Abstract
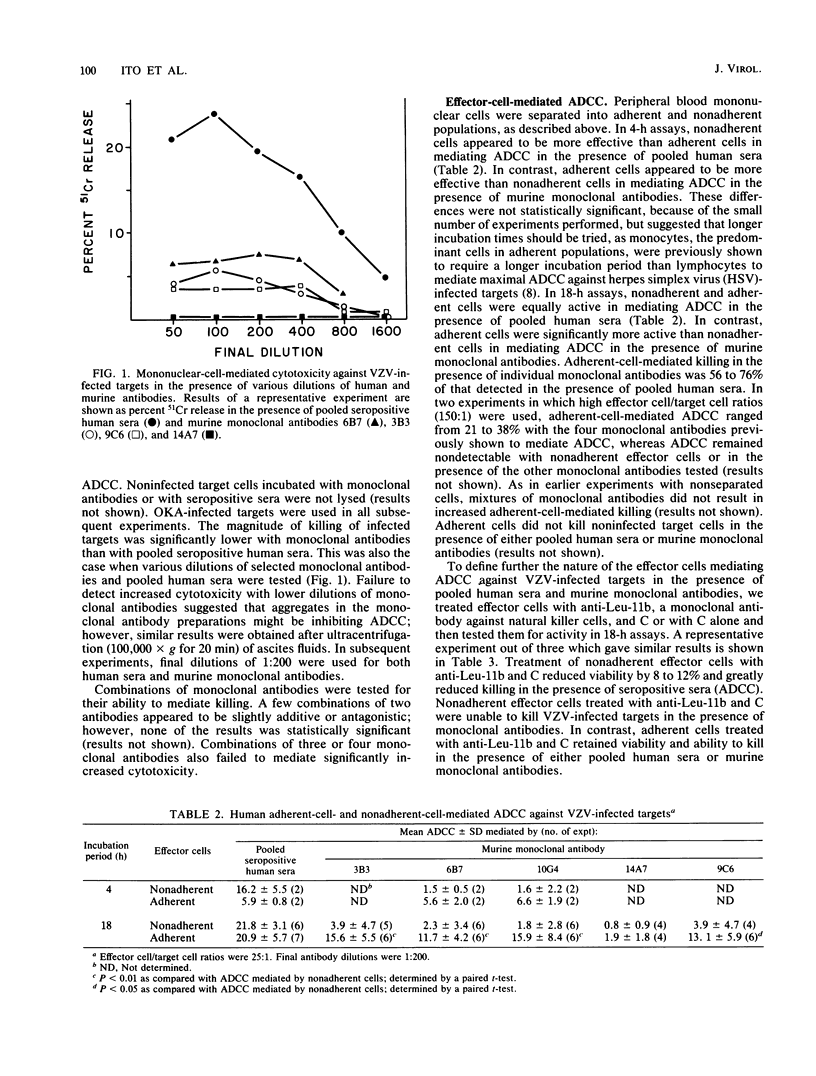
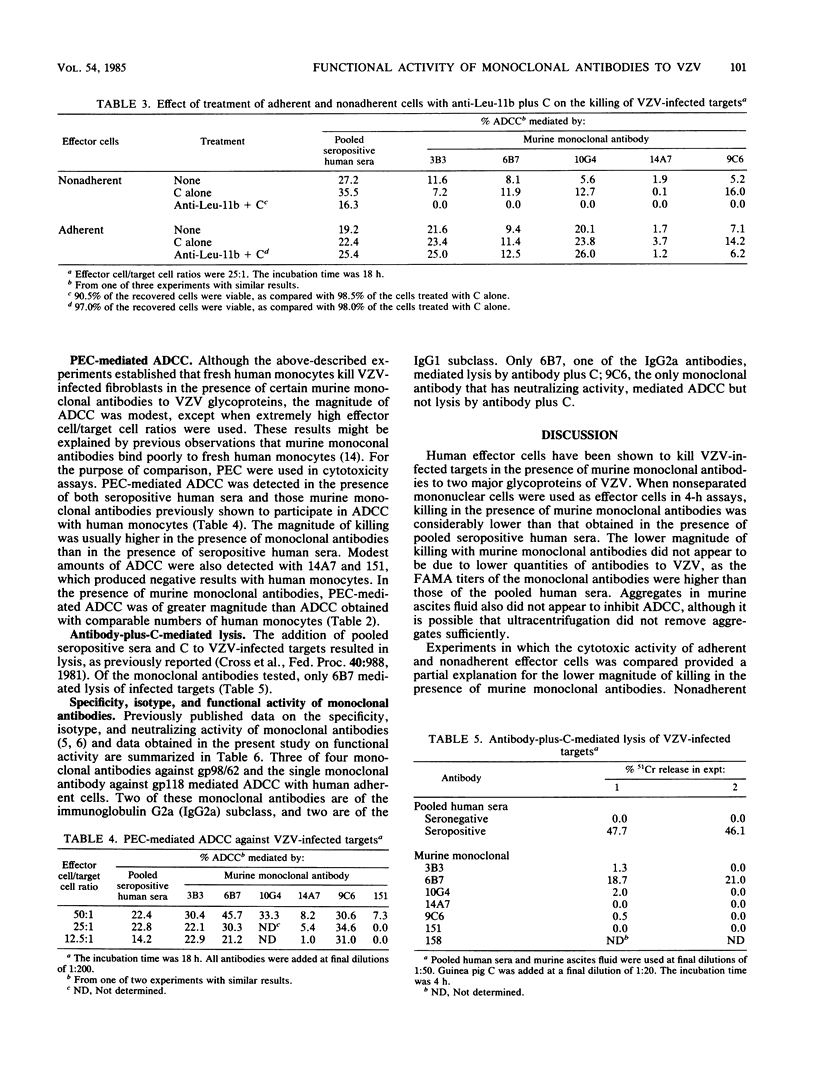
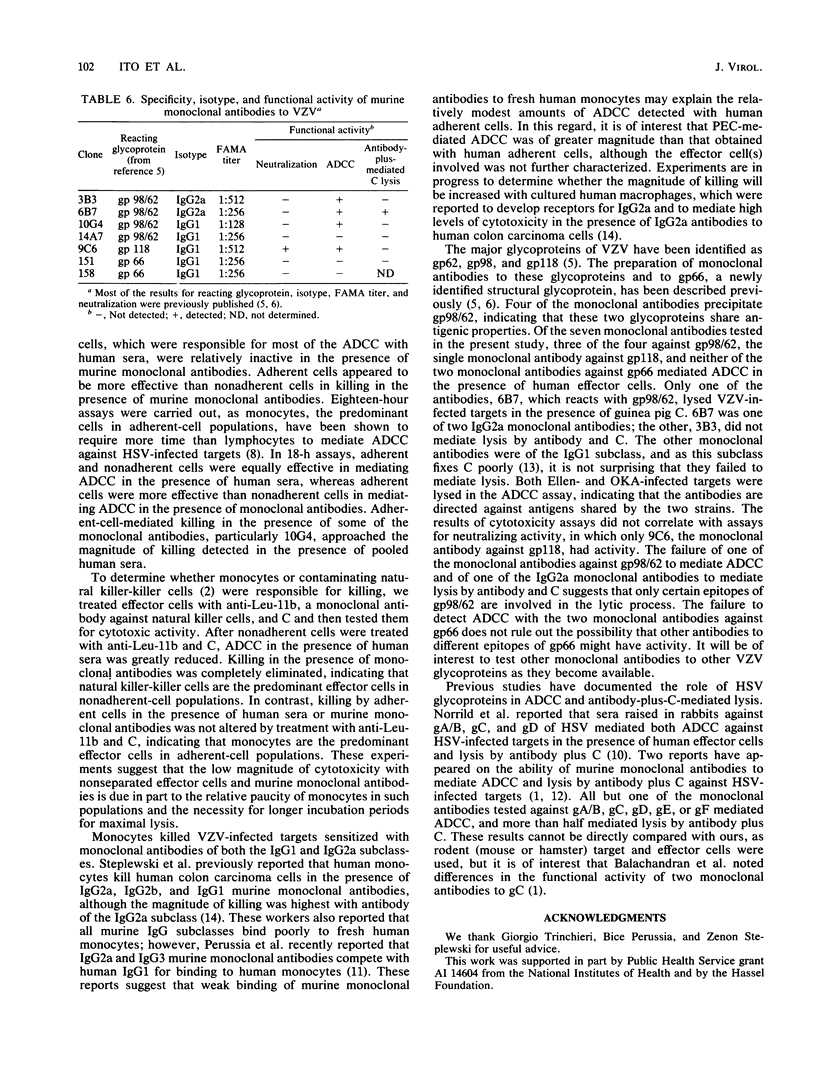
Seven murine monoclonal antibodies reacting with major glycoproteins of varicella-zoster virus were tested for functional activity in assays for antibody-dependent cellular cytotoxicity (ADCC) and antibody-plus-complement-mediated lysis. Human peripheral blood mononuclear cells killed varicella-zoster virus-infected fibroblasts in the presence of three of four monoclonal antibodies directed against gp98/62 and a single monoclonal antibody directed against gp118. Neither of two monoclonal antibodies directed against gp66 was able to mediate ADCC. In 18-h assays, adherent effector cells were more active than nonadherent effector cells in mediating ADCC. Adherent cells treated with anti-Leu-11b and complement retained their cytotoxic activity, suggesting that monocytes are responsible for most of the adherent-cell-mediated cytotoxicity. Both immunoglobulin G1 and G2a murine monoclonal antibodies were able to participate in ADCC. Of the two immunoglobulin G2a monoclonal antibodies tested, both of which reacted with gp98/62, only one mediated lysis in the presence of complement. These results indicate that some murine monoclonal antibodies against major glycoproteins of varicella-zoster virus have functional activity in cytotoxicity assays.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich B., Trinchieri G., Perussia B., Zurier R. B. The cytotoxic effector cells in preparations of adherent mononuclear cells from human peripheral blood. J Immunol. 1984 Mar;132(3):1255–1260. [PubMed] [Google Scholar]
- Gershon A. A., Steinberg S. P. Antibody responses to varicella-zoster virus and the role of antibody in host defense. Am J Med Sci. 1981 Jul-Aug;282(1):12–17. doi: 10.1097/00000441-198107000-00002. [DOI] [PubMed] [Google Scholar]
- Gershon A. A., Steinberg S. P. Inactivation of varicella zoster virus in vitro: effect of leukocytes and specific antibody. Infect Immun. 1981 Aug;33(2):507–511. doi: 10.1128/iai.33.2.507-511.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Friedrichs W. E., Weigle K. A., McGuire W. L. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect Immun. 1983 Apr;40(1):381–388. doi: 10.1128/iai.40.1.381-388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Weigle K. A., Friedrichs W. E., McGuire W. L. Varicella-zoster virus-specific gp140: a highly immunogenic and disulfide-linked structural glycoprotein. Virology. 1984 Jan 15;132(1):138–146. doi: 10.1016/0042-6822(84)90098-9. [DOI] [PubMed] [Google Scholar]
- Kamiya H., Starr S. E., Arbeter A. M., Plotkin S. A. Antibody-dependent cell-mediated cytotoxicity against varicella-zoster virus-infected targets. Infect Immun. 1982 Nov;38(2):554–557. doi: 10.1128/iai.38.2.554-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Starr S. E., oleske J. M., Shore S. L., Ashman R. B., Nahmias A. J. Human monocyte-macrophage-mediated antibody-dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977 Mar;118(3):729–735. [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Lazarus R., Fanning V., Trinchieri G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983 Oct 1;158(4):1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Use of monoclonal antibodies for analysis of antibody-dependent immunity to ocular herpes simplex virus type 1 infection. Infect Immun. 1982 Oct;38(1):168–174. doi: 10.1128/iai.38.1.168-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- Steplewski Z., Lubeck M. D., Koprowski H. Human macrophages armed with murine immunoglobulin G2a antibodies to tumors destroy human cancer cells. Science. 1983 Aug 26;221(4613):865–867. doi: 10.1126/science.6879183. [DOI] [PubMed] [Google Scholar]
- Williams V., Gershon A., Brunell P. A. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis. 1974 Dec;130(6):669–672. doi: 10.1093/infdis/130.6.669. [DOI] [PubMed] [Google Scholar]


