Abstract
Prospero is required in dividing longitudinal glia (LG) during axon guidance; initially to enable glial division in response to neuronal contact, and subsequently to maintain glial precursors in a quiescent state with mitotic potential. Only Prospero-positive LG respond to neuronal ablation by over-proliferating, mimicking a glial-repair response. Prospero is distributed unequally through the progeny cells of the longitudinal glioblast lineage. Just before axon contact the concentration of Prospero is higher in two of the four progeny cells, and after axon guidance Prospero is present only in six out of ten progeny LG. Here we ask how Prospero is distributed unequally in these two distinct phases. We show that before neuronal contact, longitudinal glioblasts undergo invaginating divisions, perpendicular to the ectodermal layer Miranda is required to segregate Prospero asymmetrically up to the four glial-progeny stage. After neuronal contact, Prospero is present in only the LG that activate Notch signalling in response to Serrate provided by commissural axons, and Numb is restricted to the glia that do not contain Prospero. As a result of this dual regulation of Prospero deployment, glia are coupled to the formation and maintenance of axonal trajectories.
Keywords: Glia, Prospero, Serrate, Numb, Notch, Miranda, Drosophila, axon guidance, neuron-glia interaction, proliferation
INTRODUCTION
Structural development and repair of the CNS requires that populations of neuronal and glial cell are produced and adjusted in an ordered manner. Generally, it is thought that all the glial cells of a given type are equivalent, glial precursors divide symmetrically producing excess progeny cells and excess glia are eliminated through apoptosis adjusting to axonal volume, enabling correct enwrapment and myelination (Raff et al., 1993; Raff, 1996; Jacobs, 2000; Miller, 2002; Freeman and Doherty, 2006). Furthermore, the proliferation of oligodendrocyte (vertebrate glia) precursors is under the control of an intrinsic timer that dictates how many cell cycles precursors go through before they either differentiate or die (Durand and Raff, 2000). However, glia are also required to aid axon guidance (Chotard and Salecker, 2004; Freeman and Doherty, 2006) and are deployed in discrete numbers to occupy choice-point positions that trigger growth cone guidance and fasciculation events (Hidalgo and Booth, 2000; Hidalgo et al., 2001; Griffiths and Hidalgo, 2004). Although symmetric proliferation ensures sufficient glial numbers for myelination, it does not explain how regulation of the number of glial cells is coupled to axon guidance.
To understand how glia divide it is necessary to look at individual glial lineages with single-cell resolution. This can be done in vivo in Drosophila. Drosophila glia originate from three types of progenitors. (1) Neuroglioblasts give rise to mixed neuronal and glial lineages, with glia specified either through the sequential activation of Notch or the asymmetric segregation of Prospero (Pros) in one of the daughter cells of the ganglion mother cell (GMC), after which the glial precursor divides symmetrically to generate glial cells (Van de Bor et al., 2000; Freeman and Doe, 2001; Udolph et al., 2001). (2) Midline glia precursors, which give rise to midline glia only. (3) Glioblasts such as GB 6-4A (Freeman and Doe, 2001) and the longitudinal glioblast (LGB), which produce only non-midline glial cells. DiI-labelling of LGBs shows that they produce between 7 and 10 progeny cells (Schmidt et al., 1997). Longitudinal Glia (LG) are produced in excess because around two LG per clone normally die (Hidalgo et al., 2001). Even taking into account apoptosis, this indicates that proliferation from the LGB does not follow a simple symmetrical profile.
Neurons influence LG proliferation, which indicates that the mitotic profile of the LGB lineage is not purely symmetrical. When neurons are ablated after axon guidance, LG proliferation decreases initially before increasing slightly (Griffiths and Hidalgo, 2004). These glial responses to neuronal loss require Pros (Griffiths and Hidalgo, 2004). The following evidence shows that Pros modulates LG proliferation. First, Pros is expressed in all dividing LG during embryogenesis. Second, Pros is required for the zygotic expression of cycE (from stage 13) in the LG. Both loss of Pros function and higher than normal levels of Pros result in the repression of cycE in the LG and the cessation of LG proliferation (Griffiths and Hidalgo, 2004). Third, in normal embryos the LG divide first into four cells, two of which then divide to result in six LG, each of which then divides to form 12 LG, whereas in pros mutants the four LG divide into eight LG and do not divide further. This means that Pros controls the timing and profile of LG proliferation. Fourth, the fact that the profile of LG division changes from 4-6-12 to 4-8 means that the division from four to six is skipped in pros mutants (Griffiths and Hidalgo, 2004). This division normally requires epidermal growth factor (EGFR)/Ras/mitogen-activated protein kinase (MAPKinase) signalling in response to Vein produced by pioneering axons but extracellular signal-related kinase (ERK) is not activated in the LG-pros mutants. Fifth, at the end of embryogenesis Pros maintains LG precursors in a quiescent state with mitotitc potential (Griffiths and Hidalgo, 2004). Only the subset of LG that are Pros-positive can re-enter the cell cycle if provided with Cyclin E, and only they over-proliferate on neuronal ablation. This means that Pros maintains the mitotitc potential of LG precursors enabling a glial-repair response. Given these roles of Pros, its distribution is of paramount importance in the control of LG proliferation.
Pros is distributed asymmetrically in the LG at two distinct times. All LG express the glial markers repo, glial cells missing (gcm) and heartless but they express pros unequally. At the beginning of axon guidance, at the 4-LG stage (stage 13), Pros is present in all four LG but the concentration is higher in the two anterior LG and lower in the two posterior LG (Hidalgo et al., 2001; Griffiths and Hidalgo, 2004). The two LG with higher Pros levels activate the EGFR in response to the ligand Vein produced by the pioneer neurons, which induces proliferation and survival (Hidalgo et al., 2001; Griffiths and Hidalgo, 2004). At the end of embryogenesis, Pros is present in only six out of ∼ten LG (stage 16/17). What controls the unequal distribution of Pros in the LG at these two times is not known.
Here, we analyse the mechanisms that regulate the distribution of Pros in LG. We show that two distinct mechanisms regulate the segregation of Pros resulting in the adjustment of LG to the formation of the axonal bundles.
OBJECTIVE
We aim to analyse the mechanisms that control the distribution of Pros in the LG lineage of the embryonic Drosophila CNS. Because Pros regulates glial proliferation in response to interactions with neurons, unravelling its regulation is essential to understand how glia are adjusted to the formation of axonal trajectories.
METHODS
Flies
LG were visualized using the reporter lacZ line F263 (Jacobs et al., 1989). Stocks were generated by conventional genetics to bear F263 in the background, except where indicated.
Mutants and other flies
(1) miraL44 F263/TM3lacZ; (2) inscP49/CyolacZ; F263; (5) dacapo04454/CyOlacZ; F263; (6) numb1/CyOlacZ; F263; (7) SerBG/TM3Ser (A. Tsakonas); (8) Su(H)lacZ (Y. Hiromi). Targeted ectopic expression was driven in all the LG with GAL4 from stage 12.2 using htlGAL4; F263 flies, or using repoGAL4 from the LGB at stage 11, which were crossed to flies driving downstream of UAS the following genes: (1) w: UAS Notchintra (A. Martinez-Arias); (2) w;UASnumb (J. Knoblich); (3) w; UASSer (A. Martinez-Arias). Balancer chromosomes had lacZ reporters and were identified with anti-βgal antibodies.
Immunohystochemistry
Antibody staining was performed following a standard protocol (Patel, 1994), except for embryos stained with dpERK, which were fixed in 8% formaldehyde for 30 min. Antibodies were used in the following dilutions for fluorescence detection: rabbit anti-βgal (Cappel) 1:2500; mouse anti-Repo 1:10 (Iowa HB); rabbit anti-Repo 1:150 (A. Travers); mouse anti-Pros 1:3 (Iowa HB); mouse FasII 1:2 (G. Tear); mouse anti-Notch-intra 1: 10 (C17.9C6, Iowa HB); rabbit anti-pHistone-H3 (Upstate Biotechnology) 1:300; mouse anti-dpERK 1:50 (Sigma); mouse anti-CyclinA 1:3 (Iowa HB); mouse anti-Cyclin B 1:3 (Iowa HB); mouse anti-CyclinE 1:1 (H. Richardson); mouse anti-Miranda 1:1 (M. Landgraf); rabbit Anti-Numb 1:100 (J. Knoblich); anti-Pon (Y.N. Jan); rat anti-Ser 1:500 (K. Irvine); mouse anti-Delta 1:10 (Iowa). As secondary antibodies we used biotinylated antibodies at 1:300 (Jackson and Vector labs) followed by streptavidin Alexa 488, Alexa 594, Alexa 546 and Alexa 660 at 1:250 or directly conjugated to Alexa 488, Alexa 546 and Alexa 594 at 1:250 (Molecular Probes). Embryos were soaked in TOTO-3 (Molecular Probes) at 1:1000 for 10 min in PBS.
Microscopy and imaging
Confocal microscopy was done using BioRad 1024, Radiance 2000 and Leica SP2 laser scanning confocal microscopes. 3-D imaging and transverse views were obtained using Volocity (Improvision). Cell number was counted by 3-D rendering of confocal stacks, followed by analysis of individual clones and rotations.
RESULTS
LG divide cell-autonomously before neuronal contact and non-autonomously after neuronal contact
In a previous report we analysed proliferation of the LG only after axon guidance, at the four progeny-cell stage (4-LG) (Griffiths and Hidalgo, 2004). Here, we trace the complete lineage of the LGB using the LG-lacZ lineage reporter (Jacobs et al., 1989) and the nuclear glial marker Repo. The LGB is large and divides first into two cells of equal size and shape, and then again into four progeny cells (stage 12.2) of equal size and only glial fate (Fig. 1A). Three progeny LG can be seen, which indicates that the first two progeny cells divide asynchronously. These divisions show that the LGB does not follow the mitotic profiles of neuroblasts. In terms of morphology, the LGB divides symmetrically up to the 4-LG stage. The four-cell clusters halt for some time before dividing again (stage 13, 4-LG stage). The two anterior cells divide first, resulting in six progeny LG (five LG can be also seen because division is not synchronous) and then they all divide symmetrically into 12 progeny cells (stage 13-14). These divisions are asynchronous, and again 7-11 progeny cells can also be seen. Some LG die in wild-type flies so, most often, there are ten progeny LG (stage 16, 10-LG stage), but the final number of LG ranges between 8 and 11 progeny cells (Hidalgo et al., 2001). Thus, the LGB does not divide with either a neuroblast or a stem-cell profile. The LGB forms a glial-only lineage that divides into 1-4-6-12 progeny cells.
Fig. 1. LG divide into progeny cells of equal size and glial fate.
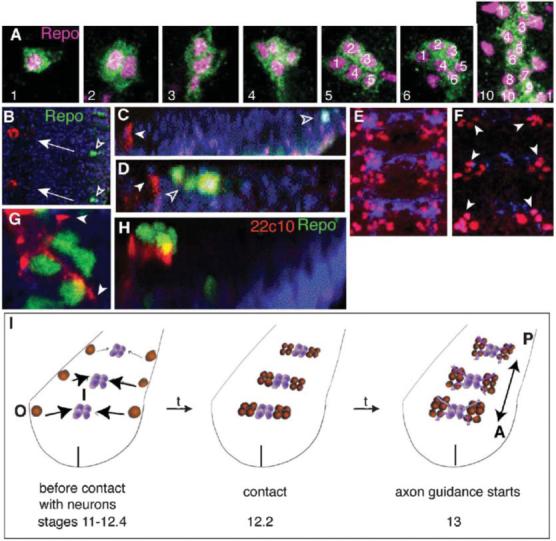
(A) LG are visualized with anti-βgal (green) in LG-reporter-lacZ background and with the nuclear glial marker anti-Repo (magenta). The LG divide into 4-6-12 LG, of which ∼two die per hemisegment resulting in 8-11 LG. Stages with three LG and five LG can be seen because division is asynchronous. (B-D,G,H) The LGB (Repo, green, empty arrowheads) originates away from the midline, at the edge ofthe neuroectoderm and migrates towards the pioneer neurons (22c10, red, white arrowheads). (B) Frontal view of two hemisegments (midline on the left; arrows indicate direction of migration). (C) Transverse view (dorsal up). (D) The LGB invaginates and divides (empty arrowheads), and it approaches the pioneer neurons (22C10, red, white arrowheads). Transverse view. (G,H) First contact of LG with pioneer neurons and axons (22c10, red, arrowheads) at 4-cell stage (stage 12.2). (G) is a frontal view and (H) is a transverse view of the same cluster of LG. (E,F) Nerve cords stained with BP102 (blue) and anti-Repo (red): (E) wild-type; (F) neuronal ablation (elavGAL4/UASricin), showing that LG are present in their normal positions and numbers at stage 13 (arrowheads). (I) Diagram illustrating the invaginating divisions of the LGB and the medial migration towards the deeper location of the neuropile (O, out; I, in), the first contact with neurons at the 4-LG stage and the start of neuron-dependent proliferation of LG during axon guidance as LG also change their direction of migration (arrows) to antero-posterior. Vertical line indicates the midline. Anterior is up and midline to the left in all images, except C,D,H where dorsal is up.
The LGB, which originates at the edge of the neuroectoderm, invaginates and migrates to reach the presumptive neuropile (Fig. 1B-D,G,H,I). This migration is an internalisation into the deeper layer of the presumptive nerve cord and towards the midline (Fig. 1B-D,G,H,I). The LG first contact neurons when there are four progeny cells per progenitor (Fig. 1G,H). From this point, LG proliferation is regulated by neurons and LG migration changes to an anterior-posterior direction as LG migrate together with extending axons (Fig. 1I). Previously, we have shown that ablation of a subset of neurons (with FTZGAL4) affects LG proliferation from the 4-LG stage (stage 13) (Griffiths and Hidalgo, 2004). Here, we show that ablation of all neurons using elavGAL4 (which drives expression of GAL4 in neurons from stage 12 onwards) does not affect LG divisions that occur before axonal contact (LGB up to the 4-LG stage) (Fig. 1F). Neuronal ablation was verified by staining all axons with BP102 as they begin to extend at stage 13 (Fig. 1E,F). In ablated embryos, anti-Repo reveals normal LG at stage 13 (Fig. 1F). Thus, in the absence of neurons the divisions of the LGB up to the 4-LG stage proceeds normally. This is not surprising because there are no axons and few mature neurons that LG could contact before stage 13. Thus, the divisions of the LGB up to 4-LG are controlled cell-intrinsically and do not require interactions with neurons. We have shown previously that Pros regulates glial proliferation in response to interactions with neurons (Griffiths and Hidalgo, 2004), so here we asked whether the distribution of Pros might underlie this switch in the control of LG proliferation.
Miranda segregates high concentrations of Pros in only two out four LG before axon contact
Pros is present in all dividing LG after neuronal contact and excluded from LG that have exited the cell cycle (stages 13-17, after the 4-LG stage) (Griffiths and Hidalgo, 2004). Here, we monitored Pros localisation in the LG before neuronal contact (stages 11-12.1). Pros occurs first in a crescent in the LGB (Fig. 2A,A”). It is then present sequentially in one out of two daughter LG cells, two out of three daughter cells and, finally, all four LG (stage 12.2, Fig. 2A-D,A”-D”). However, the concentration of Pros is higher in the two anterior LG at the 4-LG stage (Fig. 2D,D”) (see also Griffiths and Hidalgo, 2004). This profile shows that Pros is segregated asymmetrically between the first division of the LGB and the 4-cell stage (stages 11-13).
Fig. 2. Asymmetric segregation of Pros and Mira in the early LG divisions LG.
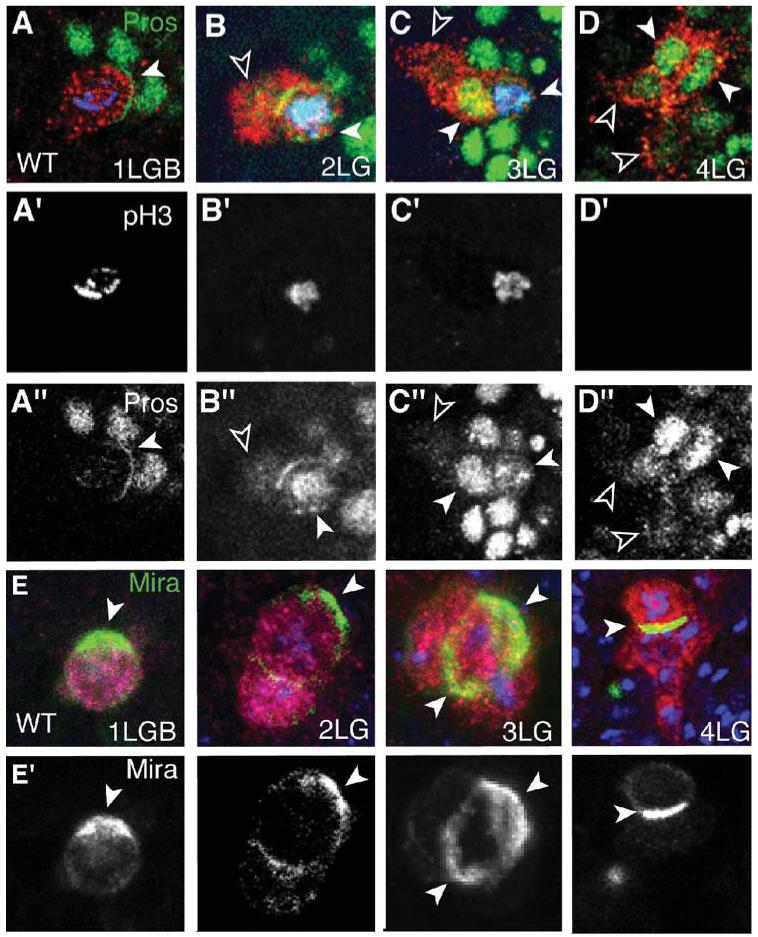
The LG are visualized with anti-βgal in the LG-lacZ reporter background (red or magenta). (A,A”-D,D”) Pros (green) is present in a crescent in the LGB (A,A”, arrowhead), in the nucleus of one of two LG (B,B” white arrowhead) and two of three LG (C,C”, white arrowheads), and in all LG at the 4-cell stage (D,D”), in higher levels in the two anterior LG (white arrowheads, empty arrowheads indicate posterior LG). (A’-D’) The nuclei of dividing LG (anti-pHistone-H3, blue in A-D) contain Pros. The LG rest for a long time at the 4-LG stage and, in this case, they are not dividing. (E,E’) Mira (green, arrowheads) is present in a crescent in the LGB, in one of two LG, in one of three LG (the two arrowheads indicate the crescent viewed from above) and one of four LG. (A’-E’ and A”-D”) are single-channel images, the rest are merged images. Anterior is up, one hemisegment shown.
Pros is segregated asymmetrically during the divisions of neuroblasts by Miranda (Mira) (Matsuzaki, 2000). Hence we asked whether Mira might control the distribution of Pros in higher levels in two of the four LG at stage 13. Mira is found first in a crescent in the LGB (Fig. 1E,E’) and, subsequently, in one crescent in one of the daughter cells up to the 4-LG stage (Fig. 1E,E’, stages 11-13). As in neuroblasts, where Pros and Mira are segregated basally by the apical complex (Betschinger and Knoblich, 2004), both Inscuteable and Bazooka are present in apical crescents in the LGB (data not shown). In mira mutants, Pros is distributed not in a crescent in the LGB but in the nucleus. In subsequent divisions, Pros is present in uniform levels in the nuclei of all daughter cells up to the 4-LG stage (Fig. 3A-D, A’-D’ 100% n = 22 hs). Thus, Pros is no longer distributed asymmetrically in the LG in mira mutants.
Fig. 3. Symmetric segregation of Pros and dpERK in Mira mutants.
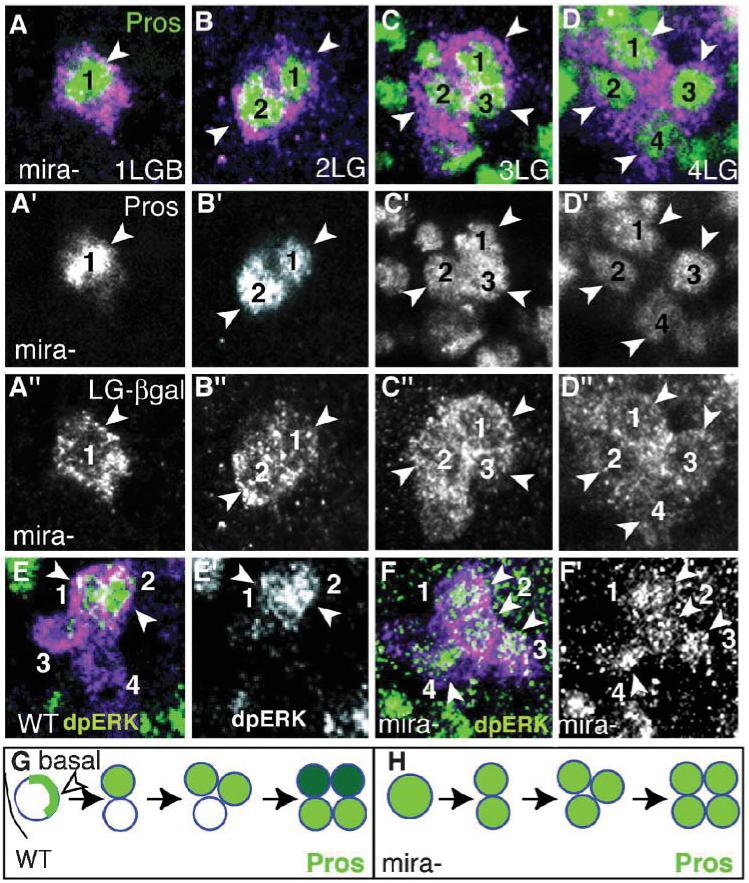
(A-D) Mira-mutant embryos stained with Pros (green) and anti-bgal (magenta) in LG expressing the LG-lacZ reporter, at the LGB stage (A) and at 2-LG (B), 3-LG (C) and 4-LG stages (stages 11-13). Pros is present in the nuclei of all progeny cells in uniform levels. (E,E”) dpERK (green) in wild type at the 4-LG stage. (F,F’) In mira mutants dpERK (green) is present in all progeny LG (arrowheads). (G,H) Diagrams illustrating the distribution of Pros (green, higher levels in dark green) in the LG up to the 4-cell stage in wild-type (G) and in mira mutants (H). (A’-D’,A”-D”,E’,F’) are single-channel images, the rest are merged images. Anterior is up, one hemisegment shown.
In wild-type embryos, the two anterior cells of each 4-LG cluster activates the EGFR and the Ras/MAPK pathway, as seen with anti-dpERK (Fig. 3E,E’) (Griffiths and Hidalgo, 2004). In mira mutants either all of the 4-LG activate ERK (Fig. 3F,F’ 9.4% n = 32 hs) or none do (71.9% n = 32 hs), and the rest of the hemisegments have dpERK in two anterior LG as normal. Presumably, in mira mutants the uniform concentration of Pros can activate ERK in all cells, although in most cases it is below a threshold preventing the activation of ERK in any of the cells. Thus, the asymmetric distribution of Pros high levels in two out of the four LG by Mira determines the fate of the two EGFR/ERK-positive LG.
Numb is distributed uniformly in all LG at the time of neuronal contact
Numb is another cell-fate determinant that is segregated asymmetrically in neuroblasts, sensory organ precursors and vertebrate retinal precursors (Matsuzaki, 2000; Knoblich, 2001). Thus, we asked how Numb might be segregated in the LG. In the LGB, Numb is present in a crescent that seems to extend beyond that of Pros (Fig. 4A,A’,A”). After division, Numb is present in crescents in the two daughter cells (Fig. 4C-E,C’-E’) whereas Pros is present in one of the two progeny LG. At the 4-LG stage, Numb is distributed uniformly throughout all four progeny cells (Fig. 4G,G’) whereas Pros is present in all LG but in higher concentrations in the two anterior LG. Thus, unlike in neuroblasts, Numb is deployed independently of Pros in the LG.
Fig. 4. Numb is not segregated asymmetrically in the LG.
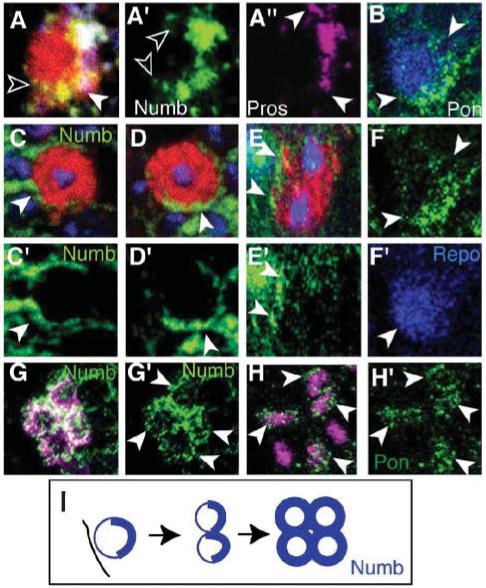
The LG are visualized with anti-βgal (red or magenta) in LG-lacZ reporter wild-type embryos. (A-A”) LGB: Numb crescent (empty arrowheads, green, A’) and Pros crescent (white arrowheads, magenta, A”). The crescent of Numb extends further than that of Pros (A, merge). (B, F,F’) Crescent of Pon (green, arrowheads, B,F) in the LGB (B merge). (C-E) At the 2-cell stage Numb (green, arrowheads, nuclei in blue with TOTO-3) is in both LG. (C-D) 3-D rendered images and rotated views of the apical (C) and the basal (D) progeny cells to show that they each have a crescent of Numb (arrowheads). (E) Transverse view generated using Volocity, of the same LG as in (C,D) to show both progeny cells, each with a crescent of Numb. (C’-E’) Numb channel only. (G,G’) At the 4-cell stage, Numb is throughout the membranes of all four LG (G’, single channel Numb). (H,H’) Pon is present in all 4 LG (stage 13, arrowheads). Here LG are visualized with anti-Repo (magenta), which stains other glia as well as LG. The four LG are identified based on their characteristic arrangement at this stage. (I) Illustration of the distribution of Numb and Pon in the early divisions of the LG. A’-A”,F,C’-F’,G’,H’ are single-channel images.
To confirm the observations with Numb, we monitored the distribution of Partner of Numb (Pon), which localizes Numb in neuroblasts (Knoblich, 2001). In the LGB, Pon is also present in a crescent (Fig. 4B, F). Like Numb, at the 4-LG stage (stage 13) Pon is also present uniformly in all four progeny LG (Fig. 4H,H’). Thus, despite starting as crescents, Pon and Numb are deployed uniformly in all progeny LG by the 4-LG stage.
Unequal distribution of Pros without prior asymmetric segregation of Numb/Pon
At stage 13 Pon, Numb, Pros and Notch coexist in all four progeny LG (see Fig. 2D,D” for Pros, Fig. 4G,H for Numb and Pon and data not shown for Notch). To understand how by the end of embryogenesis Pros becomes restricted to only six out of ten LG, we looked first at the distribution of Pon and Numb in subsequent LG divisions.
Pon is not expressed in the LG after stage 13 (Fig. 5A shows a stage 16 embryo). We detect very high concentrations of Pon at the midline and in other cells at the edge of the nerve cord after stage 13, but never again in the LG. However, whereas Numb is uniform at the 4-LG stage (stage 13), after the 6-LG stage we detect high concentrations of Numb only in the posterior, migrating LG as they exit the cell cycle (Fig. 5B). At stage 16, Numb is restricted to the four posterior, Pros-negative LG and absent from the six anterior Pros-positive LG (Fig. 5B shows 8-LG stage). Consistent with complementary distribution of Pros and Numb, ectopic expression of Numb in the LG downregulates Pros at stage 16 (except in the anterior two LG, (Griffiths and Hidalgo 2004). Similarly, Pros is not downregulated in the LG in numb mutants (except for the two posterior LG) (Fig. 5C, 65%, n = 72 hs). This means that in normal embryos, after an initial uniform distribution at stage 13, Numb is upregulated in the posterior LG, which causes downregulation of Pros.
Fig. 5. Segregation of Pros by Numb and Notch in the LG.
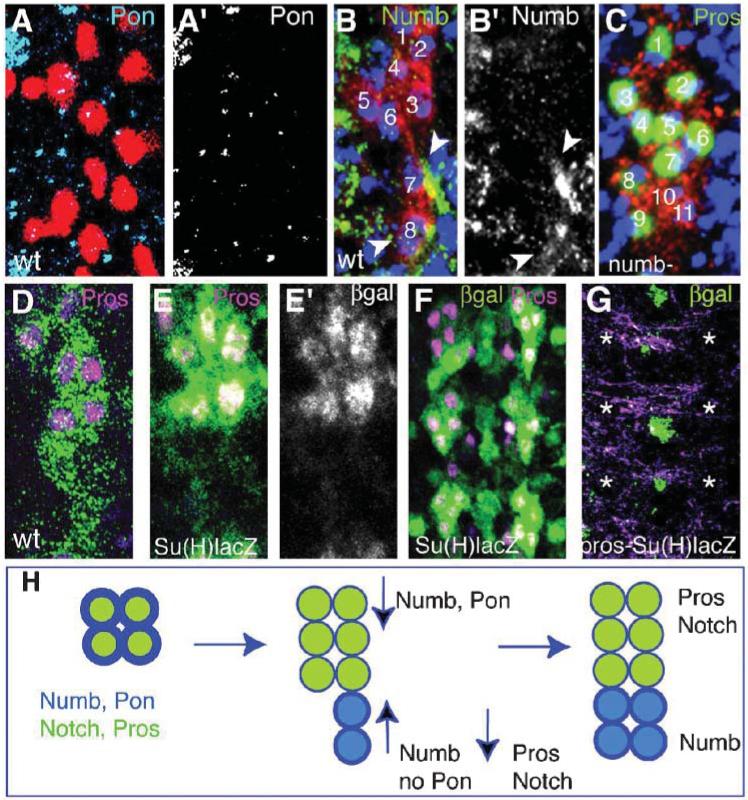
The LG are visualized with either anti-βgal (red or green) in LG-lacZ reporter (B,C,D) or as indicated. (A, A’) Pon (cyan in A and single channel A’) in wild type at stage 16, is present in midline cells but absent from the LG (visualized with anti-Repo, magenta). (B,B’) Numb in wild type at stage 13/14. Numb is upregulated in the LG that migrate posteriorly after dividing (arrowheads) and it is downregulated in the anterior six LG (there are a total of eight LG in this cluster). (C) Pros (green) is present in nine LG instead of six in a numb mutant embryo. There are two LG that do downregulate Pros (10,11). (D) Wild type Pros (magenta) at stage 16, restricted to the six anterior LG. (E,E’) βgal in Su(H)lacZ (green or alone in E’) and Pros (magenta, colocalisation in white) in wild type in the six anterior LG only at stage 16. (F) Lower magnification view of a wild-type embryo as in (E), to show three whole segments. (G) βgal (green) and Pros (magenta) in a pros mutant embryo bearing the Su(H)lacZ reporter. Pros stains axons nonspecifically, but the LG staining is missing and so is Su(H)lacZ in the LG (asterisks indicate location where Pros and β-gal should be). Three segments shown, as in (F). Su(H)lacZ (green) is clearly present at the midline. (H) The distribution of Pros and Notch (green) and Numb (blue) in the LG following the last divisions. At the 4-LG stage all LG contain Numb, Pon, Notch and Pros. As the LG divide, they migrate posteriorly, upregulate Numb and downregulate Pros. The six anterior LG upregulate Pros and Notch and downregulate Numb. Pon is no longer present after the 4-LG stage (stage 13). Anterior is up. (A’,B’,E’) are single channel images. (A-E) show one hemisegment (midline to the left) and (F,G) show three segment images.
Numb is an antagonist of Notch signalling (Schweisguth, 2004). Notch is activated in the six anterior Pros-positive LG at stage 16 as expression of the Su(H)lacZ reporter (a readout of Notch activation) is restricted to these cells (Fig. 5E). Notch maintains Pros in the LG because pros is not downregulated in the posterior LG upon expression of Notchintra (Griffiths and Hidalgo, 2004). Conversely, in pros mutants, Su(H)lacZ is not detected in the LG (Fig. 5G 100% n = 64 hs) whereas the lacZ reporter is still present at high levels at the midline), which means that Pros is also required for Notch expression in the LG. Thus, a positive-feedback loop maintains Notch and Pros expression in the anterior six LG. When they first contact neurons, all LG contain Pros, Numb and Notch. Subsequently Pros and Notch are segregated to the six anterior LG, and Numb to the four posterior LG. These findings indicate that Notch and Pros create a positive-feedback loop that amplifies Pros and Notch levels in the anterior LG, and that the segregation of Numb downregulates Pros in the posterior LG.
In neuroblasts, Numb is segregated asymmetrically, which results in downregulation of Notch in the daughter cell that inherits Numb. Asymmetric division of neuroblasts requires progression through the cell cycle. Thus, we asked whether the downregulation of Pros by Numb in the LG requires progression through the cell cycle.
The first divisions of the LG proceed without gap phases and cell division is not necessary to downregulate Pros in the LG
We first analysed how the cell cycle progresses in the LG lineage. Throughout the early embryo, cell division proceeds initially without gap phases, but different cell-cycle profiles have been predicted for CNS lineages. Gap phases are the times in the cell cycle when cells wait for and respond to signals from neighbouring cells (Roovers and Assoian, 2000). These early divisions are characterized by the broad distribution of maternal CycE, the cytoplasmic (rather than nuclear) distribution and the lack of degradation of other cyclins (Richardson et al., 1993; Knoblich et al., 1994). The first G1 is identified by the downregulation of CycE, which occurs because CycE expression becomes subject to zygotic control, and by the time in which cells arrest in the absence of Dacapo, the p21/p27 homologue that degrades CycE. Using these criteria, we asked whether the cell cycle is constant throughout the LG lineage.
In the LG lineage, before the 4-LG stage, CycA and CycB are found only in the cytoplasm, and in most progeny cells (Fig. 6A,C, stage 12.4). CycA and CycB are localized for the first time in the nucleus at the 4-cell stage (Fig. 6B,D, stage 13). This indicates that these cyclins are not functional before the 4-cell stage. CycE is present in the LGB and at the 2-cell stage (Fig. 6E,F stage 12.4) and it is first downregulated after the two LG start to divide (Fig. 6G). In cycE zygotic mutants there are never more than four LG (100% n = 63 hs at stages 15-17), meaning that from the 4-cell stage on cycE expression is exclusively under zygotic control (Griffiths and Hidalgo, 2004). In some dap mutants there are excess LG (15% hs) (Griffiths and Hidalgo, 2004) but, most often, there are only four LG per hemisegment (Fig. 6I, 70%, n = 23 hs). These observations indicate that cyclins become functional at the 4-cell stage. Thus, at the 4-cell stage LG enter their first cycle with G1 and G2 phases. In pros mutants there is no cycE expression beyond the 4-cell stage in the LG, which indicates that Pros positively regulates the zygotic expression of cycE in these cells (Griffiths and Hidalgo, 2004). Furthermore, in pros the LG divide into eight cells when, normally, there are only 4 (Griffiths and Hidalgo, 2004). The data presented here indicate that in the absence of Pros the LG divide faster because they skip the G1 phase that normally halts LG before axon contact. These findings mean that, in normal embryos, the divisions before axonal contact have no gap phases and are independent of neurons, whereas the divisions after axonal contact have gap phases and depend on extrinsic signals.
Fig. 6. Late segregation of Pros does not require asymmetric cell division.
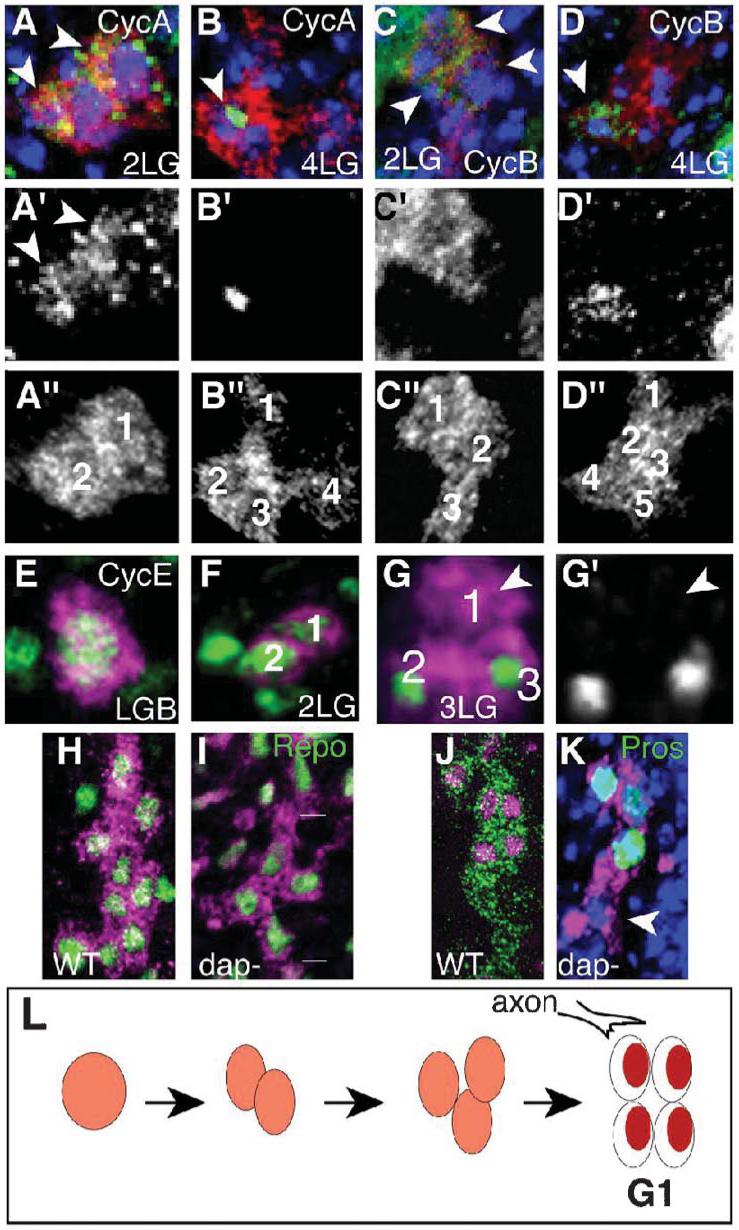
LG are visualized with anti-βgal (red or magenta) in LG-lacZ reporter embryos. (A,B) Anti-CycA (green) is (A) cytoplasmic at 2-LG (arrowheads) and (B) nuclear at the 4-LG stage (arrowheads). (C,D) Anti-CycB is first cytoplasmic (arrowhead, C) and nuclear at the 4-LG stage (arrowhead, D). (E-G) Anti-CycE. CycE is in the LGB and 2-LG stage and it is first downregulated in one LG at the 3-LG stage (G is a 3-D rendered image). (H,I) Glial nuclei are visualized with anti-Repo (green) at stage 15. (H) Wild-type embryos. (I) dacapo mutant embryos (lines indicate LG cluster from one hemisegment) with four LG instead of the normal ∼10 at this stage (stage 15). (J,K) LG stained with Pros (green) at stage 16. (J) wild-type. (K) Despite having four LG instead of ten, Pros is downregulated in one posterior LG in dacapo mutants (white arrowhead) and present in anterior LG. DNA is stained with TOTO-3 in blue. (L) Diagram illustrating the onset of the first G1 in the LG lineage. Anterior is up. (A’-D’,G’) are single channel images of the cyclins. All images represent one hemisegment and the progeny of one glioblast.
There are two dap-mutant phenotypes with variable penetrance: either there is overproliferation of LG (15% hs) (Griffiths and Hidalgo, 2004) or the LG stop dividing at the 4-cell stage and the resulting large LG stretch out along the neuropile (75% hs). Whereas in some cases all progeny LG in dap mutants express Pros (Griffiths and Hidalgo, 2004), often at least one LG downregulates Pros despite the reduction in LG number (Fig. 6K, 75%, n = 70). This means that downregulation of Notch and Pros by Numb in the posterior LG, can take place without further cell division. Although we cannot rule out that downregulation of Pros in dap mutants might be caused by abnormal cell differentiation, the data indicate that the distribution of Pros to the anterior six of the ten LG does not rely on the asymmetric segregation of either Pros or Numb during cell division. This indicates that the distribution of Pros in the LG after neuronal contact relies on extrinsic cues.
Axonal activation of Notch signalling segregates Pros in the LG
We asked whether Notch ligands might trigger the unequal distribution of Pros in the LG at the time of axon contact. A previous report has shown that both Notch ligands Delta (Dl) and Serrate (Ser) are present in neurons (Thomas and van Meyel, 2007). They also show that pan-neural expression of either Dl or Ser results in the ectopic expression of Pros in LG as a result of activation of Notch signalling. Thomas and van Meyel (Thomas and van Meyel, 2007) have proposed that Dl, but not Ser, activates Notch signalling, although Dl is not detected in axons that contact LG.
We show that Ser is expressed in commissural axons (Fig. 7A,B) and is excluded from the pioneer axons of the longitudinal pathways (Fig. 7E). Ser is detected first in commissural axons at stage 13, once the first longitudinal fascicle is formed (Fig. 7E). The division of four LG into six cells occurs as the pioneer longitudinal pathways are first formed, and the last division from six into 10-12 LG after they are already formed, during the formation of the commissures by the follower neurons. This means that Ser is present during the last LG division from six to 12 but not earlier. This is the exact time in which Pros is segregated into the six anterior progeny cells and indicates that Ser is a likely ligand to activate Notch signalling at this time.
Fig. 7. Notch signalling activated from axons triggers segregation of Pros in LG.
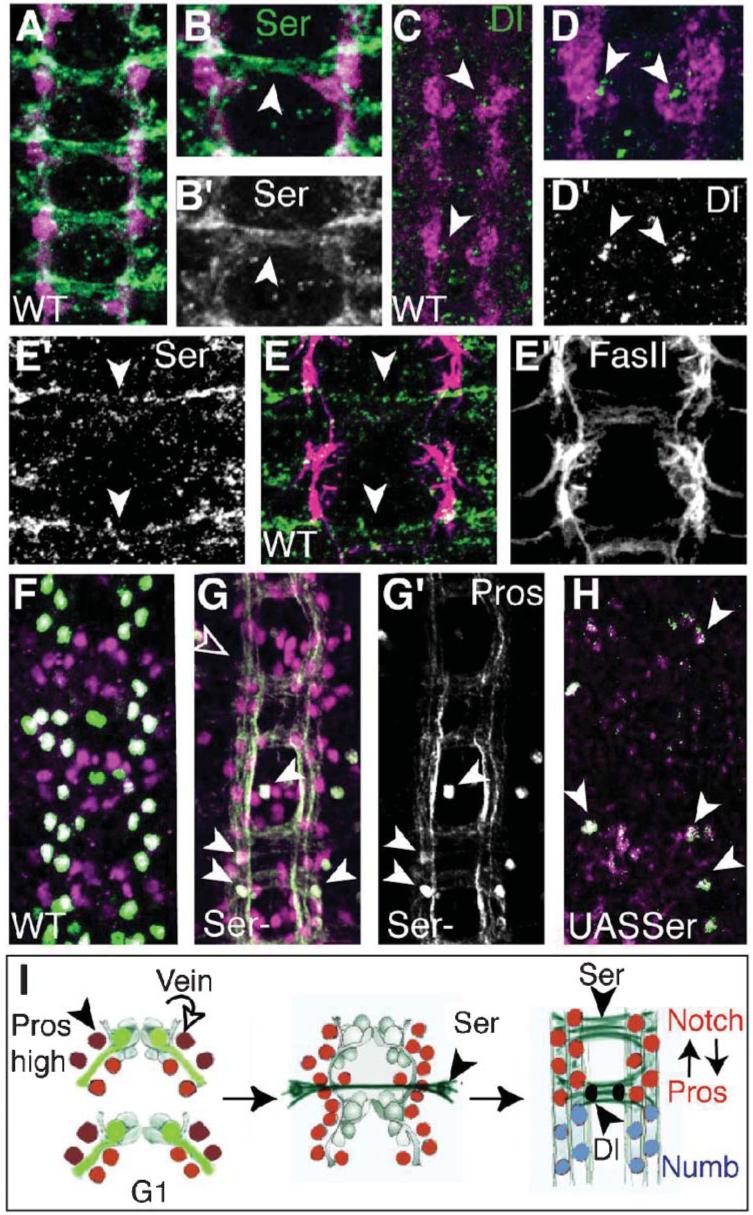
(A-D) LG visualized with βgal (magenta) in LG-LacZ reporter embryos. (A,B) Expression of Ser (green, arrowheads) in commissural axons. (C,D) Expression of Dl (green, arrowheads) adjacent to the LG. (B,D) are higher magnification views of (A,C) to show one segment. (E) Ser expression (green) is absent from pioneer axons of the longitudinal fascicles (magenta, FasII) and is present in the first projecting commissural axons (arrowheads). (E’) Ser channel alone, (F”) FasII channel alone. (F-H) LG stained with Repo (magenta) and Pros (green) (colocalisation in white). (F) Wild-type embryo. (G,G’) In Ser mutants Pros is virtually absent from the LG, arrowheads indicate a few LG that retain Pros staining, Pros staining of axons remains. Empty arrowhead, hemisegment with fewer Repo LG. (G’) Pros channel alone. (H) Ectopic expression of Ser (htlGAL4/UASSer) reduces LG number. Only a few Repo and Pros positive LG remain (arrowheads). (I) Diagram illustrating the two phases of Pros segregation during axon guidance. First, Pros is segregated asymmetrically by Mira to the two anterior LG (dark red) (stages 11-12.5), which divide in response to Vein provided by the pioneer axons of the longitudinal pathways. Subsequently, Pros (red) segregation to the anterior six LG is triggered by activation of Notch signalling by Ser from commissural axons at stage 13 and it is maintained through Notch signalling, which is confined by the source of Dl (black) and the commissural axons expressing Ser (green). Numb (blue) is restricted to the posterior LG. Anterior is up.
To verify whether Ser triggers Notch signalling in the LG as the commissural axons first contact them, we looked at the segregation of Pros in Ser mutants. For this purpose we used the allele SerBG, a dominant-negative allele of Ser that results in the secretion of a truncated Ser protein that sequesters Notch receptors (Hukriede and Fleming, 1997). SerBG-mutant embryos have been shown previously to reproduce Ser-null phenotypes. In SerBG-mutant embryos, Pros is absent from the LG (Fig. 7G, compared with wild type all hemisegments have fewer LG expressing Pros, n = 50 hs, although a few LG retain Pros). The LG still form and can be visualized with anti-Repo. However, Thomas and van Meyel found that in Ser-null mutant embryos Pros is present in six LG, as normal (Thomas and van Meyel, 2007). Their data indicate that, in the absence of Ser, Dl is sufficient to promote Pros expression in the LG, but this does not imply that normally Ser does not activate Notch signalling in the LG. In SerBG however, both Dl and Ser signalling are impaired, which results in the reduction and virtual absence of Pros from the LG.
The Notch ligand Dl is present from stage 13 at the base of the forming commissures (Fig. 7C,D), from where the LG downregulate Pros and migrate posteriorly after dividing. This indicates that the six anterior LG receive both Ser and Dl, and activate Notch sufficiently to maintain Pros. After dividing, the LG that migrate from the Ser-positive axons past the source of Dl reduce their levels of Notch signalling, resulting in upregulation of Numb and downregulation of Pros.
Activation of Notch in the LG might also regulate cell number. In fact, ectopic expression of Numb in LG at the 6-cell stage can reduce the number of progeny from the last division to 5-7 instead of the normal 10-12 (33% of hemisegments, n = 80) (Griffiths and Hidalgo, 2004). Moreover, in Ser-mutant embryos a slight reduction in the number of LG is apparent in some segments and (Fig. 7G) and cis-inactivation of Notch signalling by ectopic expression of Ser in the LG causes loss of almost all LG (Fig. 7H, 87% hs with Repo-positive LG missing, n = 74 hs). These data might indicate that activation of Notch is necessary for LG to either divide or survive.
CONCLUSIONS
Pros is distributed unequally in two distinct phases within the LGB lineage. First, it is present in higher concentrations in two of the four LG at stage 13; subsequently, it is segregated to six of the ten 10 LG at stage 17. The first segregation is cell autonomous, requires Mira and takes place during stages 11-12. The second segregation requires activation of Notch signalling by Ser and maintenance by Dl from axons (Thomas and van Meyel, 2007), the establishment of a positive-feedback loop between Pros and Notch, and the upregulation of Numb in non-Pros LG. This takes place at stages 13-14.
The dual segregation of Pros enables the LG to respond to two distinct types of neuronal signals. First, the two cells that contain more Pros at stage 13 respond to Vein, which is secreted from longitudinal pioneer axons, and secondly, LG that retain Pros respond to Ser and Dl produced by commissural axons.
After the first segregation, Pros is required in LG to divide and survive in response to Vein. However, Pros is not required for the subsequent LG divisions in contact with Ser/Dl axons. Pros is required again for LG to divide following neuronal injury in the glial-repair response. These two distinct mechanisms of Pros segregation underlie the coupling of glial number control to the formation and maintenance of axonal trajectories.
DISCUSSION
The dual segregation of Pros in the LG
We show here that the first two divisions of the LGB lineage (from one LGB to four progeny LG) (Fig. 1I) are cell-autonomous and do not require interaction with neurons. These divisions internalize the LG, directing them deep into the embryo. Insc and Baz, which promote the invagination of neuroblasts, are also present in the LGB and at the 2-LG stage (not shown). The LG progeny make contact with the pioneer neurons in clusters of four cells. As a result of these early divisions, Pros is distributed in higher levels to the two anterior-most of the four LG by Mira, but it is nevertheless present in lower levels in the nuclei of all four LG. The asymmetric segregation of Pros at the 4-LG stage enables two LG to respond to the neuregulin Vein, which is produced by the pioneer axons of the longitudinal pathways. Vein activates EGFR/ERK in the LG to promote proliferation from four to six progeny LG and maintain their survival (Hidalgo et al., 2001; Griffiths and Hidalgo, 2004) (Fig. 7I). We show here that the invaginating divisions are fast and take place without gap phases (Fig. 6L). The 4-LG stage coincides with the first G1 in the lineage, a time when cells respond to signalling molecules. Because Pros also regulates the zygotic expression of CycE and, therefore, progression through G1 (Hidalgo et al., 2001; Griffiths and Hidalgo, 2004), it also controls the timing of the interactions between LG and axons. As a result of these early LG divisions, LG are delivered in restricted numbers (four to six LG) during the formation of the first, pioneering, longitudinal fascicle. This is the first event that couples LG number control to axon guidance (Fig. 7I).
After the first longitudinal fascicle has formed, commissural axons begin to cross the midline when the 4-LG have divided into six cells (Fig. 7I). The commissural axons express Ser. We observe Dl in a cell just posterior to the cluster of six LG at this early stage. Thomas and van Meyel (Thomas and van Meyel, 2007) report expression of Dl in neurons later, at stage 15-16. The six LG at stage 13-14 are confined to a domain delimited by Ser-positive axons and a posterior source of Dl. Arrival of the first commissural axons coincides with the final round of LG division, when the six LG divide into 12 (some then die, resulting in a variable final number of 9-11). These divisions are not necessarily synchronous, and some of the LG migrate posteriorly as they exit the cell cycle, away from the Ser-commissures and past the Dl source. The migrating LG downregulate Pros and upregulate Numb. At the end of embryogenesis six of the ten anterior, Pros-positive LG are in contact with the Ser-positive commissural axons, and four Pros-negative and Numb-positive LG are in contact with the longitudinal axons only (Fig. 7I).
The distribution of Pros in the neuron-dependent divisions of the LG does not require asymmetric segregation of determinants, neither of Pros or Numb (or Pon). In the LGB, Numb and Pon are present in crescents, but by the 4-cell stage, both Numb and Pon are distributed uniformly in all progeny LG. At the 6-LG stage (stage 13), when contact with commissural axons begins, there are basal uniform levels of Pros, Notch and Numb in all LG, and Pon is switched off. Here we propose that the distribution of Pros and Numb to complementary sets of LG results from the activation of Notch by the incoming Ser axons, and that they are confined and maintained by Dl (see also Thomas and van Meyel, 2007). A positive-feedback loop between Pros and Notch amplifies Notch activation and results in the upregulation of Pros in the six anterior LG (out of ten). Notch activation in the LG that migrate away from the commissural axons is reduced, which results in downregulation of Pros and upregulation of Numb. Conversely, the anterior LG that retain Pros also downregulate Numb.
Unique features of the LG mitotic profile
We have shown that the LG follow a unique mitotic profile that is different from previously described lineages (Bernardoni et al., 1999; Gho et al., 1999; Manning and Doe, 1999; Reddy and Rodrigues, 1999; Van de Bor et al., 2000; Ceron et al., 2001; Freeman and Doe, 2001; Jan and Jan, 2001; Henrique and Scheiwsguth, 2003; Nelson, 2003; Betschinger and Knoblich, 2004), with features of both asymmetric and symmetric divisions. A previous report claimed that the LGB divides in a symmetrical fashion into final eight progeny cells (Badenhorst, 2001). However, that report did not follow the LGB cell lineage with single-cell resolution through time, so the 8-LG corresponds to a snapshot of the lineage. We show that LG divide in a 1-4-6-12 profile (see also Hidalgo et al., 2001; Griffiths and Hidalgo, 2004) and that the early divisions segregate Mira and Pros asymmetrically, as well as Inscuteable (Insc) and Bazooka (data not shown), as occurs in neuroblasts. Mira and Insc are also present in apico-basal divisions of the glial lineage of GB6-4A (Freeman and Doe, 2001).
The LG divides following a unique profile. On the one hand it is symmetrical because the LGB divides into two and then into four, there is a later symmetrical division from six into 12, and all LG progeny cells are of equal size and shape and express multiple, general, glial markers equally. Furthermore, the glioblast does not divide like a stem cell but forms two daughter cells of equal size and shape. On the other hand, the divisions of the LG result in the asymmetric segregation of Mira and Pros before neuronal contact. Later, during interactions with neurons, Pros is segregated to six out of ten LG.
The distribution of Pros in the LG lineage presents unique features: (1) in the early divisions (stage 11-12), Pros is segregated independently of Numb and Pon, unlike in neuroblasts. Furthermore, whereas Numb and Pon start off in crescents in the glioblast they are distributed uniformly and symmetrically in all LG by the 4-LG stage. These observations indicate that the first apico-basal divisions of the LGB differ mechanistically from those of neuroblasts. The details underlying these differences are not known; (2) Pros and Numb are uniform in all LG at axon contact (stage 13-14), and in the last LG divisions they are segregated to distinct LG through Notch activation. Insc and Mira are not present in these later divisions and preventing cell division in dap mutants does not interfere with the segregation of Pros. Thus, asymmetric division is not required for the later distribution of Pros (from stage 13 on). Instead, Notch activation confined by Ser and Dl (Thomas and van Meyel, 2007) segregates Pros and Numb to complementary sets of LG. Thus, the segregation of Pros and Numb is defined by cell-extrinsic, positional cues.
Our data are consistent with other reports that have shown roles of Numb that are independent of asymmetric division (Bhalerao et al., 2005). For example, in GMCs Notch signalling is activated extrinsically to the lineage by Dl from the mesoderm leading to Numb segregation to one daughter cell (Lear et al., 1999). Furthermore, Numb can specify cell fate independently of asymmetric division because blocking cell division does not affect cell-fate specification by Numb and Notch signalling in GMCs and muscle precursors (Lear et al., 1999; Wai et al., 1999; Han and Bodmer, 2003). Bhalerao et al. have suggested Numb might function independently of asymmetric cell division by the creation of a positive-feedback loop on activation of Notch that might lead to its amplification in the presence of uniform concentrations of Numb (Bhalerao et al., 2005). Our data on the LG are consistent with this. On Notch activation Numb is repressed in Notch-positive cells and upregulated in other cells. In the LG, Ser and Dl activate Notch, and a positive-feedback loop between Notch and Pros is established that results in the maintenance of Pros and Notch. Our data anticipate that the downregulation of Numb in the six anterior LG might be a direct consequence of Notch activation and/or upregulation of Pros.
Thomas and van Meyel have shown that the LG express fringe initially uniformly and subsequently restricted to the six anterior LG, but they do not explain what triggers the segregation of Fringe to the six anterior LG only (Thomas and van Meyel, 2007). In fringe mutants Pros is present initially and is lost subsequently from the LG, which indicates that Fringe is required only for the maintenance of Pros in the six anterior LG. Fringe inhibits Notch signalling by Ser, thus, it has been proposed that Dl is the ligand that enables Notch signalling in the LG (Thomas and van Meyel, 2007). However, Dl has not been detected in axons in contact with LG. In fact, the neuronal expression of either Dl or Ser can result in the upregulation of Pros in LG. Given that Dl can induce the upregulation of Pros, sufficient Dl is present in Ser loss-of-function mutant embryos to enable the upregulation of Pros. Thus, the Ser loss-of-function mutants do not show that Ser is not normally required for Notch signalling in the LG.
We have shown that: (1) axons that express Ser expressing contact the LG at exactly the time when Pros is maintained in the anterior LG (those in closer contact with Ser-positive axons) and downregulated in the LG that migrate away from the Ser axons; (2) expression of dominant-negative, truncated Ser with SerBG interferes with Notch signalling, resulting in the downregulation of Pros. This phenotype reflects the absence of both Ser and Dl. Because Thomas and van Meyel have shown that both Dl and Ser expressed in neurons can upregulate expression of Pros in LG (Thomas and van Meyel, 2007), the most parsimonious explanation of the SerBG phenotype is that both Ser and Dl activate Notch signalling in the LG; (3) ectopic expression of Ser in all LG results in the downregulation of both Pros and Repo in the LG and, possibly, loss of the LG.
We propose that Ser axons are the first to contact LG at a time when there are six LG and they have uniform distribution of Numb, Pon, Notch and Fringe (Thomas and van Meyel, 2007). Contact with Ser axons results in the activation of Notch signalling in the anterior LG, leading to the establishment of a positive-feedback loop. This loop results in the activation of Fringe and the downregulation of Numb in the six anterior LG. Fringe inhibits Ser, so maintenance of the expression of Notch signalling in the LG depends on Dl (Thomas and van Meyel, 2007). This is the most simple interpretation that accommodates our data and that of Thomas and van Meyel.
The complexity of the LG mitotic profile enables the LG to be delivered in restricted numbers coupled to the formation of axonal trajectories. Because Pros also regulates the G1 phase, which determines the duration of the cell cycle and enables cells to respond to signalling molecules, the complex Pros deployment regulates the timing of neuron-glia interactions. In general, it is possible that each cell type-specific lineage might divide with unique features that enable the attainment of cell fate, and the control and timing of cell interactions.
A potential role for a Notch/Pros feedback loop in vertebrate glial precursors?
The role of Pros and Notch signalling in the LG is to modulate their mitotic potential. Notch signalling is gliogenic or anti-gliogenic in many mixed neuro-glial lineages in vertebrates and in Drosophila (Udolph et al., 2001; Van de Bor and Giangrande, 2001; Umesono et al., 2002; Park and Appel, 2003; Bernardos et al., 2004). However, in the LG neither Pros nor Notch promote glial fate because all LG acquire glial fate (and express glial markers such as Repo and Gcm) regardless of whether they activate Notch. However, only Pros-positive LG are quiescent precursors that retain mitotic potential (Griffiths and Hidalgo, 2004). This role of controlling glial mitotic potential is similar to the roles of Numb and Notch1 in oligodendrocyte (vertebrate glia) precursors in that Notch1 prevents terminal differentiation, and maintains oligodendrocyte precursors in a quiescent, proliferative state (Wang et al., 1998; Genoud et al., 2002; Givogri et al., 2002; John et al., 2002; Park and Appel, 2003). Notch1 also responds to the Ser homologue Jagged1, also present in axons. It is compelling to verify whether analogous functions of the vertebrate homologues of Pros and Numb operate also in vertebrate glia.
ACKNOWLEDGEMENTS
We thank Y. Bellaiche, H. Bellen, W. Chia, C. Doe, J. Dunlop, C. Goodman, I. Hariharan, Y. Hiromi, Iowa Hybridoma Bank, K. Irvine, R. Jacobs, J. Knoblich, M. Landgraf, C. Lehner, A. Martinez-Arias, F. Matsusaki, N. Patel, H. Richardson, K. Saigo, G. Tear, A. Travers and H. Vaessin for reagents. R.G. held a BBSRC Studentship and A.H. a Wellcome Trust CDF, MRC CEG and EMBO Young Investigator Award.
REFERENCES
- Badenhorst P. Tramtrack controls glial number and identity in the Drosophila embryonic CNS. Development. 2001;128:4093–4101. doi: 10.1242/dev.128.20.4093. [DOI] [PubMed] [Google Scholar]
- Bernardoni R, Kammerer M, Vonesch J-L, Giangrande A. Gliogenesis depends on glide/gcm through asymmetric division of neuroglioblasts. Developmental Biology. 1999;216:265–275. doi: 10.1006/dbio.1999.9511. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Müller glia differentiation in the zebrafish retina. Developmental Biology. 2004;278:381–395. doi: 10.1016/j.ydbio.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Current Biology. 2004;14:R674–R685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Bhalerao S, Berdnik D, Török T, Knoblich JA. Localization-dependent and independent roles f numb contribute to cell-fate specification in Drosophila. Current Biology. 2005;15:1583–1590. doi: 10.1016/j.cub.2005.07.061. [DOI] [PubMed] [Google Scholar]
- Ceron J, Gonzalez C, Tejedor FJ. Patterns of cell division and expression of asymmetric cell fate determinants in post-embryonic neuroblast lineages of drosophila. Developmental Biology. 2001;230:125–138. doi: 10.1006/dbio.2000.0110. [DOI] [PubMed] [Google Scholar]
- Chotard C, Salecker I. Neurons and glia: team players in axon guidance. Trends in Neurosciences. 2004;27:655–661. doi: 10.1016/j.tins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Durand B, Raff M. A cell-intrinsic timer that operates during oligodendrocyte development. BioEssays. 2000;22:64–71. doi: 10.1002/(SICI)1521-1878(200001)22:1<64::AID-BIES11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Freeman M, Doe CQ. Asymmetric Prospero localisation is required to generate mixed neuronal/glial lineages in the Drosophila CNS. Development. 2001;128:4103–4112. doi: 10.1242/dev.128.20.4103. [DOI] [PubMed] [Google Scholar]
- Freeman M, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends in Neurosciences. 2006;29:82–90. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Genoud S, Lappe-Siefke C, Goebbels S, Radtke F, Aguet M, Scherer SS, et al. Notch1 control of oligodendrocyte differentiation in the spinal cord. Journal of Cell Biology. 2002;158:709–718. doi: 10.1083/jcb.200202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho M, Bellaiche Y, Schwiesguth F. Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development. 1999;126:3573–3584. doi: 10.1242/dev.126.16.3573. [DOI] [PubMed] [Google Scholar]
- Givogri MI, Costa RM, Schonmann N, Silva AJ, Campagnoni AT, Bongarzone ER. Central nervous system myelination in mice with deficient expression of Notch1 receptor. Journal of Neuroscience Research. 2002;67:309–320. doi: 10.1002/jnr.10128. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Hidalgo A. Prospero maintains the mitotic potential of glial precursors enabling them to respond to neurons. EMBO J. 2004;23:2440–2450. doi: 10.1038/sj.emboj.7600258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Bodmer R. Myogenic cell fates are antagonized by Notch only in asymmetric lineages of the heart, with or without cell division. Development. 2003;130:3039–3051. doi: 10.1242/dev.00484. [DOI] [PubMed] [Google Scholar]
- Henrique D, Scheiwsguth F. Cell polarity: the ups and downs of the Par6/aPKC complex. Current Opinion in Genetics and Development. 2003;13:341–350. doi: 10.1016/s0959-437x(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Booth GE. Glia dictate pioneer axon trajectories in the Drosophila embryonic CNS. Development. 2000;127:393–402. doi: 10.1242/dev.127.2.393. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Kinrade EFV, Georgiou M. The Drosophila Neuregulin Vein maintains glial survival during axon guidance in the CNS. Developmental Cell. 2001;1:679–690. doi: 10.1016/s1534-5807(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Hukriede NA, Fleming RJ. Beaded of Goldschmidt, an antimorphic allele of Serrate, encodes a protein lacking transmembrane and intracellular domains. Genetics. 1997;145:359–374. doi: 10.1093/genetics/145.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JR. The midline glia of Drosophila: a molecular genetic model for the developmental functions of glia. Progress in Neurobiology. 2000;62:475–508. doi: 10.1016/s0301-0082(00)00016-2. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Hiromi Y, Patel NH, Goodman CS. Lineage, migration and morphogenesis of longitudinal glia in the Drosophila CNS as revealed by a molecular lineage marker. Neuron. 1989;2:1621–1635. doi: 10.1016/0896-6273(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Jan Y-N, Jan LY. Asymmetric cell division in the Drosophila nervous system. Nature Reviews Neuroscience. 2001;2:772–779. doi: 10.1038/35097516. [DOI] [PubMed] [Google Scholar]
- John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, et al. Multipkle sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nature Medicine. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- Knoblich J, Sauer K, Jones L, Richardson H, Saint R, Lehner CF. Cyclin E controls S phase progression and its donw-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Asymmetric cell division during animal development. Nature Reviews Molecular Cell Biology. 2001;2:11–20. doi: 10.1038/35048085. [DOI] [PubMed] [Google Scholar]
- Kraut R, Chia W, Jan LY, Jan YN, Knoblich JA. Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature. 1996;383:50–55. doi: 10.1038/383050a0. [DOI] [PubMed] [Google Scholar]
- Lear BC, Skeath JB, Patel NH. neural cell fate in rca1 and cycA mutants: the roles of intrinsic and extrinsic factors in asymmetric divission in the Drosophila central nervous system. Mechanisms of Development. 1999;88:207–219. doi: 10.1016/s0925-4773(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Manning L, Doe CQ. Prospero distinguishes sibling cell fate without asymmetric localization in the Drosophila adult external sense oran lineage. Development. 1999;126:2063–2071. doi: 10.1242/dev.126.10.2063. [DOI] [PubMed] [Google Scholar]
- Matsuzaki F. Asymmetric division of Drosophila neural stem cells: a basis for neural diversity. Current Opinion in Neurobiology. 2000;10:38–44. doi: 10.1016/s0959-4388(99)00052-5. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Progress in Neurobiology. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-C, Appel B. delta-Notch signaling regulates oligodendrocye specification. Development. 2003;130:3747–3755. doi: 10.1242/dev.00576. [DOI] [PubMed] [Google Scholar]
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. In: Goldstein LSB, Fyrberg EA, editors. Drosophila melanogaster: Practical Uses in Cell and Molecular Biology. Academic Press; 1994. pp. 446–485. [DOI] [PubMed] [Google Scholar]
- Raff MC. Size control: the regulation of cell numbers in animal development. Cell. 1996;86:173–175. doi: 10.1016/s0092-8674(00)80087-2. [DOI] [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: Lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Reddy GV, Rodrigues V. Sibling cell fate in the Drosophila adult external sense organ lineage is specified by Prospero function, which is regulated by Numb and Notch. Development. 1999;128:2083–2092. doi: 10.1242/dev.126.10.2083. [DOI] [PubMed] [Google Scholar]
- Richardson H, O’Keele L, Reed S, Saint R. A Drosophila G1-specific cyclin E homolog exhibits different modes of expression during embryogenesis. Development. 1993;119:673–690. doi: 10.1242/dev.119.3.673. [DOI] [PubMed] [Google Scholar]
- Roovers K, Assoian RK. Integrating the MAPKinase siganl into the G1 phase cell cycle machinery. BioEssays. 2000;22:818–826. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM. The embryonic Central Nervous System lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Developmental Biology. 1997;189:186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Regulation of Notch signalling activity. Current Biology. 2004;14:R129–R138. [PubMed] [Google Scholar]
- Thomas GB, van Meyel DJ. The glycosyltransferase Fringe promotes Delta-Notch signaling between neurons and glia and is required for subtype-sepcific glial gene expression. Development. 2007;134:591–600. doi: 10.1242/dev.02754. [DOI] [PubMed] [Google Scholar]
- Udolph G, Rath G, Chia W. A requirement for Notch in the genesis of a subset of glial cells in the Drosophila embryonic central nervous system which arise through asymmetric divisions. Development. 2001;128:1457–1466. doi: 10.1242/dev.128.8.1457. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Hiromi Y, Hotta Y. Context-dependent utilization of Notch activity in Drosophila glial determination. Development. 2002;129:2391–2399. doi: 10.1242/dev.129.10.2391. [DOI] [PubMed] [Google Scholar]
- Van de Bor V, Giangrande A. Notch signalling represses the glial fate in the fly PNS. Development. 2001;128:1381–1390. doi: 10.1242/dev.128.8.1381. [DOI] [PubMed] [Google Scholar]
- Van de Bor V, Walther R, Giangrande A. Some fly sensory organs are gliogenic and require glide/gcm in a precursor that divides symmetrically and produces glial cells. Development. 2000;127:3735–3743. doi: 10.1242/dev.127.17.3735. [DOI] [PubMed] [Google Scholar]
- Wai P, Truong B, Bhat KM. Cell division genes promote asymmetric interaction between numb and Notch in the Drosophila CNS. Development. 1999;126:2759–2770. doi: 10.1242/dev.126.12.2759. [DOI] [PubMed] [Google Scholar]
- Wang J, Ghosh P, Charnay P, Burns DK, Nofsiger D. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]


