Abstract
Objective
To determine whether the major temporal lobe white matter tracts in patients with temporal lobe epilepsy manifest abnormal water diffusion properties.
Methods
Diffusion tensor MRI measurements were obtained from tractography for uncinate, arcuate, inferior longitudinal fasciculi and corticospinal tract in 13 children with left temporal lobe epilepsy and normal conventional MRI, and the data were compared to measurements in 12 age-matched normal volunteers. The relationship between tensor parameters and duration of epilepsy was also determined.
Results
All four tracts in the affected left hemisphere showed lower mean anisotropy, planar and linear indices, but higher spherical index in patients versus controls. Diffusion changes in the left uncinate and arcuate fasciculus correlated significantly with duration of epilepsy. Arcuate fasciculus showed a reversal of the normal left-right asymmetry. Various diffusion abnormalities were also seen in the four tracts studied in the right hemisphere.
Conclusion
Our findings indicate abnormal water diffusion in temporal lobe and extra-temporal lobe tracts with robust changes in the direction perpendicular to the axons. Diffusion abnormalities associated with duration of epilepsy suggest progressive changes in ipsilateral uncinate and arcuate fasciculus due to chronic seizure activity. Finally, our results in arcuate fasciculus are consistent with language reorganization to the contralateral right hemisphere.
1. Introduction
Diffusion of water molecules can be either free in all directions (isotropic diffusion) or restricted to certain directions (anisotropic diffusion). Anisotropic water diffusion is a characteristic property of brain white matter and is highly sensitive to maturational and pathological changes (Schneider et al., 2004; Sundgren et al., 2004). The water diffusion properties in cerebral white matter now can be studied in vivo using a novel MRI technique, diffusion tensor imaging (DTI). Furthermore, DTI in combination with tractography has become a powerful tool to subdivide cerebral white matter into compartments of tracts so that their diffusion properties can be studied selectively.
Anisotropy of water diffusion appears to be a sensitive indicator of the structural integrity of white matter. For example, tracts which are highly directional, such as the corticospinal tract, have high anisotropy (Snook et al., 2005). In contrast, early blind human subjects with low functional input to the visual system show low anisotropic values in the optic radiation compared to normals (Shimony et al., 2005). In patients with epilepsy, the relationship between functional activity and anisotropy is not clearly understood and may, at first glance, appear paradoxical. Indeed, several studies have found that despite excessive electrical activity in and around the seizure onset zone, there is decreased anisotropy of water diffusion (Li et al., 2003; Thivard et al., 2006). Experimental and human studies suggest that anisotropic changes in epilepsy may be related to multiple structural components such as tissue edema, breach of the blood brain barrier, gliosis, axonal demyelination, and neuronal loss (reviewed by Sutula et al., (Sutula et al., 2003). An occult focal cortical dysplasia in or adjacent to the seizure focus can also lead to decreased anisotropy (Lee et al., 2004).
In children with temporal lobe epilepsy, DTI studies have shown decreased anisotropy in the hippocampus ipsilateral as well as contralateral to the side of seizure onset (Kimiwada et al., 2006). Diffusion changes have also been reported in regions distant from the epileptic focus in adult temporal lobe epilepsy patients (Arfanakis et al., 2002). In recent studies using diffusion tensor tractography in patients with medial temporal sclerosis, loss of anisotropy was seen in the uncinate fasciculus (connecting frontal and temporal lobes) ipsilateral to the side of seizure onset (Rodrigo et al., 2007), and in bilateral fornix and cingulum (Concha et al., 2005). However, these studies did not investigate the other major temporal lobe tracts (i.e., arcuate and inferior longitudinal fasciculus) and, also, no relationship was found between DTI abnormalities and clinical variables, such as chronicity of epilepsy.
Fractional anisotropy (FA) has been the most commonly used anisotropic index in DTI studies. Although this measure is highly sensitive in detecting white matter abnormalities, it loses the directional information contained within the tensor. To overcome this problem, a few authors have used the individual component eigenvectors to measure water diffusion in the direction perpendicular ((λ2 + λ3)*0.5) and parallel (λ1) to the axons (Lazar et al., 2005; Budde et al., 2007). However, with the voxel size of (0.93*0.93*3mm3), this situation is further complicated by the high degree of axonal crossing in cerebral white matter (Zhang et al., 2006). To partially address this issue, some authors have suggested three phase description of a tensor using linear Cl, planar Cp and spherical Cs indices (Westin et al., 1997; Alexander et al., 2000). These indices provide additional directional information and also give a measure of the level of fiber crossing in a voxel.
In the present study, we evaluated changes of diffusion tensor indices in all three major white matter tracts from the temporal lobe in patients (mostly children) with temporal lobe epilepsy. In addition to FA, we also evaluated other tensor indices (Cl, Cp, and Cs) of water diffusion in the isolated white matter tracts.
2. Methods
2.1 Subjects
We selected consecutively 13 children (mean age: 10.9 years ± 6.3, range: 11 months – 19 years, 8 females and 5 males) with refractory temporal lobe epilepsy who had DTI sequences included in their clinical MRI. Selection of the children was further based on the presence of unilateral temporal lobe hypometabolism on positron emission tomography (PET) scans of glucose metabolism and based on the available clinical data, children with known cause of seizures such as head trauma or other acquired causes of seizures were excluded from the study. None of the patients had a history of febrile seizures. PET localizations were based on the clinical reading by a pediatric neurologist and epileptologist (H.T.C.), with extensive expertise in pediatric PET interpretation. We selected only patients with left temporal lobe epilepsy for this study because of the known normal asymmetry of temporal lobe tracts from previous DTI studies (Highley et al., 2002; Park et al., 2004; Nucifora et al., 2005). Most of the children had seizure semiology suggesting temporal lobe epilepsy (Table 1). Scalp ictal EEG in 10 of the patients showed seizure onset arising from the left temporal region, but showed diffuse left sided seizure onset in 3 subjects. The MRI in all 13 patients showed normal findings. Ten of the subjects were right-handed and 3 were left-handed (Table 1). Twelve normal age-matched volunteers who had undergone MRI studies with DTI were selected as control subjects (mean age: 13.2 ± 3.4, range: 7 years – 18 years, 7 females and 5 males). An independent-samples t-test showed no significant differences in the mean ages between the TLE and control groups (mean difference = 2.28 ± 2.06(SE), p=0.28). All our control subjects had been scanned with approval from the Wayne State University Human Subjects Research Committee and sedation was not used. Consent was obtained from both the parent/guardian and the subject.
Table 1.
Demographic, Clinical, and Tractographic profile of Temporal Lobe Epilepsy Patients
| ID | Sex | AGE (months) | DUR (months) | Handedness | ARF |
EEG Finding | Seizure semiology | AED History* | |
|---|---|---|---|---|---|---|---|---|---|
| Right | Left | ||||||||
| 1 | F | 227 | 86 | Right | LS | LS | Occasional sharp waves from left tempo-frontal region | Aura of colors in front of eyes, followed by staring episodes and behavioral arrest | LAM, LEV,LOR |
| 2 | M | 152 | 58 | Right | LS | LS | Frequent spike waves from left fronto-parieto-temporal region | Feeling weird followed by generalized tonic clonic activity | LEV |
| 3 | F | 111 | 99 | Right | LS | LS | Intermittent rhythmic theta activity in left temporal region | Drooling, chewing and repeated swallowing, followed by generalized stiffening, and falls asleep postictally | CAR, LEV |
| 4 | F | 86 | 81 | Left | PS | LS | Intermittent sharp wave activities at the left temporal region | Episodes of agitation with some difficulty in breathing and automatism | VAL, LEV, GAB |
| 5 | F | 40 | 35 | Right | LS | PS | Occasional Sharp wave activity in the left fronto-temporal region | Head drops, stiffness of body with arm lifting and shaking | DIV, TOP, ZON |
| 6 | F | 11 | 5 | Right | LS | PS | Rhythmic theta activity in the left parieto-temporal region | Generalized body stiffening with light twitching of the extremities | PHE, OXC, TOP |
| 7 | M | 200 | 36 | Right | LS | LS | Rare spike and sharp waves in the left subtemporal region | Visual and auditory hallucination, staring spells | DIV, ZON, TOP |
| 8 | M | 199 | 140 | Right | LS | LS | Continuous polymorphic delta slowing over left temporo-occipital region | Feeling weird followed by GTC | ZON, TOP |
| 9 | F | 138 | 114 | Left | LS | LS | Frequent spike waves from left parieto-temporal region | Eye flutters, atonic head drops | LAM, CLO |
| 10 | F | 89 | 25 | Right | LS | PS | Frequent left fronto-temporal spike wave activity as well as frequent generalized spike and wave activities | Staring episodes with behavioral arrest | OXC, LAM, ETO, DIV |
| 11 | M | 208 | 56 | Right | LS | LS | Frequent left sided polyspike bursts | Whole body stiffening, shaking and foaming of the mouth | DIV, ZON, LEV |
| 12 | M | 186 | 175 | Right | LS | LS | Occasional interictal sharp wave activity in the left temporal region during sleep | Staring episodes, eyes and head deviation to right side followed by mouth twitching, and right hand shaking | DIV, OXC, LAM |
| 13 | F | 27 | 24 | Left | LS | LS | Frequent spike and wave and sharp wave activity over the left posterior temporal region | Myoclonic jerking | LAM, OXC, VIG |
Abbreviations: F: female, M: male, DUR: duration of epilepsy, ARF: arcuate fasciculus, LS: long segment of arcuate fasciculus, PS: posterior segment of arcuate fasciculus (Catani et al., 2005). When the long segment (LS) of arcuate fasciculus was absent, the posterior segment (PS) alone was used. AED: Antiepileptic drug, LAM: Lamortigine, LEV: Levetiracetam, LOR: Lorazepam, CAR: Carbamazepine, VAL: Sodium Valporate, GAB: Gabapentin, DIV: Sodium divalproex, PHE: Phenobarbital, ZON: Zonisamide, TOP: Topiramate, OXY: Oxcarbazepine, VIG: Vigabatrin, ETO: Ethosuximide, CLO: Clobazam.
Drug history includes only the available data in our clinical database and it may be incomplete.
2.2. Imaging protocol
MRI scans were performed using a GE system with a 3 Tesla magnet. Diffusion tensor images were acquired in the axial plane with diffusion sensitization gradients applied in 6 non-collinear directions with b-value of 1000 s/mm2. The same imaging parameters were applied to acquire T2 weighted (b ~ 0 s/mm2) images to use as a reference image for signal attenuation measurement. All image volumes were acquired with six optimized directions using six repetitions to increase the number of measures. Acquiring images in each direction with six repetitions improves the image quality (geometric distortion and eddy current artifacts) and increases the signal-to-noise ratio. The echo time was 97 milliseconds, and the repetition time was 13 seconds. A set of minimum 34 axial slices of 3 millimeter thickness without gap, covering the whole brain including the cerebellum, was acquired with matrix size 128×128 and reconstructed in 256×256. Field of view is 240×240 millimeter and the approximate scanning time for the DTI acquisition was 9 minutes.
2.3 Tensor calculation and tractography
Acquired reference image and diffusion sensitized sets were transferred to a PC workstation with Intel Pentium processor and Microsoft windows operation system for further data analysis. Tensor calculation and tractography were performed using DTI-Studio software version 2.40 (Jiang et al., 2006). Tractography was carried out based on Fiber Assignment by Continuous Tracking algorithm FACT (Mori et al., 1999) with fiber propagation starting at fractional anisotropy (FA) threshold value of 0.2 and fiber propagation stopped if FA value was less than 0.2 or angle threshold was greater than 60 degrees.
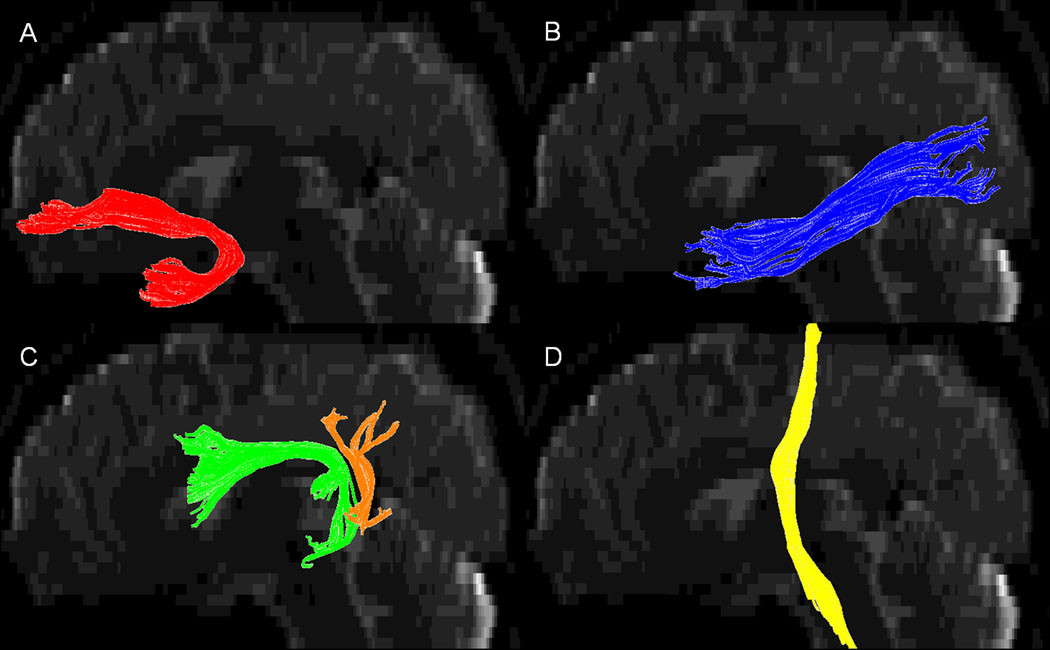
We isolated the 3 major tracts from the temporal lobe: the uncinate, the arcuate and the inferior longitudinal fasciculus (Figure 1). In addition, we also isolated corticospinal tract which contains motor fibers to serve as a ‘reference’ tract outside the temporal lobe. These tracts were isolated using simple ‘region of interest’ (ROI) drawing procedures such as a seed ‘OR’ operator, inclusive ‘AND’ operator and exclusive ‘NOT’ operator (Mori et al., 1999). The ROI drawing procedures for individual tracts are described below.
Figure 1.
3D snapshot view of left (A) uncinate fasciculus, (B) inferior longitudinal fasciculus, (C) arcuate fasciculus, (D) corticospinal tract. In cases where the long segment (green color in (C)) of arcuate fasciculus was absent, we included only the posterior segment (orange color in (C)) of arcuate fasciculus (Catani et al., 2005).
2.3.1 Uncinate fasciculus (UNF)
An initial seed ROI with an ‘OR’ operator is drawn in the coronal section of the frontal lobe with the coronal slice lying anterior to the mid section of the insula. The second ROI with an ‘AND’ is drawn on the temporal lobe in a coronal section lying anterior to the location where the uncinate makes a ‘U’ turn to end in the temporal pole. Multiple small ROIs with ‘NOT’ operator are used to remove the fibers ending close to the thalamus and caudate head.
2.3.2 Inferior longitudinal fasciculus (ILF)
An initial seed ROI with an ‘OR’ operator is drawn in a coronal section of the occipital lobe lying posterior to the splenium of the corpus callosum. A second ROI with ‘AND’ operator is drawn on the temporal lobe in a coronal section lying in the anterior temporal lobe. Multiple small ROIs with ‘NOT’ operator are then used to remove the fibers ending in the parietal lobe, thalamus and frontal lobe.
2.3.3 Arcuate fasciculus (ARF)
An initial seed ROI with an ‘OR’ operator is drawn in the posterior parietal lobe on a transaxial slice at the level of the splenium of the corpus callosum. A second ROI with an ‘AND’ operator is drawn in a coronal slice lying anterior to the arch of the arcuate fasciculus. Multiple small ROIs with ‘NOT’ operator are then used to remove the fibers ending in the thalamus and occipital lobe. However, in the absence of long segment of arcuate fasciculus we included only the posterior segment which connects the posterior temporal lobe with inferior parietal lobe (previously described by Catani et al)(Catani et al., 2005). The long segment of the ARF was absent in five out of twelve normal subjects and one out of thirteen TLE subjects on the right side and three out of thirteen TLE subjects on the left side. In the normal subjects the long segment of the ARF on the left side was easily isolated in all the subjects. To identify any difference in the tensor parameters between the long segment and posterior segment of the ARF, we compared the mean FA and Cs values of the two subgroups (normal subjects with long segment, and normal subjects with posterior segment of ARF) of the normal group on the right side. Seven normal subjects had long segment and five had posterior segment of the ARF on the right side. Independent sample t-test showed no significant differences in the mean of the tensor parameters (FA, Cs) between the two kinds of ARF tracts (FA mean difference = 0.008, p = 0.679, Cs mean difference = 0.014, p = 0.348).
2.3.4 Corticospinal tract (CST)
An initial wide seed ROI with an ‘OR’ operator is drawn in the posterior limb of internal capsule on a transaxial slice. A second ROI with ‘AND’ operator is drawn in a transaxial slice on the anterior aspect of brain stem just below the level of cerebellar efferent fibers decussation. This is followed by a third ROI with an ‘AND’ operator drawn in a transaxial slice in the subcortical white matter underlying the primary motor cortex.
For both patient and control groups, all four tracts were selected in both the right and left hemispheres. The tractography was performed by a single investigator. In our past experience with simple region drawing approach, the measured tensor parameters were highly reliable and robust (Govindan et al., 2008). Tensor parameters such as FA and eigenvalues (λ1, λ2, λ3) of all three eigenvectors (V1, V2, V3) (Figure 2) were recorded from the statistical profile function available in the DTI studio software (Mori et al., 1999). Other tensor parameters such as spherical (Cs), linear (Cl), and planar (Cp) indices were calculated from the measured eigenvalues using the following formulae (Westin et al., 1997; Alexander et al., 2000).
Figure 2.
Graphic representation of the tensor shapes in voxel containing (A) Single group of axon bundle, (B) two groups of crossing axon bundles. In voxels with crossing fibers the secondary eigenvector (V2), perpendicular to the primary eigenvector orients in the direction parallel to the plane containing the crossing fibers. However, the tertiary eigenvector (V3) remains perpendicular to both crossing axonal fibers (Wiegell et al., 2000) and hence more closely represents the water diffusion in the direction perpendicular to the axon bundles.
2.4 Statistical Analysis
Clinical data such as age of subject, date of seizure onset, and date of MRI acquisition were recorded, and the time interval (DUR) between seizure onset and date of MRI scan was calculated. Repeated measures analysis of covariance (ANCOVA) was performed with group (patients and controls) as the between-subject factor, and side (left and right) and tract (UNF, ILF, ARF, and CST) as within-subject factors. Although the comparison between the TLE group and the control group showed no significant differences in the mean ages between the two groups, we used age as a covariate in all subsequent statistical analyses since all tensor parameters undergo an age-related change. Analysis was performed for each of the tensor parameters (FA, Cp, Cl, and Cs). Lateralization index (LAT) of FA, Cl, Cp and Cs were calculated using the formula [LAT = (Left − Right) / (Left + Right)] for each of the four tracts. To identify the effect of duration from seizure onset (DUR) on the tensor parameters, partial correlation (controlled for age) was performed between tensor parameters (FA, Cl, Cp, and Cs,) and their lateralization index with DUR in all four tracts. All statistical analyses were performed using SPSS version 15.
3. Results
Comparison of patient and control groups showed significant differences between the mean values of each of the tensor parameters (FA, Cp, Cl, and Cs) in all four tracts (UNF, ARF, ILF and CST) in the affected left hemisphere. The four tracts from the left hemisphere of the patient group all showed significantly lower mean FA, Cl, and Cp values compared to the normal controls. In contrast, the mean Cs values of the patient group were significantly higher compared to controls for all four tracts in the left hemisphere.
In the contralateral right hemisphere, mean FA values were also significantly lower than controls in all four tracts. In addition, (i) mean Cp values were decreased in right UNF, ILF, and CST; (ii) mean Cl values were decreased in right UNF and CST; and, (iii) the mean Cs values were increased in right UNF, ILF and CST (Table 2) (Figure 3).
Table 2.
Comparison of tensor parameters between patients and controls
| Tract | Tensor parameter | Left Hemisphere |
Right Hemisphere |
||||
|---|---|---|---|---|---|---|---|
| Patient (n=13) |
Control (n=12) |
p-value | Patient (n=13) |
Control (n=12) |
p-value | ||
| UNF | FA | 0.437 (0.010) | 0.483 (0.011) | 0.007 | 0.444 (0.009) | 0.476 (0.009) | 0.026 |
| Cl | 0.196 (0.007) | 0.218 (0.007) | 0.049 | 0.187 (0.007) | 0.21 (0.007) | 0.046 | |
| Cs | 0.642 (0.008) | 0.593 (0.008) | 0.001 | 0.654 (0.008) | 0.607 (0.008) | 0.001 | |
| Cp | 0.162 (0.005) | 0.19 (0.005) | 0.001 | 0.159 (0.005) | 0.182 (0.005) | 0.005 | |
| ILF | FA | 0.457 (0.009) | 0.513 (0.009) | 0.001 | 0.467 (0.008) | 0.503 (0.009) | 0.009 |
| Cl | 0.184 (0.007) | 0.209 (0.007) | 0.030 | 0.195 (0.005) | 0.201 (0.005) | 0.453 | |
| Cs | 0.65 (0.010) | 0.586 (0.010) | 0.000 | 0.631 (0.007) | 0.593 (0.008) | 0.003 | |
| Cp | 0.166 (0.005) | 0.205 (0.005) | 0.000 | 0.175 (0.006) | 0.206 (0.006) | 0.004 | |
| ARF | FA | 0.464 (0.009) | 0.512 (0.010) | 0.002 | 0.47 (0.011) | 0.505 (0.011) | 0.037 |
| Cl | 0.178 (0.005) | 0.201 (0.005) | 0.008 | 0.193 (0.006) | 0.206 (0.006) | 0.193 | |
| Cs | 0.631 (0.011) | 0.564 (0.011) | 0.000 | 0.61 (0.009) | 0.59 (0.010) | 0.178 | |
| Cp | 0.192 (0.008) | 0.235 (0.009) | 0.003 | 0.197 (0.007) | 0.204 (0.008) | 0.537 | |
| CST | FA | 0.567 (0.007) | 0.612 (0.008) | 0.001 | 0.551 (0.009) | 0.602 (0.009) | 0.001 |
| Cl | 0.293 (0.009) | 0.327 (0.009) | 0.022 | 0.282 (0.010) | 0.318 (0.011) | 0.035 | |
| Cs | 0.499 (0.010) | 0.438 (0.011) | 0.001 | 0.525 (0.013) | 0.456 (0.013) | 0.002 | |
| Cp | 0.208 (0.007) | 0.235 (0.007) | 0.023 | 0.193 (0.006) | 0.227 (0.006) | 0.001 | |
All values are given as estimated marginal mean + (standard error), controlled for age. UNF: uncinate fasciculus, ILF: inferior longitudinal fasciculus, ARF: arcuate fasciculus, CST: corticospinal tract. FA : fractional anisotropy, Cl : linear index, Cs : spherical index, Cp : planar index. p value less than 0.05 were considered significant.
Figure 3.
Comparison of the tensor indices (FA, Cl, Cp, and Cs) of uncinate (UNF), inferior longitudinal fasciculus (ILF), arcuate fasciculus (ARF) and corticospinal tract (CST) in right and left hemispheres of both patients and controls.
Significant three way interaction between groups (patient-controls), sides (left-right) and tracts (UNF, ARF, ILF, CST) were seen for the spherical index (Cs) ( F = 4.9, p = 0.010). This interaction showed reversal of the normal asymmetry in ARF with a marked increase in the mean value of the spherical index (Cs) in left ARF compared to left ARF in controls and to the contralateral right ARF, whereas right ARF showed minimal difference in Cs compared to right ARF in controls. Similarly, in left ILF the mean Cs value was significantly increased compared to left ILF in controls and to contralateral right ILF. Furthermore, the mean Cs values were bilaterally increased in UNF and CST compared to the controls. However, no apparent differences were seen in the mean values of left and right UNF and CST (Figure 4).
Figure 4.

Figure showing significant (F = 4.9, p = 0.010) three way interaction between groups (patient-controls), sides (left-right) and tracts (UNF – ARF – ILF - CST) in spherical index (Cs). This interaction shows reversal of normal asymmetry in ARF with marked increase in the mean value of the spherical index (Cs) in left ARF compared to controls, whereas right ARF showed minimal difference compared to controls. In addition, comparison of patient and control groups shows marked difference between the mean values Cs in all four tracts (UNF, ARF, ILF and CST) in the affected left hemisphere.
In the patient group, the spherical index (Cs) of UNF and ARF in the epileptic hemisphere showed significant positive partial correlation (controlled for age) (r = 0.632, p = 0.027, r = 0.601, p = 0.039 respectively) with DUR (duration of epilepsy) (Figure 5). Furthermore, the lateralization index of UNF (Cs) showed an even stronger positive partial correlation with DUR (controlled for age) (r = 0.788, p = 0.002). In addition, lateralization index of UNF FA showed significant negative partial correlation with DUR (controlled for age) (r = −0.601, p = 0.039). Although mean Cs values of the ipsilateral left ILF and contralateral right UNF, ARF, and ILF tracts were significantly higher than in controls, their values did not show a correlation with DUR. The remaining tensor parameters Cl and Cp showed no significant correlation with DUR. No correlation was found between DUR and any of the tensor parameters of CST.
Figure 5.
Partial correlation Plot (controlled for age) of (A) Cs of left UNF, (B) LAT (Cs) of UNF, (C) LAT (FA) of UNF, and (D) Cs of left ARF showing significant correlation with duration of epilepsy (DUR) in the patients with left temporal lobe epilepsy.
4. Discussion
The major findings of the present study were that patients with left temporal lobe epilepsy and normal conventional MRI show decreased anisotropy in all four white matter tracts (UNF, ARF, ILF, and CST) analyzed both ipsilateral and contralateral to the hypometabolic left temporal lobe. Our results are consistent with findings from similar studies performed in both humans and animals (Arfanakis et al., 2002; Fabene et al., 2003; McMillan et al., 2004; Concha et al., 2005; Gross et al., 2006; Jupp et al., 2006; Yu et al., 2006). The present study further demonstrates a significant correlation of water diffusion changes in two of the three ipsilateral temporal lobe tracts (UNF and ARF) with duration of epilepsy. In contrast to the above mentioned adult studies with prolonged duration of epilepsy, our study is based on children with shorter duration of the disease.
4.1 Methodological issues
In our study group all the subjects showed left temporal lobe hypometabolism on the clinical read out as well as electrographic abnormalities in the left temporal region (Table 1). However, some of our subjects showed atypical seizure semiology which, in children, is not uncommon (Fontana et al., 2006; Fogarasi et al., 2007). Another limitation of our study is that we were unable to perform MRI scans on normal children less than 7 years of age because of the need for sedation. The mean age difference between the control and the TLE group is not statistically significant (p = 0.282). Nevertheless, we used age as a covariate in all statistical analyses because of the minor discrepancy between the groups at younger ages. To confirm whether this disparity in the age has affected our results, we performed an auxiliary statistical analysis after removing TLE subjects less than seven years of age (three TLE subjects with Case ID: 5, 6, 13) from the analysis to test whether this change showed any significant differences in the results. This analysis showed similar results as the original comparison with significant three way interaction with the spherical index (Cs) (F = 3.61, p = 0.035) and partial correlation of (Cs of left UNF (r = 0.677, p = 0.045), LAT(Cs) of UNF (r = 0.808, p = 0.008), LAT(FA) of UNF (r = − 0.615, p = 0.078 not significant) with duration of epilepsy (DUR).
4.2 Effect of axonal crossing on tensor parameters
To understand the effect of axonal crossing in a tensor model, it is important to recognize the orientation of the individual eigenvectors in relation to the axons in a relatively large voxel. The ‘principle’ eigenvector is the calculated main anisotropic direction of maximum diffusivity of water and this direction is the mean diffusion direction of all individual axons within the voxel. The direction of the ‘secondary’ eigenvector is assigned in the direction perpendicular to the ‘primary’ eigenvector and it can take a direction in any of the 180 degrees (in the plane) available to it around the primary eigenvector. In situations where fiber crossing is present, the secondary eigenvector will take the direction parallel to the plane of the crossing axonal bundles. Subsequently, the ‘tertiary’ eigenvector will take the only available direction which is perpendicular to both primary and secondary eigenvector (Figure 2). Thus, in situations with low planar index (λ1>> λ2 ~ λ3) where the shape of the tensor is a prolate ellipsoid (i.e., no axonal crossing in a voxel, e.g. genu or splenium of corpus callosum), the traditional scheme of using λ1 as the measure of parallel diffusivity and ((λ2 + λ3) × 0.5) as the measure of perpendicular diffusivity appears to be meaningful (Hasan et al., 2006). However, in most white matter regions we always have some measure of planar index with (λ1> λ2> λ3) and where the shape of the tensor is an oblate ellipsoid (i.e., crossing by two groups of axon bundles) (Wiegell et al., 2000; Zhang et al., 2006), the tertiary eigenvalue alone will be a close representative of the water diffusion perpendicular to both the axonal groups. This assumption is not always true in all the regions of the cerebral white matter but vary depending on the degree of axonal crossing or the planar index in that particular voxel. Given the assumption that some degree of axonal crossing is always present in cortical white matter, the tertiary eigenvalue alone is a close representative of the water diffusion perpendicular to the major groups of axon bundles in cortex. Thus, if the changes in water diffusion are primarily in the direction perpendicular to the axons, one would expect λ3 or spherical index to be a more sensitive measure than FA. This assumption is in agreement with our results where the spherical index showed a stronger relationship with the duration of epilepsy than the FA value.
Loss of anisotropy in cerebral white matter can be explained by either a decrease in water diffusion parallel to the axons or by an increase in water diffusion perpendicular to the axons. For example, during the initial segmentation phase of Wallerian degeneration of axons due to stroke or following a surgical resection, changes primarily occur in the direction parallel to the axons (Concha et al., 2006; Juan et al., 2007). Under such situations, λ1 or Cl are sensitive measures to describe the diffusion changes parallel to the direction of axons. However, in our study, although the mean Cl and Cp values were significantly different from control values, the shift in the patient values were more pronounced in the direction along the Cs axis, and this shift were particularly prominent in the left ILF and ARF. This is illustrated in Figure 6, which describes the geographical distribution of Cl, Cp and Cs in a 3-phase plot using the barycentric coordinate system (Alexander et al., 2000). These observations further suggest that loss of diffusion anisotropy with epilepsy may be primarily due to an increase in water diffusion in the direction of the tertiary eigenvector, perpendicular to the longitudinal direction of the axons.
Figure 6.
Geographical distribution of Cl, Cp and Cs in a 3-phase plot using the barycentric coordinate system (since Cl + Cp +Cs = 1). Although the mean Cl and Cp values of TLE patients were significantly different from those of controls, the shift in the patients’ values was more pronounced in the direction along the Cs axis. This shift was particularly more prominent in left ILF (C) and ARF (E).
4. 3 Analysis methods for white matter tracts
Several studies in patients with epilepsy have described loss of anisotropy and an increase in mean diffusion of water in the hippocampus and in regions close to as well as remote from the seizure focus (Arfanakis et al., 2002; Gross et al., 2006). Most of these studies have used methods of selecting representative regions by manually drawing regions in the brain, i.e., a region-of-interest (ROI) approach. Such regions were selected based on certain anatomical landmarks which could in fact vary significantly across patients. Thus, standardization of these regions in a group of patients with heterogeneous size and shape of brain is difficult. Furthermore, an ROI may contain and therefore be contaminated by fibers that are passing through and therefore not belonging to the tract of interest. Tractography, on the other hand, is a more sophisticated approach which attempts to overcome these limitations by selecting whole regions of the white matter which are connected by their direction of anisotropic water diffusion. An additional advantage of tractography is that grey matter is excluded by setting a high FA threshold (FA > 0.2) during fiber tracking.
However, tractography has its own limitations as well. Sensitivity of the fiber selection is always dependent on the drawing of an initial seed ROI. To overcome this potential bias, we used wide and generous seed ROIs in order to increase the sensitivity in our initial fiber selection. Another limitation of tractography is that visual discrimination of certain fiber groups may be difficult if several fiber groups end close to each other in the cerebral cortex. For example, in the present study, discrimination of the posterior segment of ILF which ends in occipital cortex was always difficult to distinguish from fibers terminating in the parietal cortex. Furthermore, in a few cases isolation of ARF was difficult and occasionally the posterior segment of ARF, connecting the posterior temporal lobe with the inferior parietal lobe, was included in the final tract being analyzed (Table 1) (Catani et al., 2005). Considering the limitations of tractography, we assumed this method to be a reasonable approach since in both the cases (tract with long and posterior segment and tract with posterior segment alone) the white matter tract arises from the superior posterior grey matter region of the temporal lobe and no significant difference were seen in the measured tensor parameters.
4.4 Diffuse loss of anisotropy in epilepsy patients
All the patients in our study group showed hypometabolism in the left temporal lobe and most of the patients showed seizures arising from the affected left temporal lobe. However, the decrease in anisotropic values compared to controls involved all four tracts analyzed in both right and left hemispheres, including the corticospinal tract which is outside the temporal lobe. Such extensive bilateral loss of anisotropy in limbic and nonlimbic (CST) tracts is in agreement with previous adult DTI studies (Arfanakis et al., 2002; Concha et al., 2005; Gross et al., 2006) as well as in volumetric studies (Seidenberg et al., 2005). In another recent study, the bilateral loss of anisotropy in epilepsy patients persisted even after the seizures came under control (Concha et al., 2007). Based on these observations, some investigators have suggested that the overall anisotropy reduction in these patients may be a prevailing condition present even before the onset of clinical seizures. This state of reduced anisotropy could be related to the underlying primary pathology associated with a lowered seizure threshold. It may be the first hit of the “two hit” hypothesis leading to the seizure onset (Lewis 2005). Alternatively, one could argue that the seizures themselves may result in a diffuse loss of anisotropy. Longitudinal DTI studies in epilepsy patients may be useful in elucidating the mechanisms underlying these changes in anisotropy.
Even though most tracts show overall decreased anisotropy, there is some degree of selectivity with respect to individual tracts. For example, diffusion parameters of right ARF/ILF in TLE patients is comparable to controls and this could reflect reorganization (discussed in more detail in section 4.6)
4.5 Effect of chronic seizure activity on temporal lobe tracts
Among the temporal lobe tracts, the left UNF showed a strong positive correlation between duration of epilepsy and both the spherical index Cs and lateralization index of Cs, but a negative correlation with lateralization index of FA. Only the Cs of left ARF showed a significant positive correlation with duration of epilepsy, whereas lateralization index of Cs and FA did not show any correlation. For left ILF, the mean Cs value was increased compared to the contralateral side and to control values, but did not show any correlation with duration of epilepsy. These observations may suggest the possibility that different temporal lobe tracts are affected at different stages of the underlying pathological process in epilepsy propagation. In addition, our sample size may be too small to permit these tracts to reach significance. The UNF, which is part of the limbic system connecting the temporal lobe with the inferior frontal lobe, probably plays a major role in the propagation of seizure activity to the frontal lobe (Chassoux et al., 2004), and the persistence of abnormal electrical activity along this tract could account for the diffusion changes observed.
4.6 Reorganization in the contralateral hemisphere
In a recent study of left temporal lobe epilepsy patients, fMRI and tractographic data suggested language reorganization in the contralateral hemisphere (Powell et al., 2007). The reversal of normal left-right asymmetry observed in the present study is also supportive of contralateral language reorganization. Reorganization of language function to the contralateral hemisphere might well explain why the mean Cs value of the right ARF was closer to control values than left ARF. Also supportive of language reorganization is our finding that 3 patients showed absence of the long segment of ARF on the left side (Catani et al., 2005), whereas in the control group, the long segment of ARF was never absent in the left side(Table 1). This could also reflect reduced anisotropy in the ipsilateral temporal lobe white matter causing fiber tract algorithm stopping inadvertently. However, these observations were confounded by the presence of three left-handed subjects in our patient group. Further study of ARF in our subjects comparing ARF morphology with data from functional methods, such as fMRI or Wada test, would address these issues. Similar changes were also seen in contralateral right ILF with reversal of normal left-right asymmetry although not as apparent as in ARF.
4.7 Degeneration versus Repair
In experimental studies, repeated seizures produce neuronal damage and cell death (Bengzon et al., 1997; Zhang et al., 1998; Kotloski et al., 2002). However, in addition to neuronal death, changes such as axonal demyelination, formation of axonal spines, increase in interstitial fluid volume due to edema, replacement of axons with glial cells, and astrocyte proliferation may all be associated with the damage caused by seizure activity (Sutula et al., 2003). In all these scenarios, the changes related to water diffusion are mostly in the direction of a loss of anisotropy. As mentioned earlier, this loss of anisotropy could be due to decreased parallel diffusivity, increased perpendicular diffusivity, or a combination of the two. Our results suggest that the diffusion changes are primarily in the direction perpendicular to the axons. While the relationship between epilepsy pathophysiology and decreased anisotropy due to a variety of degenerative changes is well established, more precise mechanisms in terms of parallel/perpendicular diffusivity is poorly understood. Mechanisms such as axonal swelling may preferentially increase the perpendicular diffusivity without decreasing parallel diffusivity but clearly, these notions need to be validated. Multiple tissue structural components might be involved in causing such diffusion changes, which are still poorly understood. These diffusion changes could be the result of progressive degenerative changes occurring in the epileptic cortex and underlying white matter with persistent seizures.
Since white matter tracts are always bidirectional, containing both efferent and afferent axons, the afferent axon reaching the epileptic cortex are likely to be less affected by seizures compared to the efferent axons. Moreover, proliferative adaptive changes such as inhibitory axonal sprouting and neurogenesis might also occur as adaptive mechanisms in response to seizure activity (Parent et al., 2002). However, these adaptive changes are probably small and degenerative changes might predominate overall.
Finally, it is generally accepted that persistent seizures cause progressive cognitive impairment as a function of the duration of epilepsy (Helmstaedter 2002; Jokeit et al., 2002). Such functional deficits might well be related to the white matter changes observed in the present study showing a loss of anisotropy related to seizure duration. Duration of epilepsy is only one of the clinical seizure variables that have been investigated in the present study. It is possible that other clinical variables, such as frequency or type of seizures, may also relate to diffusion changes and a more detailed study is required to assess these relationships. Moreover, our observations were made based on a relatively small number of patients and, therefore, further studies with a larger number of patients studied longitudinally and with cognitive testing should also be performed.
Acknowledgement
We sincerely acknowledge the assistance provided by Yun Wang, MAS and Joel W. Ager, PhD in the statistical analysis of the dataset.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. REFERENCES
- Alexander AL, Hasan K, Kindlmann G, Parker DL, Tsuruda JS. A geometric analysis of diffusion tensor measurements of the human brain. Magn Reson Med. 2000;44:283–291. doi: 10.1002/1522-2594(200008)44:2<283::aid-mrm16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, Meyerand ME. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002;20:511–519. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57:688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chassoux F, Semah F, Bouilleret V, Landre E, Devaux B, Turak B, Nataf F, Roux FX. Metabolic changes and electro-clinical patterns in mesio-temporal lobe epilepsy: a correlative study. Brain. 2004;127:164–174. doi: 10.1093/brain/awh014. [DOI] [PubMed] [Google Scholar]
- Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol. 2005;57:188–196. doi: 10.1002/ana.20334. [DOI] [PubMed] [Google Scholar]
- Concha L, Beaulieu C, Wheatley BM, Gross DW. Bilateral white matter diffusion changes persist after epilepsy surgery. Epilepsia. 2007;48:931–940. doi: 10.1111/j.1528-1167.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Marzola P, Sbarbati A, Bentivoglio M. Magnetic resonance imaging of changes elicited by status epilepticus in the rat brain: diffusion-weighted and T2-weighted images, regional blood volume maps, and direct correlation with tissue and cell damage. Neuroimage. 2003;18:375–389. doi: 10.1016/s1053-8119(02)00025-3. [DOI] [PubMed] [Google Scholar]
- Fogarasi A, Tuxhorn I, Janszky J, Janszky I, Rasonyi G, Kelemen A, Halasz P. Age-dependent seizure semiology in temporal lobe epilepsy. Epilepsia. 2007;48:1697–1702. doi: 10.1111/j.1528-1167.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Fontana E, Negrini F, Francione S, Mai R, Osanni E, Menna E, Offredi F, Darra F, Bernardina BD. Temporal lobe epilepsy in children: electroclinical study of 77 cases. Epilepsia. 2006;47 Suppl 5:26–30. doi: 10.1111/j.1528-1167.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- Govindan RM, Chugani HT, Makki MI, Behen ME, Dornbush J, Sood S. Diffusion tensor imaging of brain plasticity after occipital lobectomy. Pediatr Neurol. 2008;38:27–33. doi: 10.1016/j.pediatrneurol.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn Reson Med. 2006;56:130–137. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. Prog Brain Res. 2002;135:439–453. doi: 10.1016/S0079-6123(02)35041-6. [DOI] [PubMed] [Google Scholar]
- Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex. 2002;12:1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Ebner A. Effects of chronic epilepsy on intellectual functions. Prog Brain Res. 2002;135:455–463. doi: 10.1016/S0079-6123(02)35042-8. [DOI] [PubMed] [Google Scholar]
- Juan CJ, liu YJ, Chou MC, Wang CY, Liu HS, Lai TC, Tsai TT, Huang TY, Chung HW, Chen CY. Application of geometrical diffusion tensor imaging on acute ischemic cerebral infarction. Proc. Intl. Soc. Mag. Reson. Med. 2007:2273. [Google Scholar]
- Jupp B, Williams JP, Tesiram YA, Vosmansky M, O'Brien TJ. Hippocampal T2 signal change during amygdala kindling epileptogenesis. Epilepsia. 2006;47:41–46. doi: 10.1111/j.1528-1167.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- Kimiwada T, Juhasz C, Makki M, Muzik O, Chugani DC, Asano E, Chugani HT. Hippocampal and thalamic diffusion abnormalities in children with temporal lobe epilepsy. Epilepsia. 2006;47:167–175. doi: 10.1111/j.1528-1167.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- Kotloski R, Lynch M, Lauersdorf S, Sutula T. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res. 2002;135:95–110. doi: 10.1016/S0079-6123(02)35010-6. [DOI] [PubMed] [Google Scholar]
- Lazar M, Lee JH, Alexander AL. Axial asymmetry of water diffusion in brain white matter. Magn Reson Med. 2005;54:860–867. doi: 10.1002/mrm.20653. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim DI, Mori S, Kim J, Kim HD, Heo K, Lee BI. Diffusion tensor MRI visualizes decreased subcortical fiber connectivity in focal cortical dysplasia. Neuroimage. 2004;22:1826–1829. doi: 10.1016/j.neuroimage.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Lewis DV. Losing neurons: selective vulnerability and mesial temporal sclerosis. Epilepsia. 2005;46 Suppl 7:39–44. doi: 10.1111/j.1528-1167.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- Li N, Gong Z, Saucier D, Kendall EJ, Sarty GE. Water self-diffusion tensor changes in an avian genetic developmental model of epilepsy. Magma. 2003;16:121–128. doi: 10.1007/s10334-003-0020-x. [DOI] [PubMed] [Google Scholar]
- McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004;23:167–174. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport. 2005;16:791–794. doi: 10.1097/00001756-200505310-00002. [DOI] [PubMed] [Google Scholar]
- Parent JM, Valentin VV, Lowenstein DH. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci. 2002;22:3174–3188. doi: 10.1523/JNEUROSCI.22-08-03174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, Duncan JS. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36:209–221. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Golestani N, Cointepas Y, Poupon C, Semah F, Mangin JF, Le Bihan D, Meder JF. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur Radiol. 2007 doi: 10.1007/s00330-006-0558-x. [DOI] [PubMed] [Google Scholar]
- Schneider JF, Il'yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Kelly KG, Parrish J, Geary E, Dow C, Rutecki P, Hermann B. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46:420–430. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- Shimony JS, Burton H, Epstein AA, McLaren DG, Sun SW, Snyder AZ. Diffusion Tensor Imaging Reveals White Matter Reorganization in Early Blind Humans. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sundgren PC, Dong Q, Gomez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46:339–350. doi: 10.1007/s00234-003-1114-x. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Hagen J, Pitkanen A. Do epileptic seizures damage the brain? Curr Opin Neurol. 2003;16:189–195. doi: 10.1097/01.wco.0000063770.15877.bc. [DOI] [PubMed] [Google Scholar]
- Thivard L, Adam C, Hasboun D, Clemenceau S, Dezamis E, Lehericy S, Dormont D, Chiras J, Baulac M, Dupont S. Interictal diffusion MRI in partial epilepsies explored with intracerebral electrodes. Brain. 2006;129:375–385. doi: 10.1093/brain/awh709. [DOI] [PubMed] [Google Scholar]
- Westin CF, Peled S, Gudbjartsson H, Kikinis R, Jolesz FA. Geometrical diffusion measures for MRI from Tensor Basis Analysis. Proc. Intl. Soc. Mag. Reson. Med. 1997:1742. [Google Scholar]
- Wiegell MR, Larsson HB, Wedeen VJ. Fiber crossing in human brain depicted with diffusion tensor MR imaging. Radiology. 2000;217:897–903. doi: 10.1148/radiology.217.3.r00nv43897. [DOI] [PubMed] [Google Scholar]
- Yu AH, Li KC, Yu CS, Wang YP, Xue SF. Diffusion tensor imaging in medial temporal lobe epilepsy. Chin Med J (Engl) 2006;119:1237–1241. [PubMed] [Google Scholar]
- Zhang J, van Zijl PC, Mori S. Image contrast using the secondary and tertiary eigenvectors in diffusion tensor imaging. Magn Reson Med. 2006;55:439–449. doi: 10.1002/mrm.20767. [DOI] [PubMed] [Google Scholar]
- Zhang LX, Smith MA, Li XL, Weiss SR, Post RM. Apoptosis of hippocampal neurons after amygdala kindled seizures. Brain Res Mol Brain Res. 1998;55:198–208. doi: 10.1016/s0169-328x(97)00316-1. [DOI] [PubMed] [Google Scholar]