Abstract
The nucleotide sequence of a cloned cDNA copy of the mRNA coding for the hemagglutinin-neuraminidase of the paramyxovirus SV5 was determined. There was a single large open reading frame on the mRNA which encoded a protein of 565 amino acids with a molecular weight of 62,134. The deduced amino acid sequence indicated that the only major hydrophobic region in the protein sufficiently long to anchor the protein in the membrane is located near the N terminus (amino acids 18 to 36). It is suggested that, like the influenza virus neuraminidase, hemagglutinin-neuraminidase of paramyxoviruses is oriented with its N terminus inserted into the membrane.
Full text
PDF


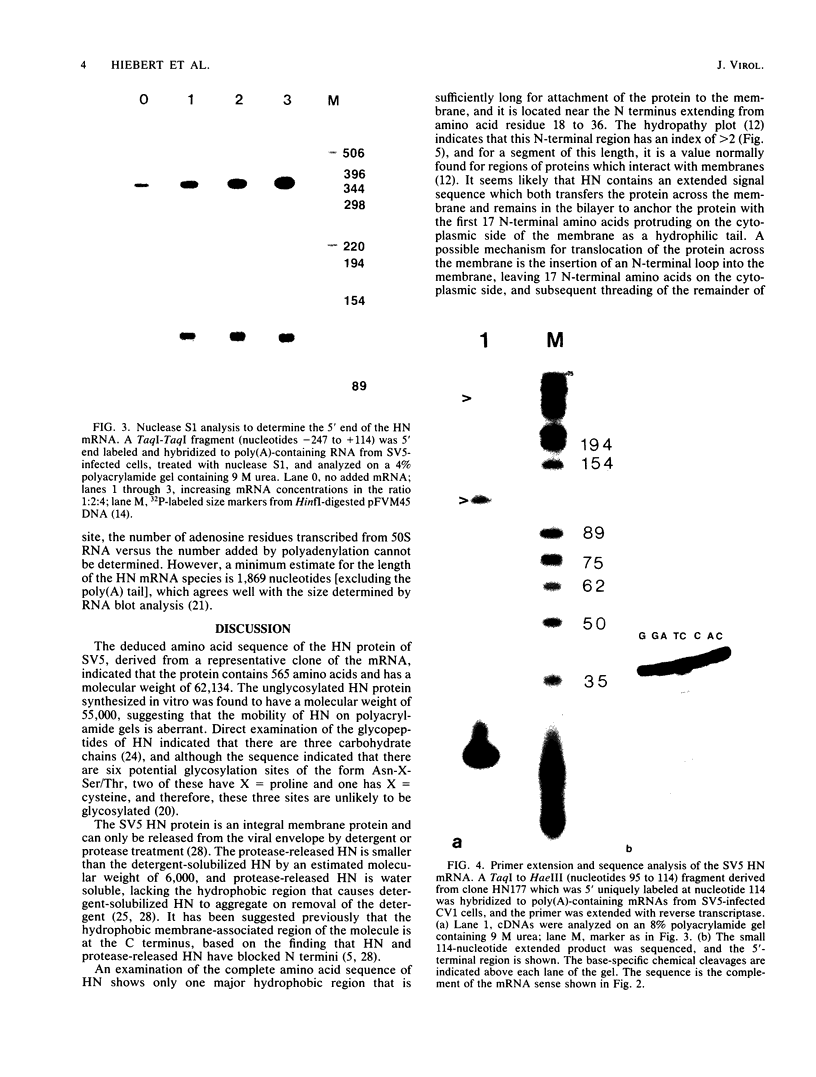
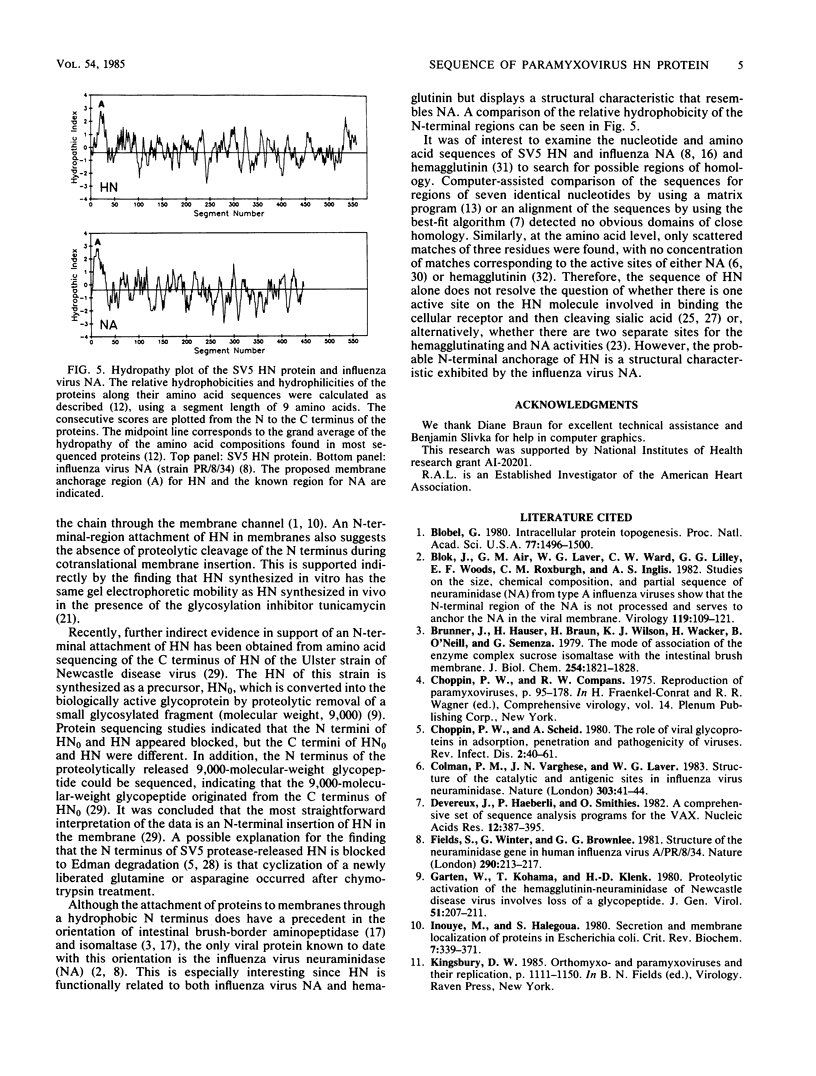

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok J., Air G. M., Laver W. G., Ward C. W., Lilley G. G., Woods E. F., Roxburgh C. M., Inglis A. S. Studies on the size, chemical composition, and partial sequence of the neuraminidase (NA) from type A influenza viruses show that the N-terminal region of the NA is not processed and serves to anchor the NA in the viral membrane. Virology. 1982 May;119(1):109–121. doi: 10.1016/0042-6822(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Braun H., Wilson K. J., Wacker H., O'Neill B., Semenza G. The mode of association of the enzyme complex sucrase.isomaltase with the intestinal brush border membrane. J Biol Chem. 1979 Mar 25;254(6):1821–1828. [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- Garten W., Kohama T., Klenk H. D. Proteolytic activation of the haemagglutinin-neuraminidase of Newcastle disease virus involves loss of a glycopeptide. J Gen Virol. 1980 Nov;51(Pt 1):207–211. doi: 10.1099/0022-1317-51-1-207. [DOI] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lagrimini L. M., Brentano S. T., Donelson J. E. A DNA sequence analysis package for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):605–614. doi: 10.1093/nar/12.1part2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Spliced and unspliced messenger RNAs synthesized from cloned influenza virus M DNA in an SV40 vector: expression of the influenza virus membrane protein (M1). Virology. 1982 Dec;123(2):237–256. doi: 10.1016/0042-6822(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Markoff L., Lai C. J. Sequence of the influenza A/Udorn/72 (H3N2) virus neuraminidase gene as determined from cloned full-length DNA. Virology. 1982 Jun;119(2):288–297. doi: 10.1016/0042-6822(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D. On the hydrophobic part of aminopeptidase and maltases which bind the enzyme to the intestinal brush border membrane. Biochim Biophys Acta. 1976 Jan 21;419(2):189–195. doi: 10.1016/0005-2736(76)90345-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Analysis and gene assignment of mRNAs of a paramyxovirus, simian virus 5. Virology. 1984 Oct 30;138(2):310–323. doi: 10.1016/0042-6822(84)90354-4. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A. The HN glycoprotein of Sendai virus: analysis of site(s) involved in hemagglutinating and neuraminidase activities. Virology. 1981 Dec;115(2):375–384. doi: 10.1016/0042-6822(81)90118-5. [DOI] [PubMed] [Google Scholar]
- Prehm P., Scheid A., Choppin P. W. The carbohydrate structure of the glycoproteins of the paramyxovirus SV5 grown in bovine kidney cells. J Biol Chem. 1979 Oct 10;254(19):9669–9677. [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. The hemagglutinating and neuraminidase protein of a paramyxovirus: interaction with neuraminic acid in affinity chromatography. Virology. 1974 Nov;62(1):125–133. doi: 10.1016/0042-6822(74)90308-0. [DOI] [PubMed] [Google Scholar]
- Schuy W., Garten W., Linder D., Klenk H. D. The carboxyterminus of the hemagglutinin-neuraminidase of Newcastle disease virus is exposed at the surface of the viral envelope. Virus Res. 1984;1(5):415–426. doi: 10.1016/0168-1702(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Fang R., Jou W. M., Devos R., Huylebroeck D., Saman E., Fiers W. Antigenic drift between the haemagglutinin of the Hong Kong influenza strains A/Aichi/2/68 and A/Victoria/3/75. Nature. 1980 Aug 21;286(5775):771–776. doi: 10.1038/286771a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]