Abstract
A long postreproductive lifespan may distinguish women from all other female primates. A long-held consensus among reproductive scientists has been that our closest living relative, the chimpanzee (Pan troglodytes), experiences menstrual cycles until death. However, a recent study of biannual assessments of gonadotropins, but lacking observations of menstruation, concluded that menopause occurs in chimpanzees between 35 and 40 yr of age. A separate report, but on wild chimpanzees, documented fertility through the 40–44 age range in all populations studied. These contradictory reports pose questions about differences between wild and captive populations and about assessments of menopause. The present study revisits this controversy by analyzing longitudinal records of anogenital swelling and menstruation in 89 female chimpanzees aged 6 to 59 yr (n = 2386 records on cycle length), monitored for most of their adult lives at the Yerkes National Primate Research Center. Twenty of these chimpanzees were observed past 39 yr of age; all 20 displayed menstrual cycles beyond this age, as confirmed by at least two observations of menses about 35 days apart. Three of these were older than 50 yr and still displayed menstrual cycles. Only the oldest female appeared menopausal, with cycles of anogenital swelling ceasing 2 yr prior to her death at age 59. Random-effects statistical modeling reveals a slight decrease in cycle length until 20 yr of age and a slight lengthening thereafter. Mean cycle length across the lifespan is 35.4 days. Our findings, based upon actual observations of menstrual cycles, suggest that menopause in the chimpanzee is rare, occurring near the end of the lifespan.
Keywords: aging, evolution, life history, longevity, longitudinal analysis, menopause, menstrual cycle, menstruation, ovulatory cycle, reproduction, sexual swelling
Menopause in the chimpanzee is likely a rare event that occurs only near the limit of the lifespan
INTRODUCTION
Women now live more than one third of their lives in a menopausal state. Because postmenopausal status has been associated with the development of a wide variety of health problems [1], understanding reproductive senescence and its impact on diverse systems is of critical importance for public health. Nonhuman primates, because of their phylogenetic proximity to humans, may serve to model aspects of human reproductive function [2].
Compared with other primates, humans' long postreproductive survival appears to be unique. For example, in macaques and baboons, menopause occurs near the end of the lifespan [3–7]. There are only a few studies on reproductive senescence in the great apes, and they have led to ambiguous conclusions. The captive gorilla experiences menopause at about 35 yr of age [8]. Feral gorillas remain fertile somewhat longer; they give birth as late as 40 yr of age and have a relatively short postreproductive survival time [9]. Findings on our closest living relative, the chimpanzee, are also inconsistent. It was first reported that menstrual cycles in captive chimpanzees persist until death [10], a finding supported by observations of FSH and LH levels [11]. In contrast, Videan and colleagues concluded that menopause in chimpanzees not only occurs, but at a relatively early age: between 35 and 40 yr. Their conclusion was not based on menstruation but on FSH and LH levels taken twice per year from 14 individuals over a period of several years [12]. They did not report menstrual bleeding, because occurrence of menses was not regularly observed in the authors' facility. Although there are no data for menopause in wild chimpanzees, a recent compilation of demographic data demonstrated age-specific fertility persisting at least through the 40- to 44-yr age range in all wild populations studied [13]. This implies that menopause does not occur until later ages in at least some females.
The goal of the present study is to resolve the controversy on the timing of menopause in chimpanzees by examining profiles of menstrual cycles, including menstrual bleeding, in females studied for an average of 20 yr at the Yerkes National Primate Research Center (YNPRC). Records of menstrual bleeding and anogenital swelling in female chimpanzees have been maintained for many decades at the YNPRC. Although the purpose of the collection of this information has varied across the decades, the basic procedure was to actually observe the occurrence of menstrual bleeding and to rate the magnitude of genital tumescence generally associated with estradiol levels [14]. The data represent a unique source of information on chimpanzee reproductive cycles across the lifespan. We analyzed these records (1) to determine whether chimpanzees displayed a cessation of menstruation that could be attributed to age, rather than to pregnancy, disease, or contraception, and (2) to describe changes in cycle length and patterns of anogenital swelling across the cycle.
MATERIALS AND METHODS
Subjects and Data Collection
Patterns of anogenital swellings and incidence of menses were used as markers of menstrual cycles. Sexual swellings in the female chimpanzee are reliable external indicators of menstrual cycle phases: Tumescence increases with rising estradiol levels during the follicular phase of the cycle, peaks at midcycle when estradiol levels are at their highest, and decreases with the rise of progesterone during the luteal phase [14–18]. Sexual swellings were rated by trained observers from 0 (no swelling) to 4 (maximum swelling), according to a well-established descriptive scale [15]. Menses were noted when overt blood flow was visible on the external perineum of the chimpanzee or on the floor or other surfaces of the housing area [11].
Data were extracted from two archival sources, both maintained at the YNPRC. First, we compiled information from the Animal Records System (ARS), a computerized database that contains detailed information on all the animals housed at the YNPRC. This computerized source provided the records of anogenital swelling and menstruation of female chimpanzees from 1990 onward. Data reported here cover the period from January 1990 through February 2006.
The second source comprised older records, spanning the period from 1967 through 1989. These handwritten records provide exceptionally rich documentation, containing daily ratings of anogenital swellings and menstruation in chimpanzees monitored for many consecutive years. For the purpose of the present study, we selected the records of females 39 yr old or older at the time of their last observation and carefully transcribed these data into a computerized format for merging with the ARS data.
The following criteria were applied in selecting cycles for analysis: (1) only cycles demarcated by recorded menstrual bleeding were considered, (2) data recorded during pregnancy were excluded, and (3) bleeding intervals more than 50 days in length or less than 20 days in length were excluded. The limitation on cycle length was imposed because cycles outside of this range might have resulted from missed observations. A total of 89 chimpanzees provided data meeting these selection criteria.
Information pertaining to health, pregnancies, oral contraceptive use, or exposure to specific experimental procedures potentially interfering with sexual swellings (e.g., hormonal treatment) was collected through ARS. Menstrual cycles containing such events were eliminated from the final dataset. In cases of pregnancy, we eliminated the menstrual cycles of the preceding 230 days [19] unless short-term birth or abortion was specified.
Median ages at death in our colony are 17 and 25.5 yr for males and females, respectively, and the maximum survivals are 45 and 59 yr [20]. The designation “old” is somewhat arbitrary; Erwin et al. [21] considered chimpanzees older than 40 yr to be aged. Of the 332 female chimpanzees that have been or are currently in the YNPRC colony, only 28 have reached the age of 39 or more yr.
The Yerkes National Primate Research Center of Emory University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Statistical Analysis
In order to statistically describe the longitudinal cycle length, we used a random-effects regression model in which the response variable was cycle length and the predictor variable was age or a linear transformation of age, rounded to the nearest year. To help identify overall trends in the highly variable original data, the continuous variable age (in years) was categorized into discrete age values ranging from 6 to 55 yr. The mean cycle length was then calculated for each animal at each age. We assumed that the mean cycle length follows a linear regression versus age for each animal with a random animal-specific intercept and with a separate population regression line. To accommodate any curvature in the data, a fractional polynomial model was fitted by a method proposed by Royston and Altman [22]. The percentage of days at maximum tumescence during each cycle was also calculated, and a similar modeling procedure was used to analyze the data.
RESULTS
Nonstatistical Observations
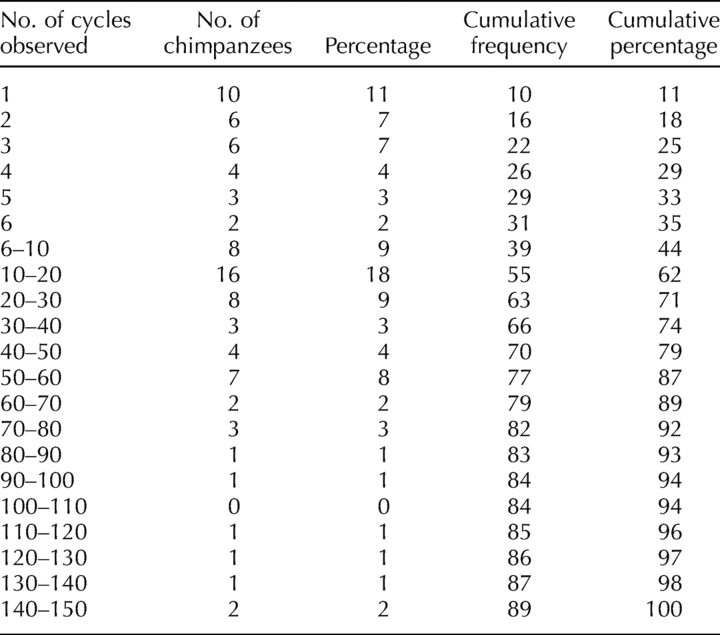
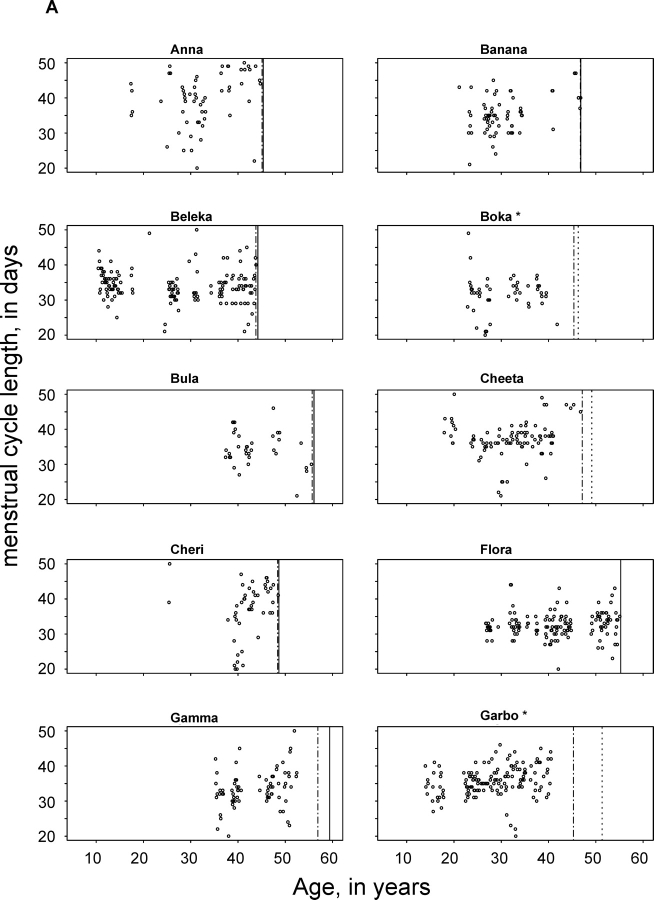
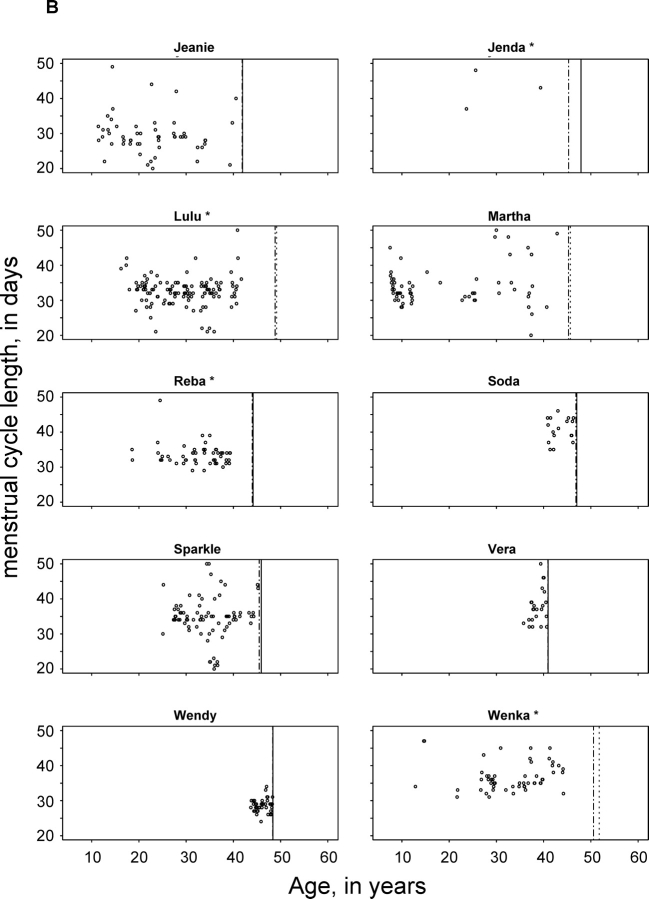
This analysis includes 2386 menstrual cycles from 89 chimpanzees aged 6 to 59 yr, totaling 664 chimpanzee-years of observation. The number of cycles observed in each chimpanzee ranged from 1 in 10 chimpanzees to 137 in a single chimpanzee (Table 1). Figure 1, A and B, depict individual cycle lengths for the 20 individual chimpanzees older than 39 yr of age. Each dot on these graphs represents the length of a cycle demarcated by menstrual bleeding (both at the beginning and at the end of the cycle). The vertical lines at the right side of each plot indicate, respectively, the date of the last observation of genital swelling and the age at last observation. The most striking feature of these plots is that most chimpanzees continued to cycle nearly until death. Some menstrual cycles were missed because of periods of hormone treatment or pregnancy. Jenda, for example, had 11 pregnancies between the ages of 10 and 38 yr, and was treated with contraceptives after this point, explaining the observation of only a small number of cycles. In other cases, bleeding may have been too light to be observed.
TABLE 1.
Menstrual cycles observed in 89 chimpanzees.
FIG. 1.
A, B) Cycle length throughout the period of observation of 20 chimpanzees having cycles after the age of 39 yr. Each dot represents the length of a cycle demarcated by menses at its beginning and end. The first vertical line indicates the last observed cycle of genital swelling. The second vertical line indicates the date of death (solid) or the last observation (dotted). *Several chimpanzees showed cessation of cycles meeting our inclusion criteria but did not meet criteria of menopause: Boka and Jenda received contraceptive treatment; Garbo, Lulu, and Riba continued to have cycles, but these were irregular and did not meet our inclusion criterion. Wenka had irregular cycles and had an enlarged uterus.
Other obvious characteristics of the data are the high degree of intraindividual variation in cycle length and the fact that menstruation continues throughout the observation period for most of these representative animals. Figure 1, A and B, includes cycle length for Flora, Wenka, and Gamma, the three individuals within our sample that lived to the oldest age. Flora showed no sign of decreased cycle frequency until her death at age 55. In Wenka, menstrual bleeding was observed until 45 yr of age, but genital swelling continued to occur until age 50, about 6 mo before the final observation for the present analysis; Wenka's cessation of menstruation cannot be confirmed as menopause, because she experienced uterine enlargement. Gamma was last observed to have menstrual bleeding at the age of 53 but continued to display anogenital swelling in a cyclic pattern until 2 yr before her death at the age of 59 yr; these last 2 yr of relatively good health in the absence of cycling can be considered postmenopausal. The continued reproductive capacity in late life is further confirmed by our observation that 5 of 20 chimpanzees had one or more full-term pregnancies after the age of 35. If not for husbandry measures (contraceptive hormone treatment, intrauterine devices, housing away from males) designed to restrict chimpanzee births, the number of chimpanzees becoming pregnant would almost certainly have been higher.
Statistical Results for Menstrual Cycle Length
A linear mixed model with a random animal-specific intercept and a separate population regression line did not adequately describe the data (P = 0.70). To accommodate the curvature in the data, a random intercept fractional polynomial model with age and its inverse as predictors was fit. This model fit the data much better than the initial mixed model, with both predictor variables achieving statistical significance (P < 0.0001). Inclusion of a random slope term did not further improve the fit. This final model is shown graphically, along with 95% reference intervals [23], in Figure 2. As is clear from this Figure, cycle length decreases gradually until around 20 yr of age (the inflection point was 19.7 yr) and increases gradually thereafter. All models were fit using SAS Proc Mixed.
FIG. 2.
Mean menstrual cycle length as a function of age in 89 chimpanzees. The solid line represents the best-fitting fractional polynomial random-effects regression model, containing age (P < 0.0001) and 1/age (P < 0.0001) as significant predictors. The dashed lines indicate the 95% reference intervals. Ŷ = 29.615 + 0.1338 × (age) + 52.106 × (1/age), Ŷ at 30 yr = 35.4 days, and the standard deviation for the fitted value Ŷ at 30 yr = 4.70.
The within-animal variance estimate (σ2W = 15.82; standard error = 0.93) accounted for 70% of the total variation in cycle length, whereas between-animal variance (σ2B = 6.64; standard error = 1.61) accounted for only about 30%.
Statistical Results for Anogenital Swelling
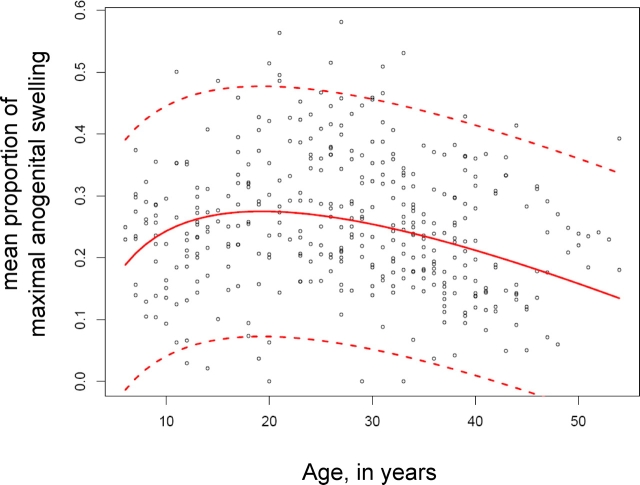
We used a similar approach to model the relationship between age and percentage of days at maximum tumescence. The best-fitting polynomial model had both the categorized age variable and its log transformation as predictors. A random intercept model was sufficient to model the data, and both predictors were found to be highly significant (P < 0.0001). The growth curve and its 95% reference intervals for this model are shown in Figure 3; the curvilinear trend is first upward and turns downward at 19.3 yr.
FIG. 3.
Mean proportion of menstrual cycle with maximal anogenital swelling as a function of age in 89 chimpanzees. The solid line represents the best-fitting fractional polynomial random-effects regression model, containing age (P < 0.0001) and log(age) (P < 0.0001) as significant predictors. The dashed lines indicate the 95% reference intervals. Ŷ = −0.08 + 0.182 × log (age) − 0.009 × (age), Ŷ at 30 yr = 0.269, and the standard deviation for the fitted value Ŷ at 30 yr = 0.103.
DISCUSSION
The present study is a retrospective analysis of archival data on chimpanzee reproductive cycles. To this end, we analyzed records of observed menstrual bleeding and genital swelling. Our results confirm a number of prior reports indicating that cycles of genital swelling continue until near the end of the lifespan in chimpanzee. This report extends the earlier findings by including decades of observation in the oldest continuously operating research colony of chimpanzees in the world. It also supplements earlier results by demonstrating that the same phenomenon of prolonged menstrual cycles is evident even when the menstrual cycles are defined in terms of observed menses rather than cyclic genital swelling. Our data sharply contradict the conclusion draw by Videan et al. [12]; they inferred from biannual measurements of serum gonadotropins that menopause in the chimpanzee occurs between 35 and 40 yr of age [12]. We conclude that menstrual cycles continue into the sixth decade. This conclusion is based upon the observation of menses in all chimpanzees over 50 yr of age. Indeed, every chimpanzee past 39 yr of age also displayed menstrual cycles. Although the contrasting conclusions of the two studies could have resulted from differences in the chimpanzee colonies, they are more likely the result of disparities in the methods employed. The biannual hormone measurements used by Videan et al. relied upon untested assumptions concerning threshold of basal levels of gonadotropins in women and which have not yet been validated in chimpanzees. Although our study lacks hormonal values, it is based upon actually observed menstruations. Because we define menstrual cycles as being demarcated by two periods of menstrual bleeding, there can be no doubt that regular menstrual cycles can continue into the fourth, fifth, and even sixth decades in chimpanzees.
Our study is a retrospective analysis of data collected for several purposes, including behavioral and physiological studies of menstrual cycles. The data were also used for breeding and fertility control because of changing chimpanzee colony maintenance goals. These factors led to some lapses in data collection and also to some periods in which menstrual cycles were suppressed by hormonal contraceptive agents (see Fig. 1 and caption). The nature of the data source made it necessary for us to eliminate apparently very long (>50 days) and very short cycles (<20 days) from analysis, because we could not assess whether such cycles were accurately recorded or simply represented missed observations. Therefore, we are not able to determine whether very long cycles suggestive of perimenopausal changes may have occurred in some chimpanzees. Despite these lapses, our data clearly indicate that at least some occurrences of menstrual cycles persisted in every individual observed past the age of 39 yr and in all three chimpanzees observed past the age of 50 yr. A total of 13 of the 20 chimpanzees observed past the age of 39 yr continued to show menstrual cycles until death or the end of data collection for this study; six of the remaining chimpanzees in this age group ceased showing cycles of bleeding that met our inclusion criteria but experienced interventions such as contraception or health problems that could explain this apparent cessation of cycling. Therefore, we consider these data as being inconclusive. In addition, several of these chimpanzees with irregular cycles continued to exhibit cyclic anogenital swelling past the last observation of a cycle defined by observed menstrual bleeding. Only one chimpanzee could be identified that ceased showing cycles of menstrual bleeding and of swelling while still in good health, thus meeting the definition of menopause provided by Burger [24].
We cannot be certain that these cycles of swelling without visible bleeding indicated continued ovulation, but evidence suggests that this was likely. Indeed, recent studies report a very tight linkage between hormonal events associated with ovulation and turgidity of the perineal skin. Approximately 10 days before the midcycle estrogen peak, genital swelling reaches maximum size and turgidity. Maximum tumescence lasts, on average, 10 days [25–27], with LH surge and ovulation occurring on the last 1 to 2 days of maximum swelling [15]. There is anogenital detumescence with decreasing estradiol (E2) levels and increasing levels of progesterone [15, 17, 28, 29]; this detumescence occurs approximately 3 days after preovulatory LH peak [28].
Emery and Whitten [27] noted that swelling begins earlier and proceeds more rapidly than the changes in E2 levels, suggesting that the relationship between swelling and E2 levels is not perfect. Nevertheless, they reported that the absolute size of swellings reflects between-cycle variation in ovarian function in chimpanzees and is an indicator of the reproductive quality of the cycle in which swelling is displayed [27]. Although swelling and ovulation are closely related in nonpregnant young adults, the possibility remains that cyclic swelling continues after menopause under some other mechanism of control. Although we consider this to be unlikely, it is a possibility that warrants additional research.
Despite our different conclusion concerning the timing of menopause, other findings of our study are similar to those reported by Videan et al. [12]. Both studies, for example, revealed that menstrual cycle length decreased after puberty, reached an inflection point, and then gradually increased during later adulthood. Both also found that the proportion of days during each cycle with maximum anogenital swelling decrease in advanced age, a change which may in part result from the increasing length of the cycle. The studies are also consistent with previous reports that the average cycle length for chimpanzees is around 35 days [28, 30]. The present study additionally reveals that the intra-animal variation in cycle length is very large, accounting for 70% of the total. This large variance within individuals emphasizes the need for repeated observations on the same individuals in order to understand age-related trends in patterns of ovarian cycles.
Our data are consistent with two reports on aging and reproductive function in chimpanzees. One of the reports [31] found that the number of follicles in chimpanzee ovaries examined histologically decreases exponentially with a log-linear slope that is not statistically different from the log-linear decline in women from birth to 47 yr of age—the limit of the chimpanzee data. The second study reported that wild chimpanzees may give birth in their 40s and 50s [13]. The three studies taken together suggest the hypothesis that female chimpanzees and women have similar rates of reproductive decline and that menopause may be rare in chimpanzees because they do not live long enough to experience it. Population-based studies of the occurrence of menopause in women reveal considerable variation in the age at which it occurs. The median age at menopause in that analysis ranged from about 45 to 50 yr of age [32–35], with 5% of women reaching menopause before ages that ranged from 39 years (Australia) [34] to 45 years (Minnesota) [33] and 5% not reaching menopause until after ages ranging from 53 years (Norway) [32] to 56 years (Mexico) [35]. This human pattern cannot be compared statistically with the chimpanzee because of the small number of very old chimpanzees available. However, the available data suggest women and chimpanzees may reach menopause near the same age.
The present analysis convincingly indicates that menopause in the chimpanzee is likely to occur late in life. The data are archival and were collected for other purposes, such as studies on reproductive behavioral patterns or to determine whether females had become pregnant. Not surprisingly, therefore, there were gaps in observations, meaning that we could not determine what percentage of individuals continue to cycle until late in life but can conclude that a large proportion of females do so. We were able to definitively observe menopause—defined as the cessation of menses for at least 12 consecutive months [24] without underlying pathology—in only the oldest subject of the colony, at about 53 yr of age (on the basis of menstrual bleeding) or 57 years (on the basis of cyclic swelling). Studies are now underway that will include regular assessments of ovarian hormones during late life menstrual cycles to determine whether they might be perimenopausal.
Acknowledgments
We thank Jill Johnson, Ashi Chhabra, Paola Espinosa, David Pierce, and Andrea Warren for their contribution to data transcription; Doris Jane Langford for assistance with manuscript preparation; Johannes Tigges and Margaret Walker for comments on the manuscript; and the Animal Resources Division of the Yerkes National Primate Research Center of Emory University for assistance in the extraction of data for this study.
Footnotes
Supported by National Institutes of Health grants P51RR000165 and P01AG026423.
REFERENCES
- Greendale GA, Lee NP, Arriola ER.The menopause. Lancet 1999; 353: 571–580. [DOI] [PubMed] [Google Scholar]
- Bellino FL, Wise PM.Nonhuman primate models of enopause workshop. Biol Reprod 2003; 68: 10–18. [DOI] [PubMed] [Google Scholar]
- Walker ML.Menopause in female rhesus monkeys. Am J Primatol 1995; 35: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL.Characterization of the onset of menopause in the rhesus macaque. Biol Reprod 1997; 57: 335–340. [DOI] [PubMed] [Google Scholar]
- Packer C, Tatar M, Collins A.Reproductive cessation in female mammals. Nature 1998; 392: 807–811. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Williams JK, Wagner JD.Naturally occurring menopause in cynomolgus monkeys: changes in hormone and carbohydrate measures with hormonal status. J Med Primatol 2005; 34: 171–177. [DOI] [PubMed] [Google Scholar]
- Walker ML, Herndon JG.Menopause in nonhuman primates? Biol Reprod 2008; 79: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsalis S, Margulis SW.Sexual and hormonal cycles in geriatric western lowland gorillas (Gorilla gorilla gorilla). Int J Primatol 2008; 27: 1663–1687. [Google Scholar]
- Robbins AM, Robbins MM, Gerald-Steklis N, Steklis HD.Age-related patterns of reproductive success among female mountain gorillas. Am J Phys Anthropol 2006; 131: 511–521. [DOI] [PubMed] [Google Scholar]
- Graham CE.Reproductive function in aged female chimpanzees. Am J Phys Anthropol 1979; 50: 291–300. [DOI] [PubMed] [Google Scholar]
- Gould KG, Flint M, Graham CE.Chimpanzee reproductive senescence: a possible model for evolution of the menopause. Maturitas 1981; 3: 157–166. [DOI] [PubMed] [Google Scholar]
- Videan EN, Fritz J, Heward CB, Murphy J.The effects of aging on hormone and reproductive cycles in female chimpanzees (Pan troglodytes). Comp Med 2006; 56: 291–299. [PubMed] [Google Scholar]
- Emery Thompson M, Jones JH, Pusey AE, Brewer-Marsden S, Goodall J, Marsden D, Matsuzawa T, Nishida T, Reynolds V, Sugiyama Y, Wrangham RW.Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol 2007; 17: 2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler RD, Dahl JF, Collins DC, Gould KG.Hormone levels and anogenital swelling of female chimpanzees as a function of estrogen dosage in a combined oral contraceptive. Proc Soc Exp Biol Med 1992; 201: 73–79. [DOI] [PubMed] [Google Scholar]
- Graham CE, Collins DC, Robinson H, Preedy JR.Urinary levels of estrogen and pregnanediol and plasma levels of progesterone during the menstrual cycle of the chimpanzee; relationship to the sexual swelling. Endocrinology 1972; 91: 13–24. [DOI] [PubMed] [Google Scholar]
- Graham CE, Warner H, Misener J, Collins DC, Preedy JRK.The association between basal body temperature, sexual swelling and urinary gonadal hormone levels in the menstrual cycle of the chimpanzee. J Reprod Fertil 1977; 50: 23–28. [DOI] [PubMed] [Google Scholar]
- Dahl JF, Nadler RD, Collins DC.Monitoring the ovarian cycles of Pan troglodytes and P. paniscus: a comparative approach. Am J Primatol 1991; 24: 195–209. [DOI] [PubMed] [Google Scholar]
- Emery MA, Whitten PL.Size of sexual swellings reflects ovarian function in chimpanzees (Pan troglodytes). Behav Ecol Sociobiol 2003; 54: 340–351. [Google Scholar]
- Dahl JF.Perineal swelling during pregnancy in common chimpanzees and puerperal pathology. J Med Primatol 1999; 28: 129–141. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM.Brain weight throughout the life span of the chimpanzee. J Comp Neurol 1999; 409: 567–572. [PubMed] [Google Scholar]
- Erwin JM, Hof PR, Ely JJ, Perl DP.One gerontology: advancing understanding of aging through studies of great apes and other primates. Erwin JM, Hof PR.Aging in Nonhuman Primates, vol. 31 New York:Basel, Karger;2002: 1–21. [Google Scholar]
- Royston P, Altman DG.Regression using fractional polynomials of continuous covariates: parsimonious parametric modeling. Appl Stat 1994; 43: 429–467. [Google Scholar]
- Royston P.Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med 1995; 14: 1417–1436. [DOI] [PubMed] [Google Scholar]
- Burger HG.The endocrinology of the menopause. J Steroid Biochem Mol Biol 1999; 69: 31–35. [DOI] [PubMed] [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C.Timing and probability of ovulation in relation to sex swelling in wild West African chimpanzees. Anim Behav 2003; 66: 551–560. [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C.Female sexual swelling size, timing of ovulation, and male behavior in the wild West African chimpanzees. Horm Behav 2004; 46: 204–215. [DOI] [PubMed] [Google Scholar]
- Emery MA, Whitten PL.Size of sexual swellings reflect ovarian function in chimpanzees (Pan troglodytes). Behav Ecol Sociobiol 2003; 54: 340–351. [Google Scholar]
- Nadler RD, Graham CE, Gosselin RE, Collins DC.Serum levels of gonadotropins and gonadal steroids, including testosterone, during the menstrual cycle of the chimpanzee (Pan troglodytes). Am J Primatol 1985; 9: 273–284. [DOI] [PubMed] [Google Scholar]
- McArthur J, Beitins I, Gorman A, Collins DC, Preedy JR, Graham CE.The interrelationships between sex skin swelling and the urinary excretion of LH, estrone, and pregnanediol by the cycling female chimpanzee. Am J Primatol 1981; 1: 265–270. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M.Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am J Primatol 2005; 67: 137–158. [DOI] [PubMed] [Google Scholar]
- Jones KP, Walker LC, Anderson D, Lacreuse A, Robson SL, Hawkes K.Depletion of ovarian follicles with age in chimpanzees: similarities to humans. Biol Reprod 2007; 77: 247–251. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Heuch I, Kvale G.Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol 2003; 157: 923–929. [DOI] [PubMed] [Google Scholar]
- Treloar AE.Menstrual cyclicity and the pre-menopause. Maturitas 1981; 3: 249–264. [DOI] [PubMed] [Google Scholar]
- Do KA, Treloar SA, Pandeya N, Purdie D, Green AC, Heath AC, Martin NG.Predictive factors of age at menopause in a large Australian twin study. Hum Biol 1998; 70: 1073–1091. [PubMed] [Google Scholar]
- Sievert LL, Hautaniemi SI.Age and menopause in Puebla, Mexico. Hum Biol 2003; 75: 205–226. [DOI] [PubMed] [Google Scholar]