Internal models are a key concept in motor control, helping explain a myriad of adaptive behaviors, including reaching, walking, and saccadic eye movements. They come in two flavors: inverse and forward models. Inverse models (also known as controllers) take as input a desired trajectory and output the corresponding motor commands. Forward models take an efferent copy of the motor commands and use it to predict the future state of the body (e.g., position, velocity). Although these concepts have been extensively used in explaining behavior in psychophysical studies, their neural correlates have remained difficult to identify.
To investigate the neural correlates of internal models, Ghasia et al. (2008) exploited one of their recent findings: extraocular motoneurons do not seem to encode for the torsion component of eye movements during horizontal and vertical smooth pursuits (Ghasia and Angelaki, 2005). Torsion corresponds to the rotation of the eye about a head-fixed front–back axis. It represents one of the three degrees of freedom along with horizontal and vertical rotations [for review of three-dimensional (3D) kinematics of the eye, see Wong (2004)]. If the center of the fovea is fixating a point on a vertical 2D plane, according to Donder's law the position of the eye corresponds to a unique vertical, horizontal, and torsion angle. As a result, when eye movements are made from one eccentric position to another, they usually contain a torsion component, even when the fovea trajectory is strictly horizontal or vertical. Listing's law, which dictates the rotation vector, predicts that the torsional component of these trajectories is influenced by the eccentricity from which the eye movement is initiated. In a nutshell, the larger the eccentricity, the larger the torsional component, with a sign change occurring at zero eccentricity.
In a previous study, Ghasia and Angelaki (2005) found that the motoneurons responsible for purely torsional movements, such as the ones induced by the roll vestibulo-ocular reflex, did not modulate their activity to account for the variation in torsion component accompanying purely horizontal or vertical smooth pursuit eye movements. That is, the motor output did not include a signal specific to the torsional component of the state of the eyes. Rather, this motion was probably a result of the intrinsic dynamics of the eye plant, which consists of the eye globe, the orbital tissues, and the extraocular muscles. The idea is analogous to the activity of arm muscles and the corresponding motion of the joints in the arm. Activation of shoulder flexors would result in not only a shoulder flexion, but also elbow extension. Therefore, Ghasia and Angelaki (2005) discovered that there exists a dissociation between the coding of the motor output and the resulting state changes in the plant. This dissociation turns out to be critical for looking for neural correlates of inverse and forward models. Premotor cells carrying a signal devoid of torsional information, consistent with that of motoneurons, should correspond to an efferent copy of ongoing motor commands. These cells would be a good candidate for output of an inverse model. On the other hand, cells that discharge with torsional information must correspond to a signal accounting for the complete state of the eye: the output of the forward model.
Ghasia et al. (2008) recorded the activity of mainly two types of premotor cells. Eighty burst-tonic (BT) neurons were recorded from areas including the prepositus/vestibular nuclei, the interstitial nucleus of Cajal, and the perioculomotor region. Forty-six eye-head (EH) neurons were recorded solely from the vestibular nuclei. The methodology was essentially the same as that presented in Ghasia and Angelaki (2005) and consisted of analyzing the spike activity of neurons while monkeys were performing purely horizontal or vertical rectilinear smooth pursuit eye movements made at different eccentricities. They quantified the modulation in activity by normalizing the peak-to-trough firing rate of each neuron with the peak-to-trough smooth pursuit velocity. The gains were then plotted against eccentricity, and a measure of slope was computed. One would expect the gains to vary with eccentricities and therefore the slopes to be different from zero in the case in which a neuron is sensitive to the torsion component of the eye movements. The slopes of each cell type were compared against that of motoneurons recorded in Ghasia and Angelaki (2005), which had been overall found to be null. Remarkably, whereas BT neurons were statistically indistinguishable from the motoneurons, on average the EH neurons had slopes that were significantly greater than zero [Ghasia et al. (2008), their Fig. 3 (http://www.jneurosci.org/cgi/content/full/28/19/5082/F3)].
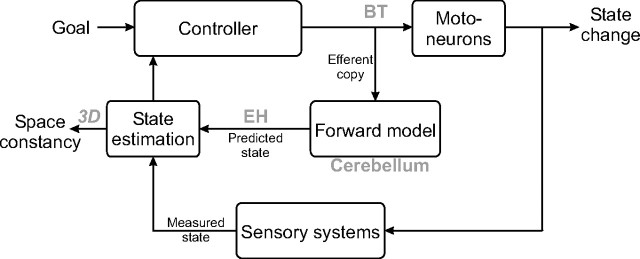
These findings represent neural evidence for the existence of an efferent copy signal carried by BT neurons and sent to various locations in the brain (BT neurons project not only to motoneurons, but also to the cerebellum and the thalamus). Therefore, they are a possible candidate for an output of the inverse model, or simply the “controller” (Fig. 1) (for review, see Shadmehr and Krakauer, 2008). The EH neurons, on the other hand, carry the signal that encodes the actual state of the eyes, including the torsion component. This makes it consistent with the output of a forward model.
Figure 1.
Schematic diagram for generating visually guided eye movements. The controller computes the motor commands that are sent both to the motoneurons and to the forward model. The forward model predicts the sensory consequence of the motor command on the state of the body. This prediction is compared with the estimation of the body state coming from the sensory organs. State estimation of the eye position is fed back to the controller. Similarly, the available 3D information (horizontal, vertical, and torsion) is sent to other subsystems such as those responsible for space constancy. The locations of the premotor cells recorded in Ghasia et al. (2008) are presented within the diagram.
What function might be served by a forward model in the context of eye movements? In Ghasia and Angelaki (2005), it was demonstrated that the torsion component of a movement is attributable to the mechanical properties of the eyeballs, or at least that it is not attributable to the motor output. The transformation from the state space, which includes a torsion coordinate, into motor commands that are devoid of a torsion signal is consequently rank deficient (i.e., the transformation matrix's rank is smaller than the size of its input), when considering a linear model of the eye plant. Therefore, the torsional information is probably irrelevant for control of the eye plant: 2D feedback should suffice to produce the appropriate motor commands. However, the knowledge about the full state of the eye, including its torsion, is essential for space constancy, i.e., the ability to maintain a stable perception of the visual world during eye movements. When the eye is moving in smooth pursuit or when it lands after a saccade, it needs to estimate the orientation of the retina in the 3D space to prevent the world from appearing to be rolling around; the forward model carries the appropriate information to play a role in this process.
We researched the efferent and afferent connections of the neurons under investigation, especially their link with the cerebellum. Consistent with the hypothesis that the cerebellum plays the role of a forward model, a number of BT neurons project to the cerebellum, and EH neurons receive projections from the flocculus and the ventral paraflocculus in the cerebellum (Langer et al., 1985; Lisberger et al., 1994). In other words, BT and EH cells can be viewed as carrying, respectively, input to and output from the cerebellum. This observation further supports the idea that the cerebellum is involved in state estimation through monitoring of efferent copy, as was suggested by recent transcranial magnetic stimulation (Miall et al., 2007) and neurophysiological experiments in reaching (Pasalar et al., 2006).
The output of the forward model, which carries the predicted sensory consequences of action, is expected to combine with the actual sensory inputs to provide a better estimate of the state of the eye. A recent study found neurons in the primary somatosensory cortex (SI) involved in the proprioceptive representation of eye position (Wang et al., 2007). Because the output of the forward model appears to contain torsional information, we predict that these neurons in SI code for torsion as well. An experiment testing this would be a nice follow-up to the authors' work.
In conclusion, Ghasia et al. (2008) presented results that are one of the very first to suggest neural correlates of inverse and forward models in the brain. The results are consistent with the hypothesis that the cerebellum plays the role of a forward model. The input to the forward model is an efferent copy of the motor commands conveyed by BT neurons. Its output, which is coded by EH neurons, is an estimate of the sensory consequences of the motor commands and includes torsion (Fig. 1).
Footnotes
This work was supported by the National Institute of Neurological Disorders and Stroke, the Belgian Program on Interuniversity Attraction Poles initiated by the Belgian Federal Science Policy Office, Fonds National de la Recherche Scientifique, Fonds de la Recherche Scientifique Medicale, Actions de Recherche Concertées, Fonds Spéciaux de Recherche (Belgium), and the European Space Agency (European Union). We thank Drs. Reza Shadmehr and David Zee for insightful comments on this manuscript.
Editor's Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.s
References
- Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293. doi: 10.1016/j.neuron.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Ghasia FF, Meng H, Angelaki DE. Neural correlates of forward and inverse models for eye movements: evidence from three-dimensional kinematics. J Neurosci. 2008;28:5082–5087. doi: 10.1523/JNEUROSCI.0513-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer T, Fuchs AF, Scudder CA, Chubb MC. Afferents to the flocculus of the cerebellum in the rhesus macaque as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1985;235:1–25. doi: 10.1002/cne.902350102. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Responses during eye movements of brain stem neurons that receive monosynaptic inhibition from the flocculus and ventral paraflocculus in monkeys. J Neurophysiol. 1994;72:909–927. doi: 10.1152/jn.1994.72.2.909. [DOI] [PubMed] [Google Scholar]
- Miall RC, Christensen LO, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol. 2007;5:e316. doi: 10.1371/journal.pbio.0050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci. 2006;9:1404–1411. doi: 10.1038/nn1783. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci. 2007;10:640–646. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
- Wong AM. Listing's law: clinical significance and implications for neural control. Surv Ophthalmol. 2004;49:563–575. doi: 10.1016/j.survophthal.2004.08.002. [DOI] [PubMed] [Google Scholar]