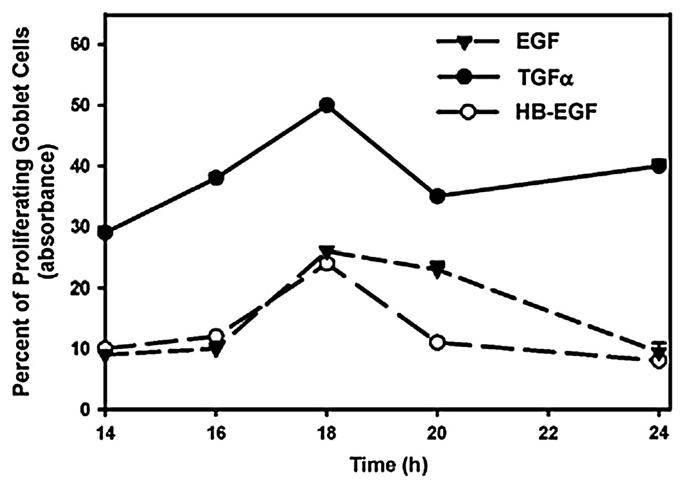
Effect of time on the EGF family of growth factors-stimulated goblet cell proliferation. Goblet cells from primary culture were stimulated with EGF (10−7 M), TGF-α (10−7 M), and HB-EGF (10−7 M) for 12–24 h. Cell proliferation was measured using WST-1. Data are mean ± SEM from 3 independent experiments.
4. Discussion
The results presented in this manuscript show that all four ErbB receptors are present in the conjunctival epithelium and in cultured goblet cells. The ligands that activate these receptor subtypes were also present. In addition, ligands that bind to the EGFR (EGF and TGF-α), but not ErbB3 or ErbB4 (heregulin), activated the signaling intermediates ERK, JNK, and p38 MAPK and stimulated goblet cell proliferation. HB-EGF that binds to the EGFR and ErbB4 was similar in effect to EGF and TGF-α. Although a role for ErbB2 and ErbB3 was not detected in the present study, these receptor subtypes could function as partners for heterodimer formation with the EGFR and ErbB4. In spite of the differential activation of signaling intermediates by EGF, TGF-α, and HB-EGF, there did not appear to be a difference in the effectiveness of the three ligands or the activation of the two types of receptors on the amount of goblet cell proliferation. Activation of ERK, JNK, and p38 MAPK appears to be important for the stimulation of proliferation by this family of growth factors as EGF, TGF-α, and HB-EGF each activated these kinases. We plan to test this in future experiments by using inhibitors of ERK, p38 MAPK, and JNK and explore these pathways in greater detail.
Measurement of protein kinases that function as signaling intermediates was performed only at 5 min of stimulation. We chose this time point as it was maximum for EGF activation of ERK in cultured goblet cells (Horikawa et al., 2003). It is possible that differential activation of ERK, p38 MAPK, JNK, and AKT would be detected if a more complete time-or concentration-dependency of protein kinase activation was measured. Additionally we could potentially detect heregulin activation of any of the protein kinases if additional times or concentrations of growth factors were used.
In all tissues of the body, except for the granular cells of the submandibular gland, the EGF family of growth factors are present as membrane bound precursors (Scott et al., 1985; Marechal et al., 1996). To be available for stimulating proliferation, these growth factors would be released from the cellular source when stimulated by activation of G protein-linked receptors. These receptors activate a matrix metalloproteinase, usually of the ADAM family, to release the active ectodomain of the growth factor that then binds to the EGFR or ErbB4 on the goblet cells. This is known as the “triple membrane passing signal” (Prenzel et al., 1999). Amphiregulin, TGF-α, and HB-EGF are known to be released by this mechanism from multiple tissues (Brown et al., 1998; Izumi et al., 1998; Peschon et al., 1998; Sahin et al., 2004). EGF is released from the lacrimal gland membranes when α1D-adrenergic agonists are added (Chen et al., 2006).
The EGF family of growth factors that stimulate proliferation could be released from several sources. The mRNA of all four members of the EGF family of ligands investigated in the present study was detected in the goblet cells in culture and in situ in the conjunctiva. Activation of goblet cells by G protein-linked agonists could release EGF, TGF-α, and HB-EGF from the cellular membranes where it could interact with the EGFR and ErbB4 present there. The stratified squamous cells of the conjunctiva contained all four ligands investigated and could also be the source of the growth factors. A similar scenario could cause release of EGF family of growth factors from the stratified squamous cells that would then interact with the EGFR or ErbB4 on the goblet cells. Alternatively, the conjunctiva need not be the source of these ligands as the lacrimal gland also contains these growth factors. The membrane spanning form of EGF that is present on the apical membranes of lacrimal gland acinar and ductal cells and released into tears could interact with conjunctival goblet cells (Chen et al., 2006). The lacrimal gland is also a potential source for TGF-α, HB-EGF, and heregulin in tears as mRNA for these growth factors has also found in the lacrimal gland, but their cellular location and secretion have not yet been studied. The corneal epithelium is another source of EGF family of growth factors. Corneal wounding causes release of HB-EGF from corneal cells (Block et al., 2004; Xu et al., 2004). Thus EGF, TGF-α, and HB-EGF that stimulate goblet cell proliferation could be released from multiple sources in the ocular surface epithelium and in tears.
The EGF family members released from the goblet, stratified squamous, lacrimal gland, or corneal epithelial cells would interact with the ErbB receptors found on the goblet cells. All four ErbB receptor subtypes were detected by Western blotting analysis in goblet cells when analyzed either in situ in the conjunctival epithelium or in culture. In contrast, ErbB4 was not detected in either the conjunctival epithelium or the cultured goblet cells when immunofluorescence microscopy was performed. The cause of this discrepancy is not clear. At least two different antibodies, including the one used for the Western blot analysis, were used for immunofluorescence experiments. Heregulin and HB-EGF both bind to ErbB4, but only HB-EGF stimulates goblet cell proliferation. Thus ErbB4 does not appear to be used to stimulate this process. This would be consistent with its absence from the conjunctiva, but we cannot definitively conclude this as ErbB4 could be involved in the regulation of other cellular functions.
The EGFR, ErbB2, and ErbB3 were localized in both goblet cells and stratified squamous cells of the conjunctiva. Although we have only measured goblet cell function, the EGF family members could also stimulate unidentified functions in the stratified squamous cells as both the growth factors and their receptors are present in these cells as well. The EGFR, ErbB2, and ErbB3 were also localized in the cultured goblet cells. Surprisingly these receptors were found in the cytoplasm of these cells rather than the plasma membrane. There are two potential explanations for this finding. First, the lack of cell polarity of the cultured cells compared to those in the conjunctiva could have altered the location of the receptors. Second, the cultured goblet cells were grown in the presence of serum, which could have activated a subpopulation of the EGF receptor subtypes and caused their internalization and perhaps degradation. If the cells were serum starved and then stimulated with the appropriate ligand we could perhaps detect receptors in the plasma membranes.
We conclude that in conjunctival goblet cells, multiple EGF family ligands EGF, TGF-α, HB-EGF, and heuregulin are present and three of the four receptor subtypes, EGFR, ErbB2, and ErbB3 are consistently are present. EGF, TGF-α, and HB-EGF that bind to the EGFR activate the protein kinases ERK, p38MAPK, and JNK, but did not significantly active AKT, and stimulate goblet cell proliferation. Heregulin that binds to ErbB3 and ErbB4 does not activate ERK, p38MAPK, JNK, or AKT and does not stimulate goblet cell proliferation.
References
- Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J. Biol. Chem. 2004;279(23):24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- Brown CL, Meise KS, Plowman GD, Coffey RJ, Dempsey PJ. Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. J. Biol. Chem. 1998;273(27):17258–17268. doi: 10.1074/jbc.273.27.17258. [DOI] [PubMed] [Google Scholar]
- Chen MS, Bermingham-McDonogh O, Danehy FT, Jr., Nolan C, Scherer SS, Lucas J, et al. Expression of multiple neuregulin transcripts in postnatal rat brains. J. Comp. Neurol. 1994;349(3):389–400. doi: 10.1002/cne.903490306. [DOI] [PubMed] [Google Scholar]
- Chen LL, Johansson JK, Hodges RR, Zoukhri D, Ghinelli E, Rios JD, et al. Differential effects of the EGF family of growth factors on protein secretion, MAPK activation, and intracellular calcium concentration in rat lacrimal gland. Exp. Eye Res. 2005;80(3):379–389. doi: 10.1016/j.exer.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Chen L, Hodges RR, Funaki C, Zoukhri D, Gaivin RJ, Perez DM, et al. Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells. Am. J. Physiol. Cell Physiol. 2006;291(5):C946–C956. doi: 10.1152/ajpcell.00014.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog. Retin. Eye Res. 2002;21(6):555–576. doi: 10.1016/s1350-9462(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Dartt DA, Rios JD, Kanno H, Rawe IM, Zieske JD, Ralda N, et al. Regulation of conjunctival goblet cell secretion by Ca(2+) and protein kinase C. Exp. Eye Res. 2000;71(6):619–628. doi: 10.1006/exer.2000.0915. [DOI] [PubMed] [Google Scholar]
- Dogru M, Okada N, Asano-Kato N, Tanaka M, Igarashi A, Takano Y, et al. Atopic ocular surface disease: implications on tear function and ocular surface mucins. Cornea. 2005;24(8 Suppl):S18–S23. doi: 10.1097/01.ico.0000178741.14212.53. [DOI] [PubMed] [Google Scholar]
- Dvorak B, Kolinska J, McWilliam DL, Williams CS, Higdon T, Zakostelecka M, et al. The expression of epidermal growth factor and transforming growth factor-alpha mRNA in the small intestine of suckling rats: organ culture study. FEBS Lett. 1998;435(1):119–124. doi: 10.1016/s0014-5793(98)01050-3. [DOI] [PubMed] [Google Scholar]
- Hayase Y, Higashiyama S, Sasahara M, Amano S, Nakagawa T, Taniguchi N, et al. Expression of heparin-binding epidermal growth factor-like growth factor in rat brain. Brain Res. 1998;784(1–2):163–178. doi: 10.1016/s0006-8993(97)01325-5. [DOI] [PubMed] [Google Scholar]
- Hellyer NJ, Kim HH, Greaves CH, Sierke SL, Koland JG. Cloning of the rat ErbB3 cDNA and characterization of the recombinant protein. Gene. 1995;165(2):279–284. doi: 10.1016/0378-1119(95)00436-a. [DOI] [PubMed] [Google Scholar]
- Horikawa Y, Shatos MA, Hodges RR, Zoukhri D, Rios JD, Chang EL, et al. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest. Ophthalmol. Vis. Sci. 2003;44(6):2535–2544. doi: 10.1167/iovs.02-1117. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Mekada E. ErbB and HB-EGF signaling in heart development and function. Cell Struct. Funct. 2006;31(1):1–14. doi: 10.1247/csf.31.1. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, et al. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998;17(24):7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno H, Horikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, et al. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am. J. Physiol. Cell Physiol. 2003;284(4):C988–C998. doi: 10.1152/ajpcell.00582.2001. [DOI] [PubMed] [Google Scholar]
- Kaser MR, Lakshmanan J, Fisher DA. Comparison between epidermal growth factor, transforming growth factor-alpha and EGF receptor levels in regions of adult rat brain. Brain Res. Mol. Brain Res. 1992;16(3–4):316–322. doi: 10.1016/0169-328x(92)90241-3. [DOI] [PubMed] [Google Scholar]
- Lemp MA. The Dry Eye: A Comprehensive Guide. Springer Verlag; Heidelberg, Germany: 1992. [Google Scholar]
- Li Z, Szabolcs M, Terwilliger JD, Efstratiadis A. Prostatic intraepithelial neoplasia and adenocarcinoma in mice expressing a probasin-Neu oncogenic transgene. Carcinogenesis. 2006;27(5):1054–1067. doi: 10.1093/carcin/bgi324. [DOI] [PubMed] [Google Scholar]
- Marechal H, Jammes H, Rossignol B, Mauduit P. EGF receptor mRNA and protein in rat lacrimal acinar cells: evidence of its EGF-dependent phosphotyrosilation. Am. J. Physiol. 1996;270(4 Pt 1):C1164–C1174. doi: 10.1152/ajpcell.1996.270.4.C1164. [DOI] [PubMed] [Google Scholar]
- McCulley JP, Moore MB, Matoba AY. Mucus fishing syndrome. Ophthalmology. 1985;92(9):1262–1265. doi: 10.1016/s0161-6420(85)33873-3. [DOI] [PubMed] [Google Scholar]
- Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin. Cancer Res. 2006;12(21):6326–6330. doi: 10.1158/1078-0432.CCR-06-1732. [DOI] [PubMed] [Google Scholar]
- Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, et al. The ErbB receptors and their ligands in cancer: an overview. Curr. Drug Targets. 2005;6(3):243–257. doi: 10.2174/1389450053765879. [DOI] [PubMed] [Google Scholar]
- Park JM, Borer JG, Freeman MR, Peters CA. Stretch activates heparin-binding EGF-like growth factor expression in bladder smooth muscle cells. Am. J. Physiol. 1998;275(5 Pt 1):C1247–C1254. doi: 10.1152/ajpcell.1998.275.5.C1247. [DOI] [PubMed] [Google Scholar]
- Perez-Vilar J, Mabolo R. Gel-forming mucins. Notions from in vitro studies. Histol. Histopathol. 2007;22(4):455–464. doi: 10.14670/HH-22.455. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999;19(3):201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Roat MI, Ohji M, Hunt LE, Thoft RA. Conjunctival epithelial cell hypermitosis and goblet cell hyperplasia in atopic keratoconjunctivitis. Am. J. Ophthalmol. 1993;116(4):456–463. doi: 10.1016/s0002-9394(14)71404-7. [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 2004;164(5):769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J, Patterson S, Rall L, Bell GI, Crawford R, Penschow J, et al. The structure and biosynthesis of epidermal growth factor precursor. J. Cell Sci. Suppl. 1985;3:19–28. doi: 10.1242/jcs.1985.supplement_3.3. [DOI] [PubMed] [Google Scholar]
- Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest. Ophthalmol. Vis. Sci. 2001;42(7):1455–1464. [PubMed] [Google Scholar]
- Shatos MA, Rios JD, Horikawa Y, Hodges RR, Chang EL, Bernardino CR, et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest. Ophthalmol. Vis. Sci. 2003;44(6):2477–2486. doi: 10.1167/iovs.02-0550. [DOI] [PubMed] [Google Scholar]
- Sundaresan S, Roberts PE, King KL, Sliwkowski MX, Mather JP. Biological response to ErbB ligands in non-transformed cell lines correlates with a specific pattern of receptor expression. Endocrinology. 1998;139(12):4756–4764. doi: 10.1210/endo.139.12.6378. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Hirst LW, Maumenee AE, Kenyon KR, Sun TT, Green WR. Possible mechanisms for the loss of goblet cells in mucin-deficient disorders. Ophthalmology. 1984;91(6):545–552. doi: 10.1016/s0161-6420(84)34251-8. [DOI] [PubMed] [Google Scholar]
- Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2004;45(3):813–820. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yokoe S, Takahashi M, Asahi M, Lee SH, Li W, Osumi D, et al. The Asn418-linked N-glycan of ErbB3 plays a crucial role in preventing spontaneous heterodimerization and tumor promotion. Cancer Res. 2007;67(5):1935–1942. doi: 10.1158/0008-5472.CAN-06-3023. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest. Ophthalmol. Vis. Sci. 2000;41(6):1346–1355. [PubMed] [Google Scholar]


