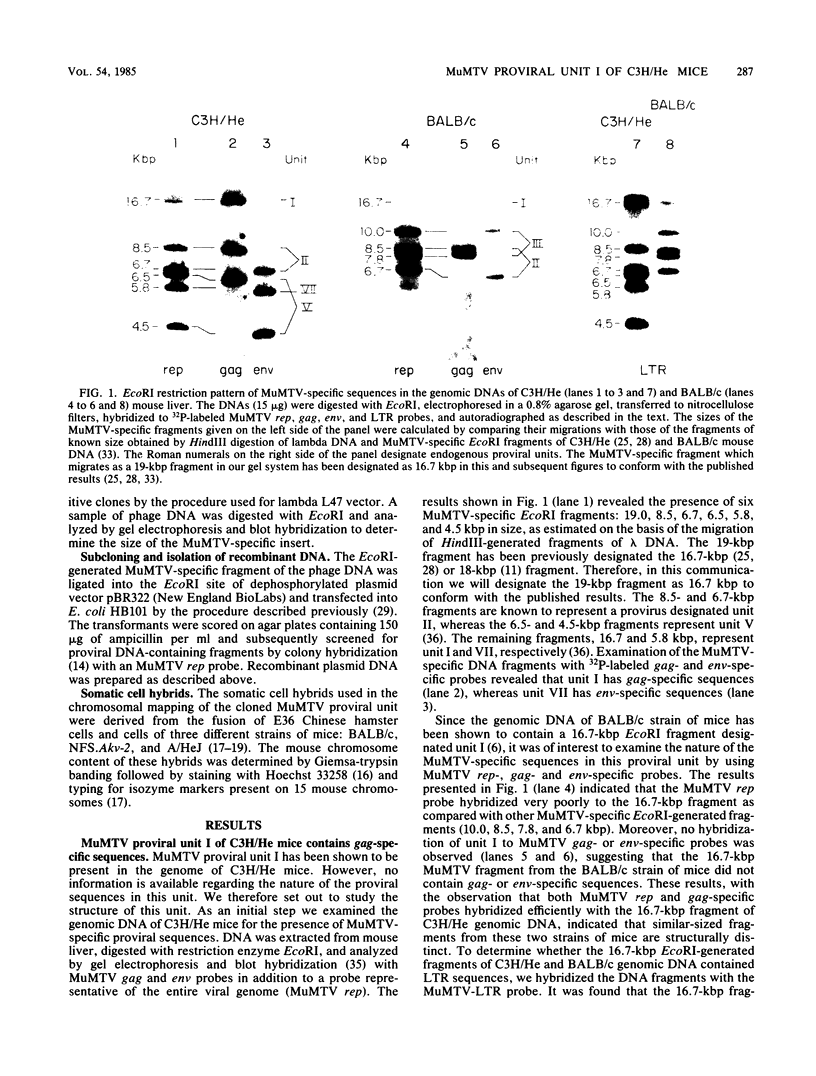
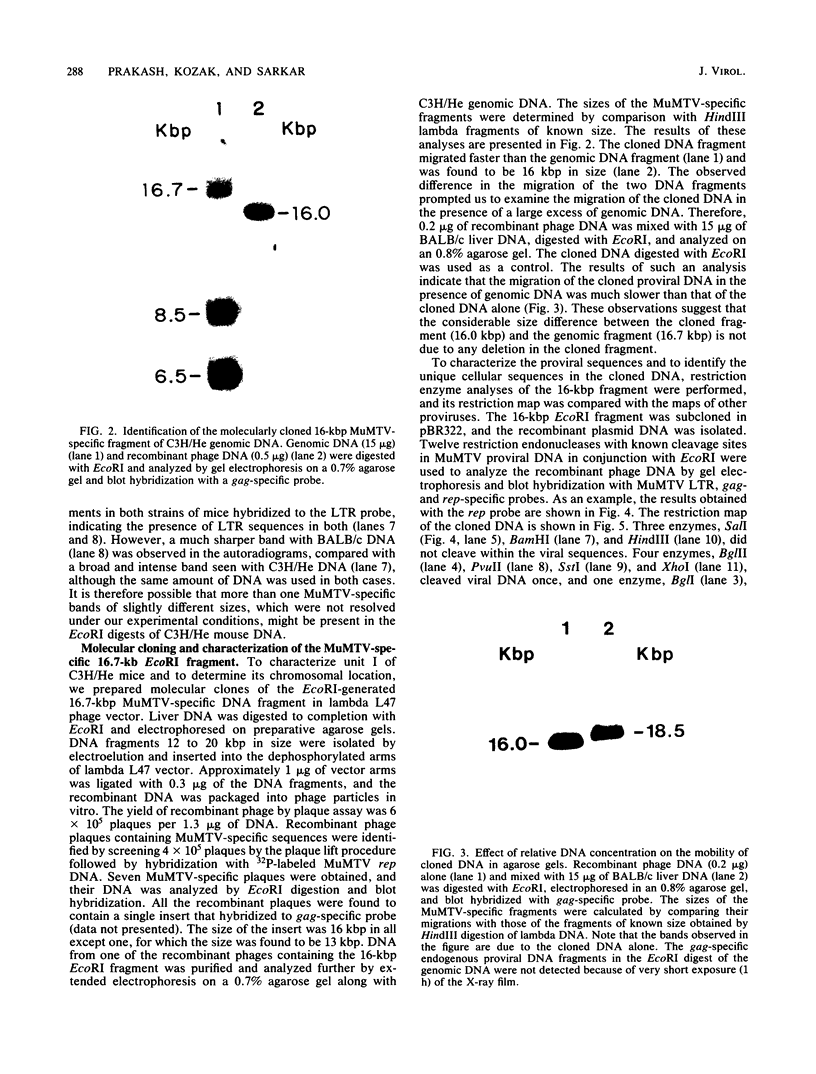
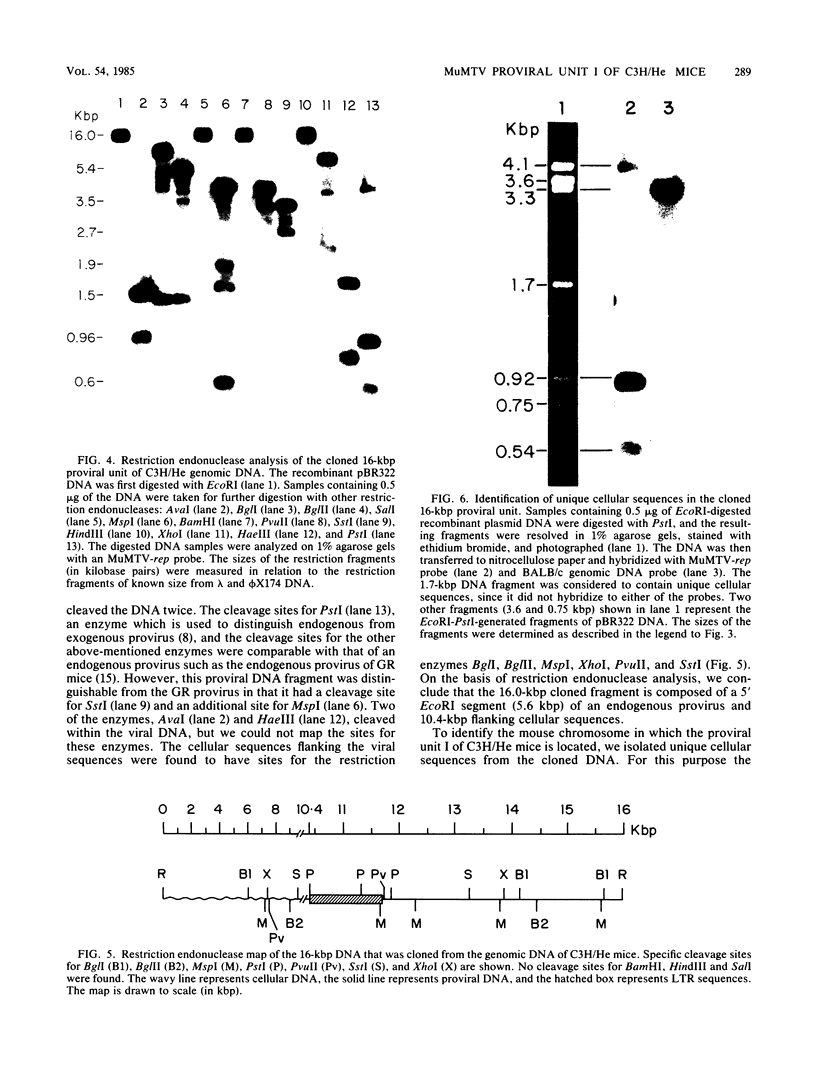
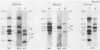Abstract
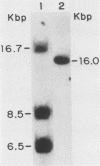
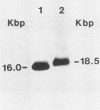
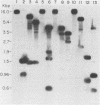
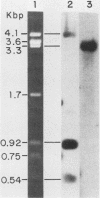
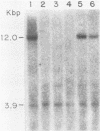
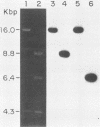
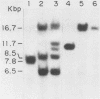
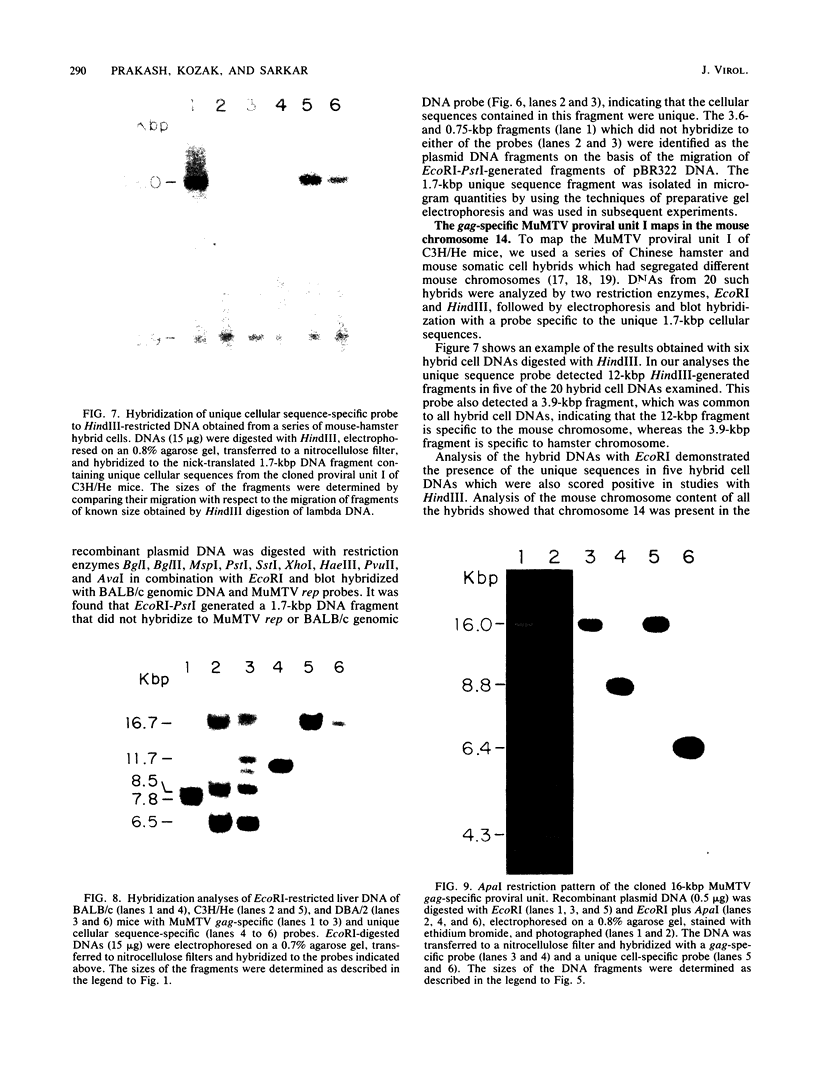
We have cloned and characterized a novel endogenous murine mammary tumor virus proviral unit of the C3H/He strain of mice. The cloned proviral unit is 16 kilobase pairs (kbp) in size and is composed of a 5.6-kbp 5' EcoRI segment of an endogenous provirus with 10.4-kbp flanking cellular sequences. A comparison of the restriction map of the cloned proviral DNA with that of an endogenous provirus of the GR strain of mice has revealed minor differences in restriction sites on the two proviruses. The restriction enzyme SstI, which does not cleave the 5' EcoRI fragment of GR DNA, cleaves the C3H/He proviral sequences once; MspI has an additional site in the C3H/He proviral sequences. By using a subcloned fragment containing unique cellular sequences as a hybridization probe, we (i) mapped the C3H/He proviral unit to chromosome 14 by using mouse-hamster somatic cell hybrids, and (ii) demonstrated that this proviral unit is also present in the genome of DBA/2 mice. From these results we conclude that the C3H/He strain of mice acquired this proviral unit from DBA stock by genetic transmission. Our data also indicate that the murine mammary tumor virus sequences present in the gag-specific proviral unit of C3H/He mice extend at least 2.45 kbp downstream of the EcoRI site in the genomic DNA. Since the structural organization and chromosomal location of this proviral unit are distinct from those of previously reported proviral units represented by similar-sized (16.7-kbp) EcoRI fragments, we tentatively propose to designate this proviral unit Mtv-7a.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Callahan R., Gallahan D., Kozak C. Two genetically transmitted BALB/c mouse mammary tumor virus genomes located on chromosomes 12 and 16. J Virol. 1984 Mar;49(3):1005–1008. doi: 10.1128/jvi.49.3.1005-1008.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol. 1980 Aug;35(2):298–305. doi: 10.1128/jvi.35.2.298-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggelmann H., Vessaz A. L., Buetti E. Cloned endogenous mouse mammary tumor virus DNA is biologically active in transfected mouse cells and its expression is stimulated by glucocorticoid hormones. Virology. 1982 Oct 30;122(2):332–341. doi: 10.1016/0042-6822(82)90233-1. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Sarkar N. H. Integration of new endogenous mouse mammary tumor virus proviral DNA at common sites in the DNA of mammary tumors of C3Hf mice and hypomethylation of the endogenous mouse mammary tumor virus proviral DNA in C3Hf mammary tumors and spleens. J Virol. 1983 Jan;45(1):114–123. doi: 10.1128/jvi.45.1.114-123.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N., Buetti E., Firzlaff J., Pearson K., Diggelmann H. Nucleotide sequence of the 5' noncoding region and part of the gag gene of mouse mammary tumor virus; identification of the 5' splicing site for subgenomic mRNAs. Nucleic Acids Res. 1983 Oct 25;11(20):6943–6955. doi: 10.1093/nar/11.20.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic murine leukemia virus-inducing locus of BALB/c mouse to chromosome 5. Science. 1979 Apr 6;204(4388):69–71. doi: 10.1126/science.219475. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of the ecotropic virus-inducing locus Akv-2 of the AKR mouse. J Exp Med. 1980 Nov 1;152(5):1419–1423. doi: 10.1084/jem.152.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C., Nichols E., Ruddle F. H. Gene linkage analysis in the mouse by somatic cell hybridization: assignment of adenine phosphoribosyltransferase to chromosome 8 and alpha-galactosidase to the X chromosome. Somatic Cell Genet. 1975 Oct;1(4):371–382. doi: 10.1007/BF01538668. [DOI] [PubMed] [Google Scholar]
- MacInnes J. I., Morris V. L., Flintoff W. F., Kozak C. A. Characterization and chromosomal location of endogenous mouse mammary tumor virus loci in GR, NFS, and DBA mice. Virology. 1984 Jan 15;132(1):12–25. doi: 10.1016/0042-6822(84)90087-4. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequencing of an apparent proviral copy of env mRNA defines determinants of expression of the mouse mammary tumor virus env gene. J Virol. 1983 Sep;47(3):495–504. doi: 10.1128/jvi.47.3.495-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nuss R. R., van Nie R. Identification of the Mtv-2 gene responsible for the early appearance of mammary tumors in the GR mouse by nucleic acid hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2368–2372. doi: 10.1073/pnas.75.5.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., de Moes J., Hilkens J., van Nie R. Localization of a gene for expression of mouse mammary tumor virus antigens in the GR/Mtv-2- mouse strain. J Exp Med. 1980 Sep 1;152(3):712–719. doi: 10.1084/jem.152.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippsen P., Kramer R. A., Davis R. W. Cloning of the yeast ribosomal DNA repeat unit in SstI and HindIII lambda vectors using genetic and physical size selections. J Mol Biol. 1978 Aug 15;123(3):371–386. doi: 10.1016/0022-2836(78)90085-2. [DOI] [PubMed] [Google Scholar]
- Prakash O., Guntaka R. V., Sarkar N. H. Evidence for a prokaryotic promoter in the murine mammary tumor virus long terminal repeat. Gene. 1983 Aug;23(2):117–130. doi: 10.1016/0378-1119(83)90043-4. [DOI] [PubMed] [Google Scholar]
- Redmond S. M., Dickson C. Sequence and expression of the mouse mammary tumour virus env gene. EMBO J. 1983;2(1):125–131. doi: 10.1002/j.1460-2075.1983.tb01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Varmus H. E., Ring J., Yamamoto K. R. Integration and transcription of mouse mammary tumor virus DNA in rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):665–669. doi: 10.1073/pnas.76.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Traina-Dorge V., Cohen J. C. Molecular genetics of mouse mammary tumor virus. Curr Top Microbiol Immunol. 1983;106:35–56. doi: 10.1007/978-3-642-69357-1_2. [DOI] [PubMed] [Google Scholar]
- Traina V. L., Taylor B. A., Cohen J. C. Genetic mapping of endogenous mouse mammary tumor viruses: locus characterization, segregation, and chromosomal distribution. J Virol. 1981 Dec;40(3):735–744. doi: 10.1128/jvi.40.3.735-744.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. A., Butel J. S., Medina D., Cardiff R. D., Hager G. L. Transcription of mouse mammary tumor virus: identification of a candidate mRNA for the long terminal repeat gene product. J Virol. 1983 Apr;46(1):42–49. doi: 10.1128/jvi.46.1.42-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., Michalides R. J., Nusse R. Structural analysis of a 1.7-kilobase mouse mammary tumor virus-specific RNA. J Virol. 1983 May;46(2):362–370. doi: 10.1128/jvi.46.2.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]