Abstract
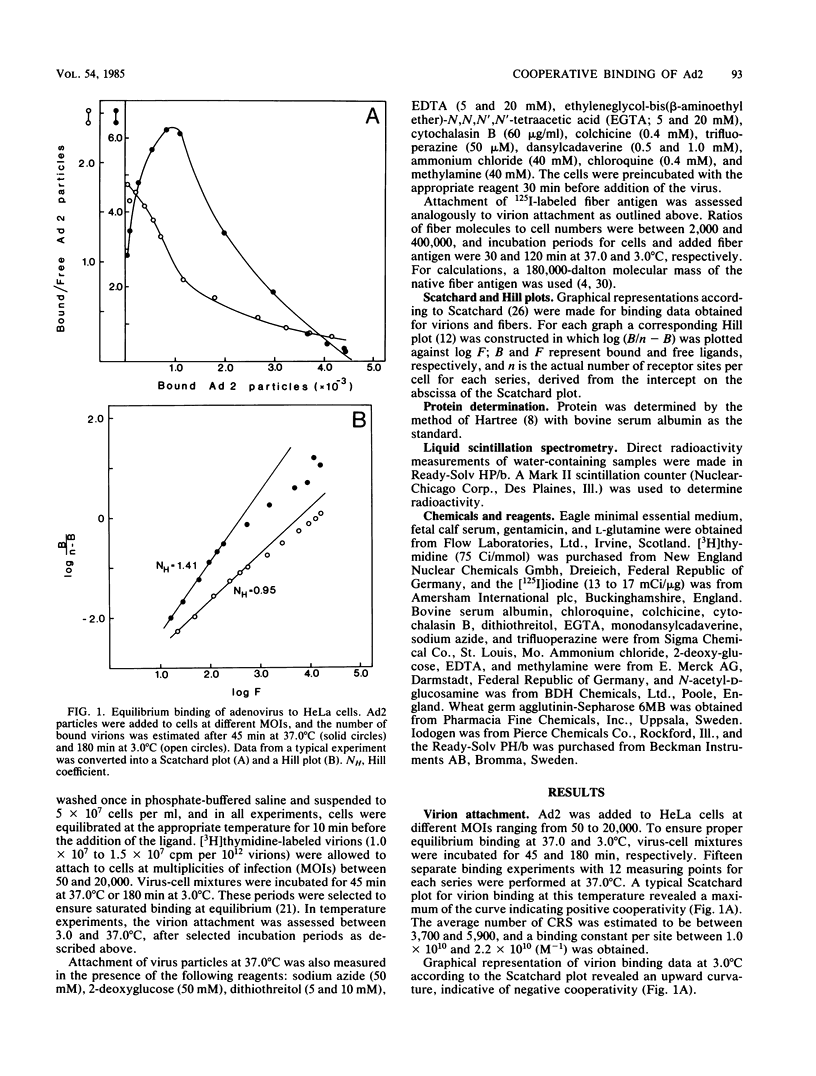
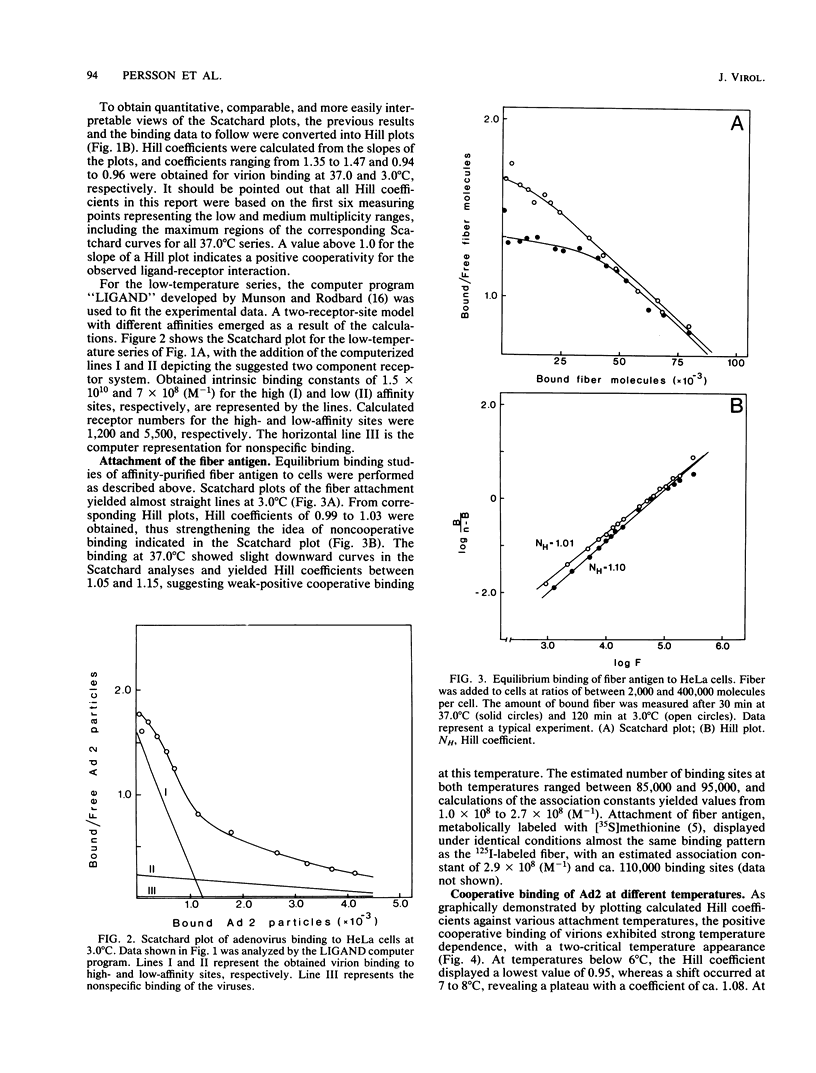
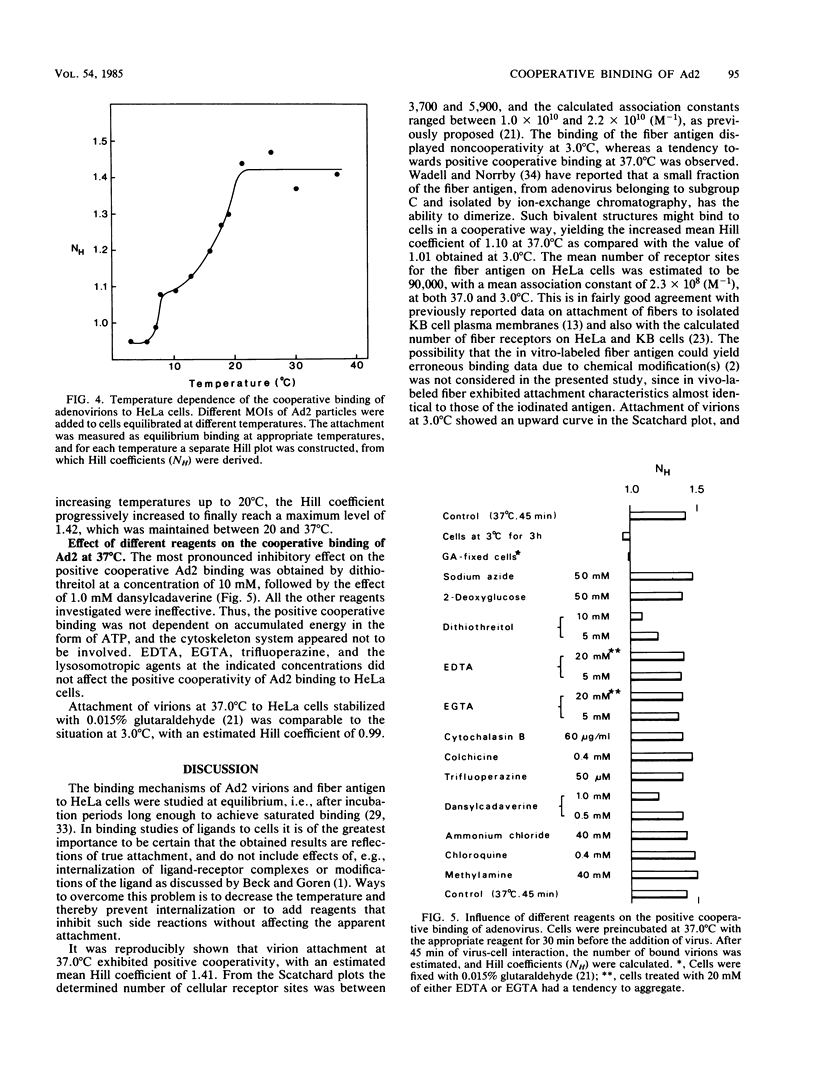
The established positive cooperativity of adenovirus 2 binding to HeLa cells revealed a strong temperature dependence. The degree of cooperativity, quantified by means of Hill coefficients, progressively increased from 10 degrees C to reach a maximum level, which was maintained between 20 and 37 degrees C. On the other hand, negative cooperativity of virion attachment was apparent at 3.0 degrees C and on glutaraldehyde-stabilized cells. The corresponding monovalent ligand of the system, the fiber antigen, demonstrated only weak-positive cooperativity of the binding at 37.0 degrees C, which was absent at 3.0 degrees C. Dithiothreitol and dansylcadaverine, reagents inhibiting clustering of ligand-receptor complexes in the plasma membrane, markedly reduced the degree of positive cooperative binding at 37.0 degrees C. Evidently, the positive cooperative binding of adenovirus to HeLa cells at 37.0 degrees C is a consequence of both the multivalency of virus attachment proteins, i.e., fibers, on the virion and of the capacity of the receptor sites to migrate in the plane of the plasma membrane, forming local aggregates of virus-receptor site complexes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. S., Goren H. J. Simulation of association curves and 'Scatchard' plots of binding reactions where ligand and receptor are degraded or internalized. J Recept Res. 1983;3(5):561–577. doi: 10.3109/10799898309041948. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deman J. J., Bruyneel E. A. Thermal transitions in the adhesiveness of HeLa cells: effects of cell growth, trypsin treatment and calcium. J Cell Sci. 1977;27:167–181. doi: 10.1242/jcs.27.1.167. [DOI] [PubMed] [Google Scholar]
- Devaux C., Zulauf M., Boulanger P., Jacrot B. Molecular weight of adenovirus serotype 2 capsomers. A new characterization. J Mol Biol. 1982 Apr 25;156(4):927–939. doi: 10.1016/0022-2836(82)90148-6. [DOI] [PubMed] [Google Scholar]
- Edvardsson B., Everitt E., Jörnvall H., Prage L., Philipson L. Intermediates in adenovirus assembly. J Virol. 1976 Aug;19(2):533–547. doi: 10.1128/jvi.19.2.533-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Role of disulphide and sulphydryl groups in clustering of enkephalin receptors in neuroblastoma cells. Nature. 1979 Dec 6;282(5739):626–628. doi: 10.1038/282626a0. [DOI] [PubMed] [Google Scholar]
- Hennache B., Torpier G., Boulanger P. Adenovirus adsorption and sterol redistribution in KB cell plasma membrane. Exp Cell Res. 1982 Feb;137(2):459–463. doi: 10.1016/0014-4827(82)90052-0. [DOI] [PubMed] [Google Scholar]
- Hennache B., Torpier G., Boulanger P. Freeze-fracture study of adenovirus-induced KB cell surface alterations. Exp Cell Res. 1979 Nov;124(1):139–150. doi: 10.1016/0014-4827(79)90264-7. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Neet K. E. Cooperativity in enzyme function: equilibrium and kinetic aspects. Methods Enzymol. 1980;64:139–192. doi: 10.1016/s0076-6879(80)64009-9. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Patterson S., Russell W. C. Ultrastructural and immunofluorescence studies of early events in adenovirus-HeLa cell interactions. J Gen Virol. 1983 May;64(Pt 5):1091–1099. doi: 10.1099/0022-1317-64-5-1091. [DOI] [PubMed] [Google Scholar]
- Persson R., Svensson U., Everitt E. Virus receptor interaction in the adenovirus system. II. Capping and cooperative binding of virions on HeLa cells. J Virol. 1983 Jun;46(3):956–963. doi: 10.1128/jvi.46.3.956-963.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Philipson L., Höglund S. Structural proteins of adenoviruses. I. Purification and characterization of the adenovirus type 2 hexon antigen. Virology. 1967 Dec;33(4):575–590. doi: 10.1016/0042-6822(67)90057-8. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Prujansky A., Ravid A., Sharon N. Cooperativity of lectin binding to lymphocytes, and its relevance to mitogenic stimulation. Biochim Biophys Acta. 1978 Mar 21;508(1):137–146. doi: 10.1016/0005-2736(78)90195-5. [DOI] [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Scheinberg I. H. Scatchard plots. Science. 1982 Jan 15;215(4530):312–313. doi: 10.1126/science.215.4530.312. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Chang K. J., Jacobs S., Cuatrecasas P. Modulation of binding and bioactivity of insulin by anti-insulin antibody: relation to possible role of receptor self-aggregation in hormone action. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2720–2724. doi: 10.1073/pnas.76.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunquist B., Pettersson U., Thelander L., Philipson L. Structural proteins of adenoviruses. IX. Molecular weight and subunit composition of adenovirus type 2 fiber. Virology. 1973 Jan;51(1):252–256. doi: 10.1016/0042-6822(73)90389-9. [DOI] [PubMed] [Google Scholar]
- Svensson U., Persson R. Entry of adenovirus 2 into HeLa cells. J Virol. 1984 Sep;51(3):687–694. doi: 10.1128/jvi.51.3.687-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson U., Persson R., Everitt E. Virus-receptor interaction in the adenovirus system I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981 Apr;38(1):70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., Norrby E. The soluble hemagglutinins of adenoviruses belonging to Rosen's subgroup 3. II. The slowly sedimenting hemagglutinin. Arch Gesamte Virusforsch. 1969;26(1):53–62. doi: 10.1007/BF01241175. [DOI] [PubMed] [Google Scholar]



