Abstract
A Nonidet P-40 extract of HSV-1-purified virions was fractionated by reversed-phase high-performance liquid chromatography (RP-HPLC). The first peak fraction eluted at 25% organic solvent. Polyacrylamide gel electrophoresis showed that it contained a 57,000-dalton polypeptide. The polypeptide was characterized by determination of the amino acid composition and the N-terminal amino acid sequence. Adsorption of the detergent extract before RP-HPLC showed that the polypeptide reacted with monoclonal antibodies LP1 directed against herpes simplex virus polypeptide VP-16.
Full text
PDF
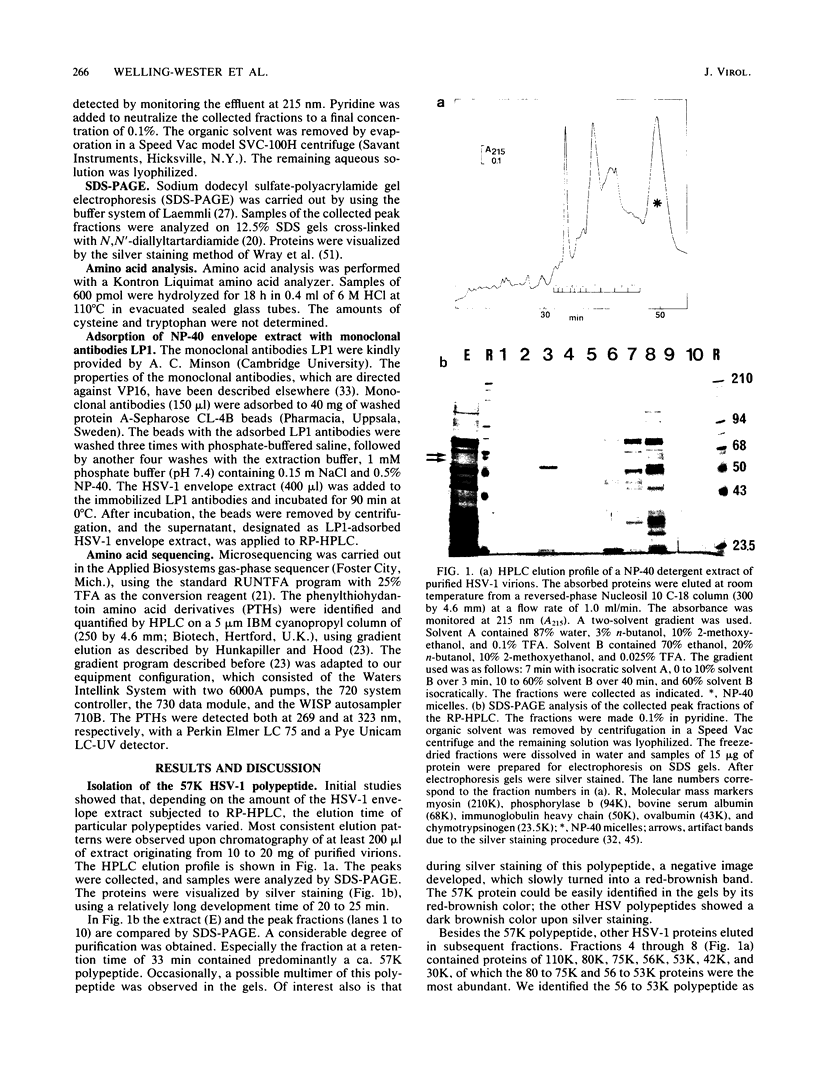
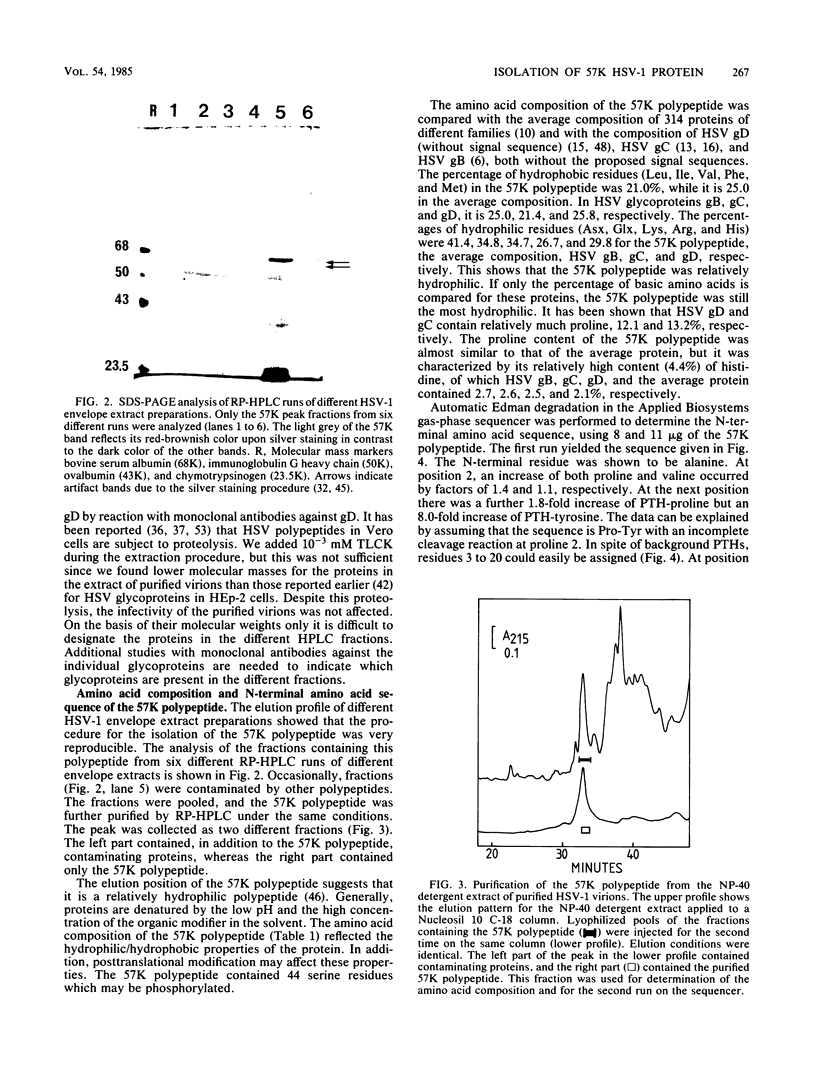


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. L., Corey L. Effect of acyclovir treatment of primary genital herpes on the antibody response to herpes simplex virus. J Clin Invest. 1984 Mar;73(3):681–688. doi: 10.1172/JCI111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Killington R. A., Bacchetti S., Rawls W. E. Monoclonal antibodies to two glycoproteins of herpes simplex virus type 2. J Virol. 1981 Aug;39(2):438–446. doi: 10.1128/jvi.39.2.438-446.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Rawls W. E., Bacchetti S. Glycoproteins of herpes simplex virus type 2 as defined by monoclonal antibodies. J Virol. 1982 Oct;44(1):344–355. doi: 10.1128/jvi.44.1.344-355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhown A. S., Bennett J. C., Mole J. E., Hunter E. Purification and characterization of the gag gene products of avian-type C retroviruses by high-pressure liquid chromatography. Anal Biochem. 1981 Mar 15;112(1):128–134. doi: 10.1016/0003-2697(81)90269-4. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. R., Zaia J. A., Balce-Directo L., Ting Y. P. Isolation and partial chemical characterization of a 64,000-dalton glycoprotein of human cytomegalovirus. J Virol. 1984 Jan;49(1):279–282. doi: 10.1128/jvi.49.1.279-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernick R., Heukeshoven J., Hilbrig M. Induction of neutralizing antibodies by all three structural poliovirus polypeptides. Virology. 1983 Oct 15;130(1):243–246. doi: 10.1016/0042-6822(83)90134-4. [DOI] [PubMed] [Google Scholar]
- Done J. N., Kennedy G. J., Knox J. H. Revolution in liquid chromatography. Nature. 1972 May 12;237(5350):77–81. doi: 10.1038/237077a0. [DOI] [PubMed] [Google Scholar]
- Dowbenko D. J., Lasky L. A. Extensive homology between the herpes simplex virus type 2 glycoprotein F gene and the herpes simplex virus type 1 glycoprotein C gene. J Virol. 1984 Oct;52(1):154–163. doi: 10.1128/jvi.52.1.154-163.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Mou S. W. Relative titers of antibodies to individual polypeptide antigens of herpes simplex virus type 1 in human sera. J Infect Dis. 1983 Sep;148(3):436–444. doi: 10.1093/infdis/148.3.436. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Hogue-Angeletti R., Cohen G. H. Amino-terminal sequence of glycoprotein D of herpes simplex virus types 1 and 2. J Virol. 1984 Jan;49(1):265–268. doi: 10.1128/jvi.49.1.265-268.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. C., Docherty J. J., Rawls W. E. Antibody responses in humans to individual proteins of herpes simplex viruses. Infect Immun. 1981 Dec;34(3):880–887. doi: 10.1128/iai.34.3.880-887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Brackmann K. H. The application of high-performance liquid chromatography for the resolution of proteins encoded by the human adenovirus type 2 cell transformation region. Anal Biochem. 1982 Jul 15;124(1):209–216. doi: 10.1016/0003-2697(82)90239-1. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M., Kent S., Caruthers M., Dreyer W., Firca J., Giffin C., Horvath S., Hunkapiller T., Tempst P., Hood L. A microchemical facility for the analysis and synthesis of genes and proteins. Nature. 1984 Jul 12;310(5973):105–111. doi: 10.1038/310105a0. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Wittels M., Spear P. G. Binding to cells of virosomes containing herpes simplex virus type 1 glycoproteins and evidence for fusion. J Virol. 1984 Oct;52(1):238–247. doi: 10.1128/jvi.52.1.238-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W., Kaerner H. C. Virus-specific basic phosphoproteins associated with herpes simplex virus type a (HSV-1) particles and the chromatin of HSV-1-infected cells. J Gen Virol. 1980 Feb;46(2):405–414. doi: 10.1099/0022-1317-46-2-405. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaster S., Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980 Sep;35(3):798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiken J., Van der Zee R., Welling G. W. Structure and activity of proteins after reversed-phase high-performance liquid chromatography. J Chromatogr. 1984 Feb 3;284(2):482–486. doi: 10.1016/s0021-9673(01)87851-1. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall T., Williams K. M. Artifacts associated with 2-mercaptoethanol upon high resolution two-dimensional electrophoresis. Anal Biochem. 1984 Jun;139(2):502–505. doi: 10.1016/0003-2697(84)90041-1. [DOI] [PubMed] [Google Scholar]
- McLean C., Buckmaster A., Hancock D., Buchan A., Fuller A., Minson A. Monoclonal antibodies to three non-glycosylated antigens of herpes simplex virus type 2. J Gen Virol. 1982 Dec;63(2):297–305. doi: 10.1099/0022-1317-63-2-297. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., West M., Issel C. J. High-performance gel permeation chromatography of proteins in denaturing solvents and its application to the analysis of enveloped virus polypeptides. Anal Biochem. 1981 Jul 1;114(2):398–406. doi: 10.1016/0003-2697(81)90501-7. [DOI] [PubMed] [Google Scholar]
- Para M. F., Zezulak K. M., Conley A. J., Weinberger M., Snitzer K., Spear P. G. Use of monoclonal antibodies against two 75,000-molecular-weight glycoproteins specified by herpes simplex virus type 2 in glycoprotein identification and gene mapping. J Virol. 1983 Mar;45(3):1223–1227. doi: 10.1128/jvi.45.3.1223-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Dondero D., Norrild B., Roizman B. Differential immunologic reactivity and processing of glycoproteins gA and gB of herpes simplex virus types 1 and 2 made in Vero and HEp-2 cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5202–5206. doi: 10.1073/pnas.78.8.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Dondero D., Roizman B. Herpes simplex virus glycoprotein gA/B: evidence that the infected Vero cell products comap and arise by proteolysis. J Virol. 1982 Oct;44(1):88–97. doi: 10.1128/jvi.44.1.88-97.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan M. A., Cohen K. A. Gradient optimization principles in reversed-phase high-performance liquid chromatography and the separation of influenza virus components. J Chromatogr. 1983 Aug 26;266:55–66. doi: 10.1016/s0021-9673(01)90879-9. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Regnier F. E. High-performance liquid chromatography of biopolymers. Science. 1983 Oct 21;222(4621):245–252. doi: 10.1126/science.6353575. [DOI] [PubMed] [Google Scholar]
- Roizman B., Norrild B., Chan C., Pereira L. Identification and preliminary mapping with monoclonal antibodies of a herpes simplex virus 2 glycoprotein lacking a known type 1 counterpart. Virology. 1984 Feb;133(1):242–247. doi: 10.1016/0042-6822(84)90447-1. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasheva B., Dessev G. Artifacts in sodium dodecyl sulfate-polyacrylamide gel electrophoresis due to 2-mercaptoethanol. Anal Biochem. 1983 Feb 15;129(1):98–102. doi: 10.1016/0003-2697(83)90057-x. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Welling-Wester S., Vos J., Wilterdink J. B. Differences in antigenic properties of Fc-binding activity during infection with herpes simplex virus type 1. Arch Virol. 1984;80(2-3):183–193. doi: 10.1007/BF01310658. [DOI] [PubMed] [Google Scholar]
- Welling G. W., Nijmeijer J. R., van der Zee R., Groen G., Wilterdink J. B., Welling-Wester S. Isolation of detergent-extracted Sendai virus proteins by gel-filtration, ion-exchange and reversed-phase high-performance liquid chromatography and the effect on immunological activity. J Chromatogr. 1984 Aug 3;297:101–109. doi: 10.1016/s0021-9673(01)89033-6. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Characterization of a herpes simplex virus type 2 75,000-molecular-weight glycoprotein antigenically related to herpes simplex virus type 1 glycoprotein C. J Virol. 1983 Sep;47(3):553–562. doi: 10.1128/jvi.47.3.553-562.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Limited proteolysis of herpes simplex virus glycoproteins that occurs during their extraction from vero cells. J Virol. 1984 Apr;50(1):258–262. doi: 10.1128/jvi.50.1.258-262.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Stanton L. W. Immune response to herpes simplex virus infections: virus-specific antibodies in sera from patients with recurrent facial infections. Infect Immun. 1981 Feb;31(2):624–630. doi: 10.1128/iai.31.2.624-630.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Showalter S. D., Bladen S. V., Heilman C. J., Jr, Hampar B. Herpes simplex virus type 2 glycoprotein gF and type 1 glycoprotein gC have related antigenic determinants. J Virol. 1983 Jul;47(1):185–192. doi: 10.1128/jvi.47.1.185-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]