Abstract
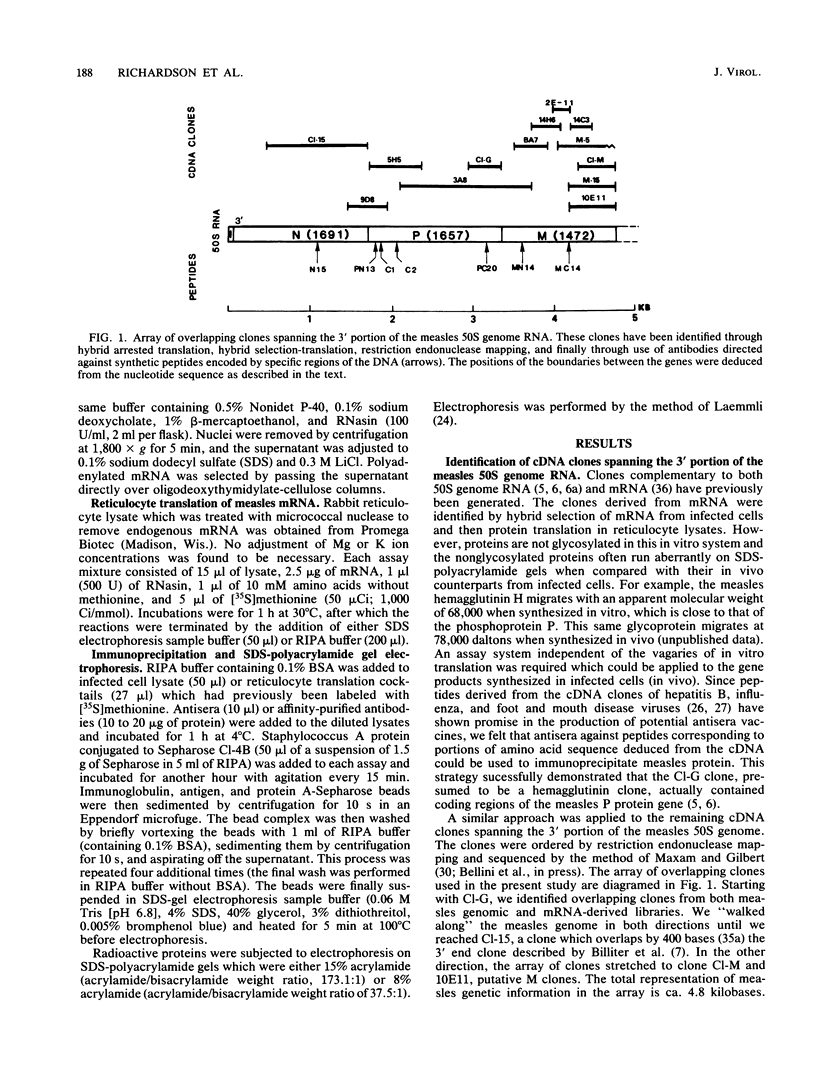
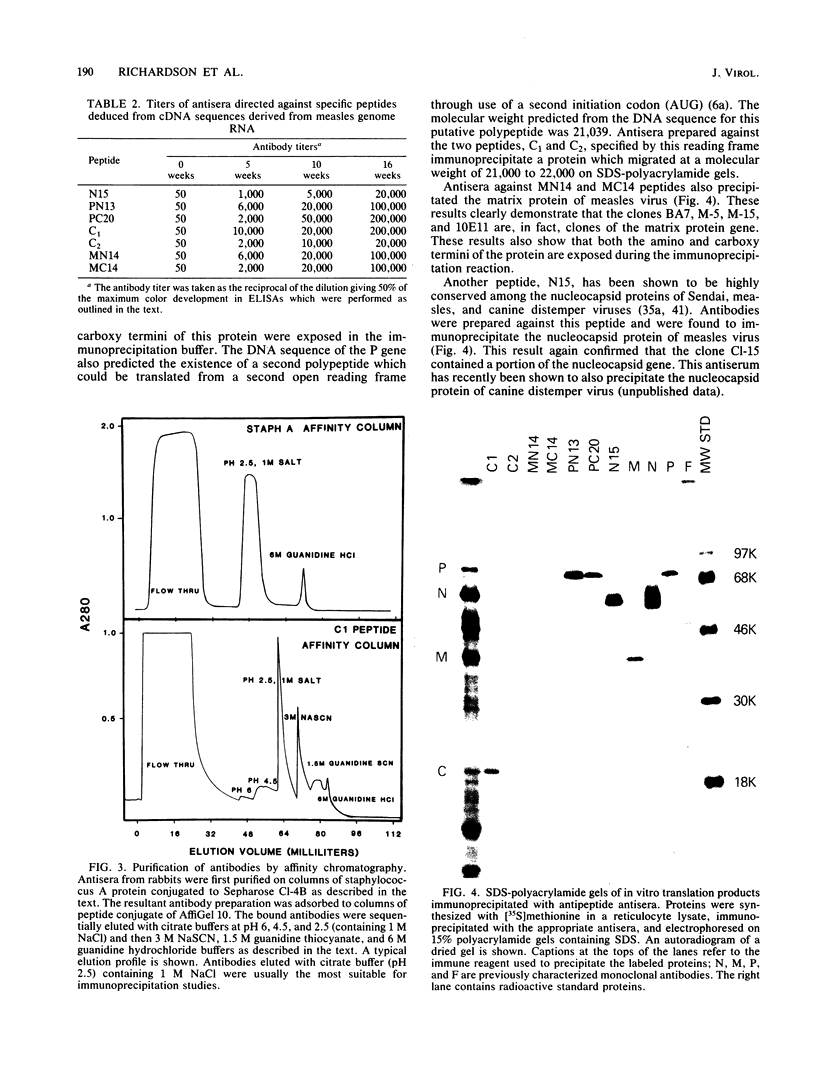
A number of cDNA clones complementary to measles virus mRNA and 50S genome RNA have been generated. These clones have been mapped by restriction enzyme analysis and were subsequently sequenced by the method of Maxam and Gilbert (A. M. Maxam and W. Gilbert, Methods Enzymol. 65:499-560, 1980). Computer analysis of these DNA sequences revealed open reading frames which potentially could code for a number of gene products. Portions of these putative polypeptides were synthesized, and rabbit antibodies directed against peptide-hemocyanin conjugates were produced. These antibodies were used to immunoprecipitate virus-specific polypeptides which were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. For each of the antisera tested, a unique protein was precipitated whose migration on polyacrylamide gels corresponded to standard gene products identified by monoclonal antibodies and antisera against measles virus. By using this method, we were able to assign the coding regions of cDNA clones to specific protein products and, subsequently, to order the genes of the 3'-terminal third of measles genome RNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R. Chemically defined antiviral vaccines. Annu Rev Microbiol. 1980;34:593–618. doi: 10.1146/annurev.mi.34.100180.003113. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Webster R. G. Localization, synthesis, and activity of an antigenic site on influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1983 Feb;80(3):840–844. doi: 10.1073/pnas.80.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K., Billeter M., ter Meulen V. Purification and molecular weight determination of measles virus genomic RNA. J Gen Virol. 1983 Jun;64(Pt 6):1409–1413. doi: 10.1099/0022-1317-64-6-1409. [DOI] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Richardson C. D., Rozenblatt S. Positive identification of a measles virus cDNA clone encoding a region of the phosphoprotein. J Virol. 1984 Jun;50(3):939–942. doi: 10.1128/jvi.50.3.939-942.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Baczko K., Schmid A., Ter Meulen V. Cloning of DNA corresponding to four different measles virus genomic regions. Virology. 1984 Jan 15;132(1):147–159. doi: 10.1016/0042-6822(84)90099-0. [DOI] [PubMed] [Google Scholar]
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W., Ball L. A., Hightower L. E. Coding assignments of the five smaller mRNAs of Newcastle disease virus. J Virol. 1982 Sep;43(3):1024–1031. doi: 10.1128/jvi.43.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L., Kolakofsky D. In vitro synthesis of the nonstructural C protein of Sendai virus. J Virol. 1983 Apr;46(1):321–324. doi: 10.1128/jvi.46.1.321-324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling P. C., Giorgi C., Roux L., Dethlefsen L. A., Galantowicz M. E., Blumberg B. M., Kolakofsky D. Molecular cloning of the 3'-proximal third of Sendai virus genome. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5213–5216. doi: 10.1073/pnas.80.17.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesman G. R., Sanchez Y., Ionescu-Matiu I., Sparrow J. T., Six H. R., Peterson D. L., Hollinger F. B., Melnick J. L. Antibody to hepatitis B surface antigen after a single inoculation of uncoupled synthetic HBsAg peptides. Nature. 1982 Jan 14;295(5845):158–160. doi: 10.1038/295158a0. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Cross R. K., Lamb R. A., Merz D. C., Choppin P. W. In vitro synthesis of structural and nonstructural proteins of Sendai and SV5 viruses. Virology. 1980 Jan 15;100(1):22–33. doi: 10.1016/0042-6822(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Silver S. M., Choppin P. W. Measles virus polypeptides synthesis in infected cells. Virology. 1978 May 1;86(1):254–263. doi: 10.1016/0042-6822(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Lucher L. A., Symington J. S., Kramer T. A. Human adenovirus 2 E1B-19K and E1B-53K tumor antigens: antipeptide antibodies targeted to the NH2 and COOH termini. J Virol. 1983 Dec;48(3):604–615. doi: 10.1128/jvi.48.3.604-615.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Complete sequences of the intergenic and mRNA start signals in the Sendai virus genome: homologies with the genome of vesicular stomatitis virus. Nucleic Acids Res. 1984 May 11;12(9):3829–3841. doi: 10.1093/nar/12.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. The polypeptides of canine distemper virus: synthesis in infected cells and relatedness to the polypeptides of other morbilliviruses. Virology. 1980 Jan 30;100(2):433–449. doi: 10.1016/0042-6822(80)90534-6. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Adler S., Lazzarini R. A., Colonno R. J., Banerjee A. K., Westphal H. Intervening polyadenylate sequences in RNA transcripts of vesicular stomatitis virus. Cell. 1978 Oct;15(2):587–596. doi: 10.1016/0092-8674(78)90027-2. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Schubert M., Keene J. D., Lazzarini R. A. Polycistronic vesicular stomatitis virus RNA transcripts. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4662–4665. doi: 10.1073/pnas.77.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. Respiratory syncytial virus mRNA coding assignments. J Virol. 1983 May;46(2):667–672. doi: 10.1128/jvi.46.2.667-672.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Hamagishi Y., Segawa K., Dalianis T., Appella E., Willingham M. Antibodies against a nonapeptide of polyomavirus middle T antigen: cross-reaction with a cellular protein(s). J Virol. 1983 Dec;48(3):709–720. doi: 10.1128/jvi.48.3.709-720.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Determination by peptide mapping of the unique polypeptides in Sendai virions and infected cells. Virology. 1978 Feb;84(2):469–478. doi: 10.1016/0042-6822(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Sutcliffe J. G., Shinnick T. M. Antibodies to chemically synthesized peptides predicted from DNA sequences as probes of gene expression. Cell. 1981 Feb;23(2):309–310. doi: 10.1016/0092-8674(81)90126-4. [DOI] [PubMed] [Google Scholar]
- Lerner R. A. Synthetic vaccines. Sci Am. 1983 Feb;248(2):66–74. doi: 10.1038/scientificamerican0283-66. [DOI] [PubMed] [Google Scholar]
- Lerner R. A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature. 1982 Oct 14;299(5884):593–596. doi: 10.1038/299592a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. Structure of the gene N:gene NS intercistronic junction in the genome of vesicular stomatitis virus. Cell. 1979 Jul;17(3):673–681. doi: 10.1016/0092-8674(79)90274-5. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Martin S. J. Effect of undiluted passage on the polypeptides of measles virus. J Gen Virol. 1979 Jul;44(1):135–144. doi: 10.1099/0022-1317-44-1-135. [DOI] [PubMed] [Google Scholar]
- Rima B. K., Roberts M. W., McAdam W. D., Martin S. J. Polypeptide synthesis i mumps virus-infected cells. J Gen Virol. 1980 Feb;46(2):501–505. doi: 10.1099/0022-1317-46-2-501. [DOI] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Eizenberg O., Ben-Levy R., Lavie V., Bellini W. J. Sequence homology within the morbilliviruses. J Virol. 1985 Feb;53(2):684–690. doi: 10.1128/jvi.53.2.684-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Gesang C., Lavie V., Neumann F. S. Cloning and characterization of DNA complementary to the measles virus mRNA encoding hemagglutinin and matrix protein. J Virol. 1982 Jun;42(3):790–797. doi: 10.1128/jvi.42.3.790-797.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Venkatesan S. Nucleotide sequence of the gene encoding respiratory syncytial virus matrix protein. J Virol. 1984 Apr;50(1):92–99. doi: 10.1128/jvi.50.1.92-99.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Herman R. C., Lazzarini R. A. Site on the vesicular stomatitis virus genome specifying polyadenylation and the end of the L gene mRNA. J Virol. 1980 May;34(2):550–559. doi: 10.1128/jvi.34.2.550-559.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Hanecak R., Dorner L. F., Wimmer E. A membrane-associated precursor to poliovirus VPg identified by immunoprecipitation with antibodies directed against a synthetic heptapeptide. Cell. 1982 Feb;28(2):405–412. doi: 10.1016/0092-8674(82)90358-0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Sutcliffe J. G., Green N., Lerner R. A. Synthetic peptide immunogens as vaccines. Annu Rev Microbiol. 1983;37:425–446. doi: 10.1146/annurev.mi.37.100183.002233. [DOI] [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Lerner R. A. Antibodies that react with predetermined sites on proteins. Science. 1983 Feb 11;219(4585):660–666. doi: 10.1126/science.6186024. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Liu F. T., Niman H. L., Lerner R. A. Chemical synthesis of a polypeptide predicted from nucleotide sequence allows detection of a new retroviral gene product. Nature. 1980 Oct 30;287(5785):801–805. doi: 10.1038/287801a0. [DOI] [PubMed] [Google Scholar]
- Udem S. A., Cook K. A. Isolation and characterization of measles virus intracellular nucleocapsid RNA. J Virol. 1984 Jan;49(1):57–65. doi: 10.1128/jvi.49.1.57-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Elango N., Chanock R. M. Construction and characterization of cDNA clones for four respiratory syncytial viral genes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1280–1284. doi: 10.1073/pnas.80.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A., Morrison T. Structural and functional characterization of Newcastle disease virus polycistronic RNA species. J Virol. 1984 Jul;51(1):71–76. doi: 10.1128/jvi.51.1.71-76.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. Synthetic peptide fragment of src gene product inhibits the src protein kinase and crossreacts immunologically with avian onc kinases and cellular phosphoproteins. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7412–7416. doi: 10.1073/pnas.78.12.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman A. J. Developing synthetic vaccines. Nature. 1982 Jan 14;295(5845):98–99. doi: 10.1038/295098a0. [DOI] [PubMed] [Google Scholar]