Abstract
Cigarette smoking is schizophrenia is prevalent and may be due to self-medicating attempts to improve cognitive deficits related to α7 and α4β2 nicotinic receptor dysregulation. Galantamine is an acetylcholinesterase inhibitor that acts as a positive allosteric modulator of nicotine acetylcholine receptors including both the α4β2 and α7 subunits. In a double blind randomized clinical trial galantamine was compared to placebo for its effects on cognitive functioning in people with schizophrenia. This manuscript reports findings for galantamine's effect on smoking behavior in people from this 12-week trial who were smokers (18 galantamine, 25 placebo). Expired CO was measured every 2 weeks and the Fagerström Test for Nicotine Dependence (FTND) was administered at baseline and endpoint. Expired CO measures in galantamine subjects were 23.0 ± 9.7 ppm and 21.1 ± 10.3 ppm at baseline and Week 12, respectively, compared to 20.1 ± 8.5 ppm and 21.0 ±10.3 ppm at baseline and Week 12 in placebo subjects. The mean tau-b correlation between expired CO level and visit was −0.05 ± 0.41 in the galantamine group and 0.13 ± 0.42 in the placebo group (F=0.73, df=1,38, p=0.40), suggesting that there were no trends toward increased or decreased smoking in either group. Mean FTND scores in the galantamine group were 4.9 ± 2.5 at baseline and 5.2 ± 2.2 at week 12, compared to 4.1 ± 2.6 at baseline and 3.7 ± 2.6 at Week 12 in the placebo group (Mantel-Haenszel Χ2= 5.53, df=1, p=0.019), for an effect size of 0.4. These results suggest that galantamine has no effect on cigarette smoking and that during galantamine treatment nicotine dependency scores worsen.
Keywords: galantamine, smoking, nicotine, acetylcholine, schizophrenia
Background
People with schizophrenia have a high prevalence of cigarette smoking with recently reported rates of approximately 60−70% (Goff et al 2005, Ucok et al 2004). This is considerably higher than smoking rates of about 35% in the general public (Goff et al 2005). Additionally, people with schizophrenia who smoke are heavier smokers than smokers in the general population (Kelly and McCreadie 1999). While the causes of this high prevalence are not known, this behavior is believed to be due to a complex interaction of psychological, behavioral and environmental factors that may lead to a higher likelihood to abuse substances.
Heavy and frequent cigarette smoking in people with schizophrenia was originally attributed to boredom and cigarette rewards for good behavior on treatment units (Goff et al 1992, Masterson and O'Shea 1984, Gopalaswamy and Morgan 1986). However, more recently is it has been recognized that cigarette smoking represents more than ”something to do.” Cigarette smoking may partially represent an attempt at self-medication to control symptoms not addressed by antipsychotic medications (Simosky et al 2002). Studies of the effects of nicotine in people with schizophrenia have shown improvements in numerous areas including learning and memory, eye tracking, prepulse inhibition of acoustic startle response and P50 sensory inhibition (Levin 1992, Rezvani and Levin 2001, Olincy et al 1998, Hong et al. 2007, Adler et al 1993) or to control medication side effects (Barnes et al 2006). There is evidence implicating the nicotinic cholinergic and specifically the α7 and α4β2 nicotinic receptor systems in the pathophysiology of schizophrenia (Martin and Freedman 2007, Levin and Rezvani 2007). Activation of the nicotinic acetylcholine receptors can increase glutamate release onto layer V pyramidal neurons of the prefrontal cortex. This is important as schizophrenia pathology also involves abnormal cortical activation, NMDA hypofunction and difficulty with cognition (Lambe et al 2003). Thus, nicotinic modulation through cigarette smoking may play a critical role in the rationale for smoking in people with schizophrenia that is potentially different from the factors driving smoking in normal controls. This may partially explain the significantly higher rate of smoking in people with schizophrenia.
Smoking, however, is the single greatest preventable cause of death in our society and may help account for an estimated 20% reduction in life expectancy among persons with schizophrenia compared to the general population. In people with schizophrenia, apart from suicide, the most common causes of death are cardiovascular and respiratory disease, both of which may be mediated by smoking (Mortensen and Juel 1993). Until recently the health risks of smoking have been neglected among people with severe mental illness.
To date, pharmacotherapeutic options for smoking cessation have been largely ineffective in people with schizophrenia, with most treatments reporting less than 10% effectiveness (Addington 1998, Horowitz et al 1985, Lyon et al 1999, Ziedonis and George 1997, de Leon et al 2005, Baker et al 2006). This is in contrast to cessation rates from alcohol and drug use disorders of over 40% (de Leon et al 2005) in this population. Furthermore, even when interventions are been found to be modestly effective in double-blind trials, relapse is very common once the intervention is discontinued (Evins et al 2005, Evins et al 2007). Others have reported decreases in total cigarette consumption but little evidence is available to suggest that total abstinence is easily achieved even with combined pharmacologic agents currently available and behavioral interventions (Baker et al 2006, Evins et al 2007, Weiner et al 2001).
Galantamine is an acetylcholinesterase inhibitor that increases the effect of acetylcholine. Additionally this drug acts as a positive allosteric modulator of nicotine acetylcholine receptors (nAchR). Galantamine is not selective across the nAchRs and is known to modulate α4β2 and α7 subunits that are broadly expressed in the central nervous system (Broad et al 2006). Since nicotine is active at both the α4β2 and α7 nicotinic receptors and long term exposure to nicotine leads to an increased expression and upregulation of neuronal nAChRs (Buisson and Ertrand 2001, Pakkanen et al 2005), it has been suggested that drugs that modulate nAchR may have utility in the management of nicotine dependence (Papke et al 2007). In fact, one recent study in alcohol dependent subjects found that galantamine treated subjects had a 20% lower cumulative number of smoked cigarettes and 15% lower cumulative number of smoking days as compared to placebo, suggesting that galantamine may have utility as a strategy for smoking reduction or cessation (Diehl et al 2006). However, because the neuropathological etiology of people with schizophrenia may involve dysregulation of nicotinic receptors and because people with schizophrenia smoke at higher rates, it remains unknown if galantamine may decrease cigarette smoking in this population.
Methods
Study Overview
In a double-blind 12-week randomized trial, adjunctive galantamine treatment was compared to placebo for measures of cognition, smoking and symptom improvement. This paper presents data on galantamine's effect on cigarette smoking; a more comprehensive summary of study design and results is reported elsewhere (Buchanan et al 2007, Conley et al 2008).
Subjects included were inpatients or outpatients between the ages of 18 to 60 years with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder as assessed by the Structured Clinical Interview (SCID-IV) (First et al 1997). Subjects were required to be in a chronic stable phase of their illness and were being treated with an antipsychotic agent other than clozapine (second generation or low dose first generation). Subjects were not receiving anticholinergic treatment and they were required to have a Simpson-Angus Extrapyramidal Symptom Score (SAS) (Simpson and Angus 1970) total score ≤ 4. Subjects were excluded if they had organic brain disease (e.g. seizure disorder, stroke), mental retardation, a history of second or third degree AV block, or a chronic unstable medical condition. Subjects were also excluded if they were pregnant or had a DSM-IV diagnosis of alcohol or substance abuse or dependence in the last six months (other than nicotine dependence). Subjects receiving other acetylcholinesterase inhibitors were excluded.
Prior to randomization, subjects underwent a two-week evaluation to assess medical health, baseline symptom levels, and stability of symptoms. The institutional review boards of the University of Maryland and the State of Maryland's Department of Health and Mental Hygiene approved this protocol. Written informed consent was obtained from all subjects after study procedures and risks had been fully explained and prior to participation in the study. Subject ability to provide valid informed consent was documented using study specific procedures.
Galantamine subjects were initially dosed at 8 mg/day followed by an increase of 8 mg/day every four weeks to a maximum dose of 24 mg/day. Medication was given twice a day, half in the morning and half in the evening. Matching placebo was given at the same intervals to the control group. Adherence was measured by weekly pill counts and medication review and adherence of >75% was required to remain in the study.
Assessment of Smoking Measures
Smoking status was ascertained by expired CO levels at baseline and during follow-up in participants with schizophrenia randomly assigned to receive adjunctive treatment with galantamine or placebo. Subjects with baseline expired CO levels <8 ppm were considered non-smokers at baseline and excluded from further analysis of the impact of galantamine on smoking habits (Chatkin et al 2007). Baseline expired CO was measured during the screening phase and/or at the first double blind visit, prior to the start of drug treatment, and every two weeks thereafter during 12 weeks of double blind treatment. The smoking status for a few subjects, for whom baseline expired CO was not collected, was determined from the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al 1991) and/or chart review. Baseline smoking status was determined by the mean of both pre-treatment expired CO measurements to reduce the effect of measurement error. CO measurements were obtained between 12:00 pm and 2:00 pm to establish consistency in the time of day and enable subjects to exhibit normal smoking behavior in the morning and early afternoon. The FTND was administered at baseline and at week 12.
Statistical Analysis
Means and standard deviations by visit and treatment were examined for expired CO levels and nicotine dependency scores for subjects who were smokers at baseline. The percentage of baseline smokers who were considered non-smokers (expired CO<8 ppm) at week 12 of double blind treatment is also reported. Because of the small sample size and non-normal distribution of these data, a procedure proposed by McMahon, Arndt and Conley (2005) was used to describe treatment-related trends in expired CO. Briefly, for each subject, the Kendall tau-b rank correlation was calculated between outcome and visit, and the distribution of these correlations was compared using the Conover-Salsburg rank test (1988). This procedure can accommodate varying numbers of follow-up visits among subjects, and has superior power to mixed models for repeated measures ANOVA for outcomes with non-normal distributions where only a subgroup of subjects may respond to treatment. Nicotine dependency scores take on a few discrete values, whose distribution is not well approximated by the normal distribution. Accordingly, we stratified the analysis of week 12 nicotine dependency scores by the baseline nicotine dependency score, and used a Mantel-Haenszel chi-square test (Mantel, 1963) to test whether average nicotine dependency scores differed between treatments, after controlling for baseline score. Spearman rank correlation coefficients were used to examine association of age, baseline symptom ratings and neuropsychological measures altered by galantamine treatment (WAIS IV-R digit symbol, California verbal learning test, and Gordon distractibility test; Buchanan et al, 2008) with changes in expired CO and FTND scores.
Results
During this study 42 subjects were randomized to galantamine and 44 subjects were randomized to placebo. Expired CO measures were available for 33 subjects randomized to galantamine and for 40 subjects randomized to placebo. Smoking status was determined from FTND interview or extended direct observation of presence or absence of smoking behavior on inpatient wards by study staff in 7 galantamine subjects (2 smokers, 5 non-smokers) and 2 placebo subjects (both non-smokers) whose baseline CO levels were not collected. Thus, 18/40 (45%) of subjects randomized to galantamine and 25/42 (40%) of subjects randomized to placebo were smokers. These two groups of smokers are the focus of the remainder of this report. The mean demographic and clinical information for the groups of smokers are listed by treatment in table 1.
Table 1.
Galantamine Trial: Baseline Characteristics of Smoking Participants By Treatment Assignment
| Baseline Characteristics | Galantamine (N=18) |
Placebo (N=23) |
|||||
|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | t | df | P-value | |
| Age, years | 50.4 | 6.0 | 45.3 | 11.3 | 1.89 | 38.1 | 0.066* |
| Education, years | 12.0 | 1.2 | 12.4 | 2.2 | −0.83 | 38.2 | 0.41* |
| BPRS total score | 33.7 | 8.1 | 37.2 | 10.5 | 01.17 | 41 | 0.25 |
| BPRS psychosis | 9.9 | 4.9 | 11.9 | 4.8 | −1.28 | 41 | 0.21 |
| BPRS anxiety/depression | 8.1 | 2.9 | 8.4 | 3.6 | −0.28 | 41 | 0.78 |
| SANS total score | 30.2 | 13.0 | 28.0 | 10.5 | −0.60 | 40 | 0.55 |
| CGI Severity score | 4.3 | 0.7 | 4.4 | 0.8 | −0.29 | 41 | 0.77 |
| n | % | n | % | χ2 | df | P-value** | |
| Female gender | 1 | 5.6 | 1 | 4.0 | 0.06 | 1 | 1.00* |
| Race: | 1.18 | 2 | 0.73* | ||||
| White | 7 | 38.9 | 7 | 28.0 | |||
| Black | 11 | 61.1 | 17 | 68.0 | |||
| Other | 0 | 0.0 | 1 | 4.0 | |||
| Antipsychotic: | 4.46 | 4 | 0.46* | ||||
| Olanzapine | 12 | 66.7 | 14 | 56.0 | |||
| Risperidone | 5 | 28.8 | 7 | 30.4 | |||
| Quetiapine | 0 | 0.0 | 2 | 8.7 | |||
| Aripiprazole | 1 | 5.6 | 0 | 0.0 | |||
| Polypharmacy | 0 | 0.0 | 2 | 8.0 | |||
t-tests for age and education level performed using Satterthwaite's approximation due to evidence of inequality of variances in the two group.
P-values calculated from Fisher's exact test due to small n's in some cells.
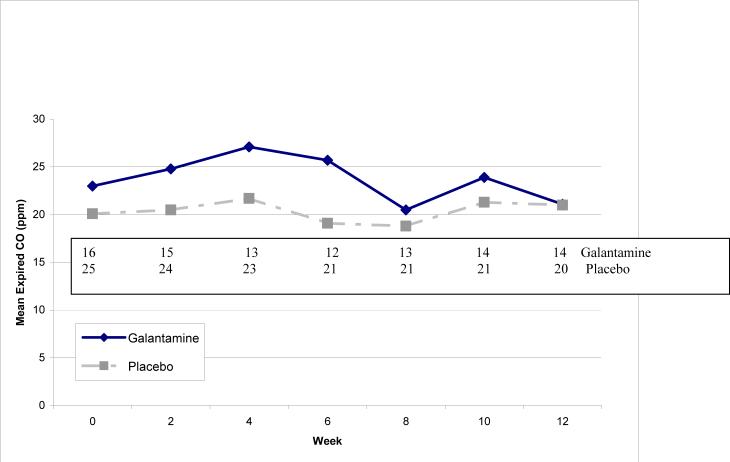
Among smokers, at least 1 follow-up expired CO measurement was available for 18 subjects randomized to galantamine and 24 subjects randomized to placebo. Among this group of smokers, mean ± s.d. expired CO measures in galantamine subjects were 23.0 ± 9.7 ppm at baseline and 21.1 ± 10.3 ppm at Week 12, compared to 20.1 ± 8.5 ppm at baseline and 21.0 ±10.3 ppm at Week 12 in placebo subjects. Figure 1 shows the mean expired CO levels by week and treatment, together with the number of subjects with expired CO measurements at each week. The mean tau-b correlation between expired CO level and visit was −0.05 ± 0.41 in the galantamine group and 0.13 ± 0.42 in the placebo group (Conover-Salsburg test for differences in trend, F=0.73, df=1,38, p=0.40), suggesting that there were no trends toward increased or decreased smoking in either group. Expired CO levels for a few subjects sporadically fell below 8 ppm; no subject maintained levels below 8 ppm for more than 2 successive biweekly visits.
Figure 1. Mean Expired CO Levels (ppm) by Treatment and Week.
Sample size by week listed above for galantamine and placebo groups. Conover-Salsburg test for treatment differences in trend with time, F=0.53, df=1,37, p=0.47
FTND scores were available at both baseline and end of study for 14 subjects assigned to galantamine and 17 assigned to placebo. Mean FTND scores in the galantamine group were 4.9 ± 2.5 at baseline and 5.2 ± 2.2 at week 12, compared to 4.1 ± 2.6 at baseline and 3.7 ± 2.4 at Week 12 in the placebo group (Mantel-Haenszel Χ2= 5.53, df=1, p=0.019 for difference at Week 12 after stratifying on baseline value), for an effect size (mean difference in change scores divided by pooled s.d.) of 0.7/2.4=0.3. The distribution of changes in FTND score by treatment group is shown in Figure 2. The distribution of cigarettes smoked per day reported on the FTND at baseline and end of study is shown in Figure 3. After stratifying on baseline value, there was a weak trend toward less reduction in cigarettes per day in the galantamine than the placebo group (Mantel-Haenszel Χ2=2.61, df=1, p=0.11). Data on other FTND items at baseline and follow-up are available in online supplemental tables; no differences were statistically significant on any of these items, although there were weak trends for more reduction from baseline among placebo compared to galantamine subjects in positive responses on item 2, difficulty refraining from smoking where it is forbidden (p=0.09) and item 3, smoking in bed when sick all day (p=0.11).
Figure 2.
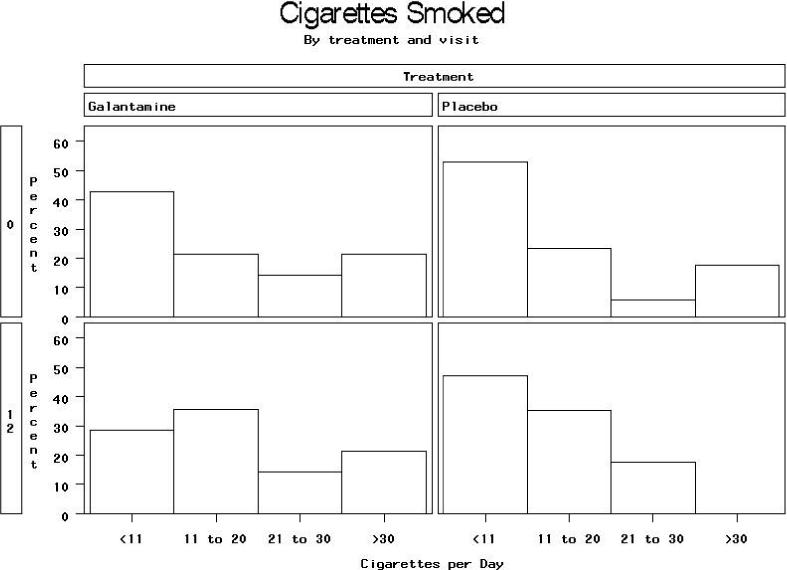
Mantel-Haenszel Χ2=2.61, df=1, p=0.11 for treatment difference in Week 12 cigarettes per day, stratified on baseline level of smoking.
Figure 3.
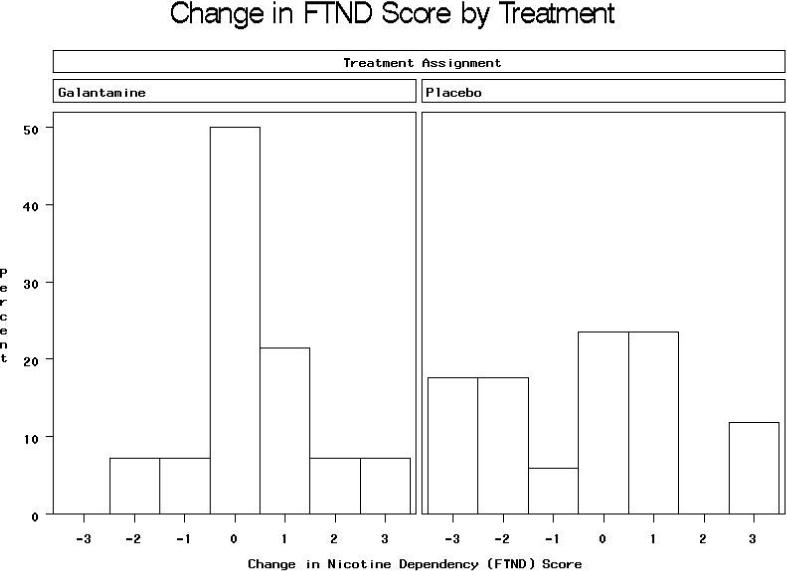
Mantel-Haenszel Χ2= 5.53, df=1, p=0.019 for treatment difference in FTND score at Week 12 after stratifying on baseline value.
Age, education level, baseline symptom scores and changes in the three neuropsychological scores, which significantly differed in galantamine versus placebo groups (Buchanan et al 2008), were not significantly correlated with 12-week change in expired CO or FTND score (all p>0.13).
Discussion
To our knowledge, this is the first study to assess changes in cigarette smoking in people with schizophrenia treated with galantamine. We failed to find any evidence that galantamine treatment reduced smoking intensity or nicotine dependency during the 12-week double blind treatment period. Subjects treated with galantamine actually showed a moderate increase (effect size = 0.4 × baseline s.d.) in nicotine dependency scores relative to placebo. Smokers in the galantamine group had higher symptom scores and were slightly older. However, these group differences likely had no effects on smoking behavior as the galantamine treatment arm did not significantly improve symptoms (Boggs et al 2007) and symptom severity has not been previously associated with smoking in schizophrenia (Barnes et al 2006). In addition, symptom severity was not associated with changes in expired CO or FTND scores in the current study.
Due to the known modulatory effects of galantamine on the nicotine acetylcholine receptors (nAchR), it has been postulated that galantamine may affect smoking behavior similarly to nicotine replacement therapies. Galantamine was found to be effective for reducing smoking behavior in a population of people diagnosed with alcohol dependent (Diehl et al, 2006), yet in our study no decrease in smoking was noted in people with schizophrenia. However, in the current study, participants were not selected based on expressed interest in smoking cessation, and no psychosocial component or smoking cessation program was included in the study. It has been noted that combined pharmacologic and psychosocial programs may offer the best choice for smoking cessation in people with schizophrenia and the psychosocial reinforcement may be necessary to see cigarette smoking reduction (Baker et al 2006). We did not record the actual numbers of cigarettes smoked daily and did not measure urine cotinine. We did not measure changes in other substance abuse and alcohol consumption, quality of life, financial status and perceived stress throughout the study. These factors are all known to contribute and mediate the need or want to smoke a cigarette (Dixon et al 2007). Additionally, the study by Diehl et al (2006) in subjects with alcohol dependence, was 24 weeks in length and some of the most notable effects occurred between 12 and 24 weeks, suggesting that a longer period of galantamine treatment may be necessary to see significant changes in smoking behavior. Finally, galantamine differs pharmacologically from selective nicotinic acetylcholine partial agonists, such as varenicline, which have been shown to reduce smoking behavior. Since it is an acetylcholinesterase inhibitor and allosteric modulator, the pharmacological effects of galantamine are primarily observed in the context of acetylcholine release. In contrast, varenicline functions as a selective nicotinic partial agonist at α4β2 receptors and it exerts its effects, including the reduction of feelings of craving to smoke, regardless of the presence of acetylcholine (or nicotine) (Villarroya et a. 2007; Lam and Patel 2007). Lastly, in vivo data suggest that chronic galantamine binding at nAChRs may decrease subsequent functional responses to acute stimulation with nicotine (Barik et al. 2005). This may lead to an increased dependency or craving to smoke to achieve the same effects rather than actually decrease the desire to smoke.
One interesting finding in this study was the moderate increase in nicotine dependency scores as rated by the FTND in subjects treated with galantamine as compared to the placebo group. While expired CO levels did not change, people with schizophrenia were reporting a higher degree of dependency scores to cigarettes during galantamine treatment. Since galantamine could function partially as a substitute for nicotine, withdrawal should not theoretically be a significant concern (Diehl et al. 2006). As mentioned above, the binding of galantamine at nAchRs may theoretically lead to an increased craving or dependence. Nevertheless, it is not known why nicotine dependency scores are increasing and specific measures of cigarette craving such as cue reactivity were not performed to assess if these scores are being driven by changes in craving.
In summary, this pilot data suggests that despite the fact that galantamine increases acetylcholine at nicotinic receptors and is an allosteric modulator of these receptors, this agent used adjunctively with antipsychotics failed to improve smoking behavior. In fact, treatment with galantamine led to increased scores of dependency or craving ratings in this population. While this data is not encouraging, these findings should be replicated in a controlled trial that more fully characterizes smoking behavior.
Supplementary Material
Acknowledgements
The authors would like to thank the staff in the Treatment Research Program and the Outpatient Research Program for all their work on this project and to Patricia Ball and Stephanie Feldman for research coordination. Also thanks to Matthew Nelson and James Gold for their participation in the study. The work was supported in part by the VA Capital Network (VISN 5) Mental Illness, Research, Education and Clinical Center, by the Stanley Medical Research Institute (PI, RW Buchanan) and by NIMH grant P30 068580 (PI, RW Buchanan). Double blind medications were supplied by Ortho McNeil Neurologics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155:974–76. doi: 10.1176/ajp.155.7.974. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Wiser A, Freedman R. Transient Normalization of a defect in auditory sensory processing in schizophrenics following cigarette smoking. Am J Psychiatry. 150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, Jansons S, Wilhelm K. A randomized controlled trial of a smoking cessation intervention among people with psychotic disorder. Am J Psychiatry. 2006;163:1934–1942. doi: 10.1176/ajp.2006.163.11.1934. [DOI] [PubMed] [Google Scholar]
- Barik J, Dajas-Bailador F, Wonnacott S. Cellular responses to nicotinic activation are decreased after prolonged exposure to galantamine in human neuroblastoma cells. Br. J Pharmacol. 2005;145:1084–92. doi: 10.1038/sj.bjp.0706278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M, Lawform BR, Burton SC, Heslop KR, Noble EP, Hausdorf K, Young RM. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust NZJ Psychiatry. 2006;40:575–80. doi: 10.1080/j.1440-1614.2006.01841.x. [DOI] [PubMed] [Google Scholar]
- Broad LM, Zwart R, Pearson KH, Lee M, Wallace L, McPhie GI, Emkey R, Hollinshead SP, Dell CP, Baker SR, Sher E. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J Pharm Exp Ther. 2006;318:1108–1117. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold J, McMahon RP. Galantamine for the treatment of cognitive impairments in schizophrenia. Am J Psychiatry. 2008;165:82–9. doi: 10.1176/appi.ajp.2007.07050724. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human alpha4beta2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley RR, Boggs DL, Kelly DL, McMahon RP, Feldman S, Ball P, Buchanan RW. The Effects of Galantamine on Psychopathology in Chronic Stable Schizophrenia. Clinical Neuropharmacology. 2008 doi: 10.1097/WNF.0B013E31816F2795. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ, Salsburg DS. Locally most powerful tests for detecting treatment effects when only a subset of patients are expected to ‘respond’ to treatment. Biometrics. 1988;44:189–196. [PubMed] [Google Scholar]
- De Leon J, Susce MT, Diaz FJ, Rendon DM, Velasquez DM. Variable associated with alcohol, drug and daily smoking cessation in patients with severe mental illness. J Clin Psychiatry. 2005;66:1447–55. doi: 10.4088/jcp.v66n1112. [DOI] [PubMed] [Google Scholar]
- Diehl A, Nakovics H, Croissant B, Smolka MN, Batra A, Mann K. Galantamine reduces smoking in alcohol-dependent patients: a randomized, placebo controlled trial. Int J Clin Pharmaocl Ther. 2006;44:614–22. doi: 10.5414/cpp44614. [DOI] [PubMed] [Google Scholar]
- Dixon L, Medoff DR, Wohlheiter K, DiClemente C, Goldberg R, Kreyenbuhl J, Adams C, Luckstead A, Davin C. Correlates of severity of smoking among persons with severe mental illness. Am J Addict. 2007;16:101–10. doi: 10.1080/10550490601184415. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Deckersbach T, Freudenreich O, et al. A double-blind placebo-controlled trial of bupropion sustained release for smoking cessation in schizophrenia. J Clin Psychopharmacol. 2005;25:218–25. doi: 10.1097/01.jcp.0000162802.54076.18. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, Freudenreich O, Henderson DC, Schoenfeld DA, Rigotti NA, Goff DC. A 12-week double-blind, placebo-controlled study of buproprion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. 2007;27:380–6. doi: 10.1097/01.jcp.0b013e3180ca86fa. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structural Clinical Interview for DSM-IV Axis Disorders (SCID-IV) Biometrics Research Department: New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry. 1992;149:1189–94. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, DAgostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-yer risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Gopalaswamy AK, Morgan R. Smoking in chronic schizophrenia. Br J Psychiatry. 1986;149:523. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Gagerstrom Tolerance Questionnaire. Br J Addictions. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hong LE, Wonodi I, Lewis J, Thaker GK. Nicotine effect on prepulse inhibition and prepulse facilitation in schizophrenia patients. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301601. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MD, Hindi-Alexander M, Wagner TJ. Psychosocial mediators of abstinence, relapse, and continued smoking: a one year follow up of a minimal intervention. Addictive Behaviors. 1985;10:29–39. doi: 10.1016/0306-4603(85)90050-4. [DOI] [PubMed] [Google Scholar]
- Kelly C, McCreadie RG. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry. 1999;156:1751–1757. doi: 10.1176/ajp.156.11.1751. [DOI] [PubMed] [Google Scholar]
- Kume T, Sigoimoto M, Takada Y, Yamaguchi T, Yonezawa A, Katsuki H, Sugimoto H, Akaike A. Up-regulation of nicotinic acetylcholine receptors by central-type acetylcholinesterase inhibitor in rat cortical neurons. Eur J Pharmacol. 2005;527:77–85. doi: 10.1016/j.ejphar.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Lam S, Patel PN. Varenicline; a selective alpha4beta2 nicotinic acetylchonie receptor partial agonist approved for smoking cessation. Cariol Rev. 2007;15:154–61. doi: 10.1097/01.crd.0000260270.12829.45. (Review) [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciototto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacol. 2003;28:216–25. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic systems and cognitive function. Psychopharmacol. 1992;108:417–31. doi: 10.1007/BF02247415. (Review) [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74:1182–91. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon ER. A review of the effects of nicotine on schizophrenia and antipsychotic medications. Psychiatric Services. 1999;50:1346–1350. doi: 10.1176/ps.50.10.1346. (Review) [DOI] [PubMed] [Google Scholar]
- Mantel N. “Chi-square Tests with One Degree of Freedom: Extensions of the Mantel-Haenszel Procedure,”. Journal of the American Statistical Association. 1963;58:690–700. [Google Scholar]
- Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–46. doi: 10.1016/S0074-7742(06)78008-4. (Review) [DOI] [PubMed] [Google Scholar]
- Masterson E, O'Shea B. Smoking and malignancy in schizophrenia. Br J Psychiatry. 1984;145:29–32. doi: 10.1192/bjp.145.4.429. [DOI] [PubMed] [Google Scholar]
- McMahon RP, Arndt S, Conley RR. More powerful two-sample tests for differences in repeated measures of adverse effects in psychiatric trials when only some patients may be at risk. Stat Med. 2005;24:11–21. doi: 10.1002/sim.1837. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Juel K. Mortality and cause of death in first admitted schizophrenic patients. Br J Psychiatry. 1993;163:183–189. doi: 10.1192/bjp.163.2.183. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Young DA, Roath M, Freedman R. Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenia patients. Neuropychopharmacol. 1998;18:175–85. doi: 10.1016/S0893-133X(97)00095-X. [DOI] [PubMed] [Google Scholar]
- Pakkanen JS, Jokitalo E, Tuominen RK. Upregulation of beta2 and alpha7 sbuunits containing nicotine acetylchoine receptors in mouse striatum at cellular level. Eur J Neurosci. 2005;212:2681–2691. doi: 10.1111/j.1460-9568.2005.04105.x. [DOI] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAchRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem. 2007;101:160–7. doi: 10.1111/j.1471-4159.2006.04355.x. (Review) [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–67. doi: 10.1016/s0006-3223(00)01094-5. (Review) [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Freedman R. Nicotinic agonists and psychosis. Curr Drug Targets CNS Neurol Disord. 2002;1:149–62. doi: 10.2174/1568007024606168. (Review) [DOI] [PubMed] [Google Scholar]
- Ucok A, Polat A, Bozkurt O, Meteris H. Cigarette smoking among patients with schizophrenia and bipolar disorders. Psychiatry Clin Neurosci. 2004;58:434–7. doi: 10.1111/j.1440-1819.2004.01279.x. [DOI] [PubMed] [Google Scholar]
- Weiner E, Ball MP, Summerfelt A, Gold J, Buchanan RW. Effects of sustained release buproprion and supportive group therapy on cigarette consumption in patients with schizophrenia. Am J Psychiatry. 2001;158:635–7. doi: 10.1176/appi.ajp.158.4.635. [DOI] [PubMed] [Google Scholar]
- Zeodonis DM, George TP. Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophr Bull. 1997;23:247–54. doi: 10.1093/schbul/23.2.247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.