Abstract
The receptor for advanced glycation end-products (RAGE) has been implicated in numerous disease processes including: atherosclerosis, diabetic nephropathy, impaired wound healing, and neuropathy to name a few. Treatment of animals with a soluble isoform of the receptor (sRAGE) has been shown to prevent and even reverse many disease processes. Isolating large quantities of pure sRAGE for in vitro and in vivo studies has hindered its development as a therapeutic strategy in other RAGE mediated diseases that require long-term therapy. This article provides an improvement in both yield and detail of a previously published method to obtain 10 mg of pure, endotoxin free sRAGE from 65 g of lung tissue.
Keywords: RAGE, sRAGE, purification, mouse, bovine, lung
INTRODUCTION
The receptor for advanced glycation end products (RAGE) is a member of the immunoglobulin superfamily of cell surface receptors.[1] Activation of RAGE by its ligands (including advanced glycation end products (AGEs), HMGB1/amphoterin, S100/calgranulins, and amyoid-β peptide) often leads to pro-inflammatory signaling, as well as up regulation of RAGE itself.[2] This process has been shown to lead to numerous disease states including: atherosclerosis, diabetic nephropathy, impaired wound healing, and neuropathy to name a few.[2–6] However, RAGE appears to have a protective function in pulmonary fibrosis.[7] The disease pathogenesis can be blocked and sometimes even reversed by administration of soluble RAGE (sRAGE), a naturally occurring non-signaling decoy receptor. Unfortunately, isolating large quantities of the pure protein has proven challenging and has hindered investigations into other therapeutic benefits of sRAGE. One limitation is that many of the RAGE mediated disease models in mice require long-term treatment with recombinant sRAGE. This can be complicated by the development of immune responses to this protein. Using sRAGE purified from mice would prevent this potential complication, but until now obtaining sufficient quantities for long term dosing has been difficult and costly. In this article, we describe a 5-step isolation and purification protocol for obtaining 10 mg of pure, endotoxin free sRAGE from 65 g of fresh-frozen lung tissue. This protocol eliminates the burdensome task of cloning and expressing the sRAGE gene for each animal of interest. Additionally, the protein does not need to be altered with an amino acid tag for easier purification. Purifying the protein from E. coli[8], yeast[9], and insect cells[6] may not result in all of the post-translational modifications that occur in mammals, particularly glycosylation, potentially altering its structure, function, and immunogenicity. This protocol results in a pure and unaltered protein for both in vitro and in vivo investigations. Additionally, it greatly improves the yield, purity, and ease of purification of a previously published protocol.[10]
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted. Fast protein liquid chromatography was performed using an FPLC System, FPLC Director Software, and empty columns from Pharmacia (now GE Healthcare Life Sciences, Pittsburgh, PA). All buffers were filtered through a 0.22 µm membrane filter (Corning, Lowell, MA) before use.
Lung Tissue Homogenization
65 g of fresh frozen mouse lungs (300 pairs, Pel-Freez Biologicals, Rogers, AR) were blended in 600 mL of ice-cold homogenization buffer (50 mM K2HPO4, 300 mM KBr, 3 mM EDTA, 1 mM PMSF, 0.01 mM E-64 (trans-Epoxysuccinyl-leucylamido-[ 4-guanidino] butane), pH 7.4). The homogenate was centrifuged in 250-mL polycarbonate centrifuge bottles at 20,000 × g for 20 min. at 4 °C to pellet insoluble material. The supernatants were then pooled. Polyethyleneimine was added to a final concentration of 0.01% and the homogenate was stirred for 10 min. at 4 °C to precipitate nucleic acids. The homogenate was centrifuged again as above. The supernatant was vacuum-filtered through a Büchner funnel with a coarse (40–60 µm) fritted disc to remove any unpelleted debris.
Concanavalin A Sepharose Chromatography
One hundred milliliters of Concanavalin A Sepharose (Sigma) was rinsed with 400 mL of wash buffer (50 mM HEPES, 250 mM NaCl, pH 7.0) and added to the lung homogenate in a 1-L polypropylene beaker containing a floating magnetic stir bar. The suspension was stirred for 16 hours at 4 °C and then poured into a 150-mL Büchner funnel with coarse fritted disc. The Con-A was washed in the funnel with 1 L of ice-cold wash buffer until the absorbance of the flow through at 280 nm was < 0.05. The bound protein was batch eluted (~5–6 mL/min) with 1 L of elution buffer (50 mM HEPES, 250 mM NaCl, 200 mM methyl α-D-mannopyranoside, pH 7.0) or until the Abs280 < 0.05. In order to elute the maximum amount of protein in the smallest amount of buffer, the elution buffer was put over the column twice. To accomplish this, 250 mL of buffer was eluted from the column and then poured over the column again to elute even more protein into the fraction. After the second elution, a fresh 250 mL of buffer was put onto the column and the above steps were repeated. The eluates were pooled and filtered through a 0.22 µm vacuum filter.
Heparin Sepharose
An XK-16 column was packed with 60 mL of Affi-Gel Heparin Sepharose (Bio-Rad, Hercules, CA). Using a Pharmacia FPLC system, the column was washed with 250 mL of 80% Buffer A (20 mM Tris-HCl, 50 mM NaCl, pH 7.5) and 20% Buffer B (20 mM Tris-HCl, 1 M NaCl, pH 7.5). The Concanavalin A eluate (not dialyzed) was applied to the column at a flow rate of ~ 0.5 mL/min at 4 °C. After loading, the column was attached to an FPLC system and washed with 80% Buffer A / 20% Buffer B. The following elution profile was used: flow rate = 4.5 mL/min, gradient = 1% B/min starting at 20% Buffer B, fraction collector = 1 fraction/min (Figure 1). Fractions containing the 45 kDa sRAGE protein (19–41), as determined by coomassie blue staining of SDS-PAGE, were pooled (volume = 148.5 mL) and dialyzed into 10 L of Buffer A at 4 °C for 4 hrs and then switched to fresh Buffer A overnight. The eluate was filtered through a 0.22 µm vacuum filter to remove any debris and to degas the solution.
Figure 1. The eluate from the Concanavalin A column was filtered and applied to a column containing Heparin Sepharose.
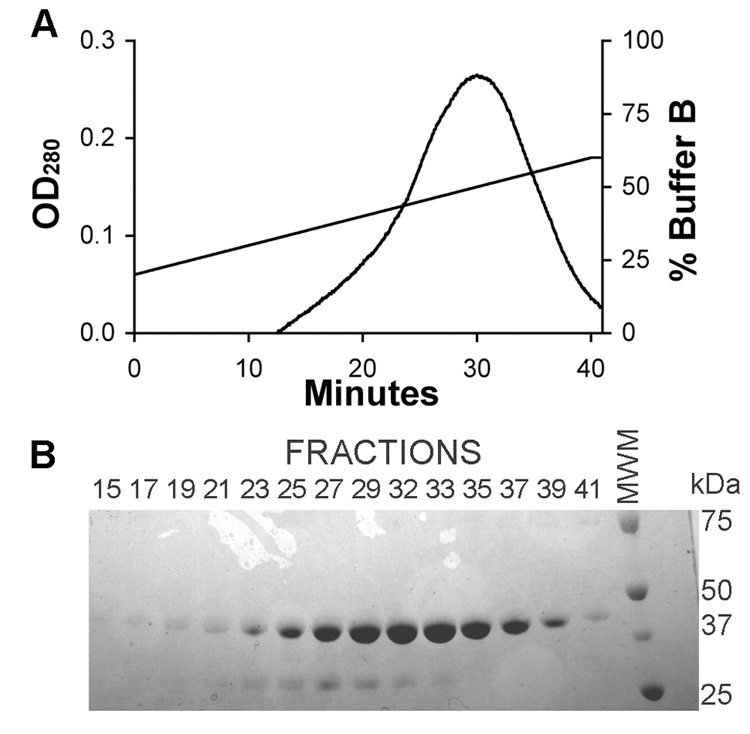
(A) The bound protein was eluted with a NaCl gradient while measuring the absorbance at 280 nm. Fractions were collected every minute and samples were subjected to reducing SDS-PAGE. (B) The gel was stained with coomassie blue and fractions 19–41 contained sRAGE as well as a lower molecular weight contaminate. Fractions 19–41 were pooled.
Anion Exchange Chromatography
A 5-mL HiTrap Q column (GE Healthcare) was attached to the FPLC and washed with 25 mL of Buffer A at 1 mL/min. Using a peristaltic pump (Pharmacia), the protein was applied to the column at 1 mL/min. The protein was eluted with an isocratic elution (35% Buffer B) at 1 mL/min and the eluate was pooled from 3–20 min. (Figure 2). The protein was dialyzed into PBS as described above.
Figure 2. The pooled and dialyzed eluate from the Heparin Sepharose column was applied to a HiTrap Q anion exchange column.
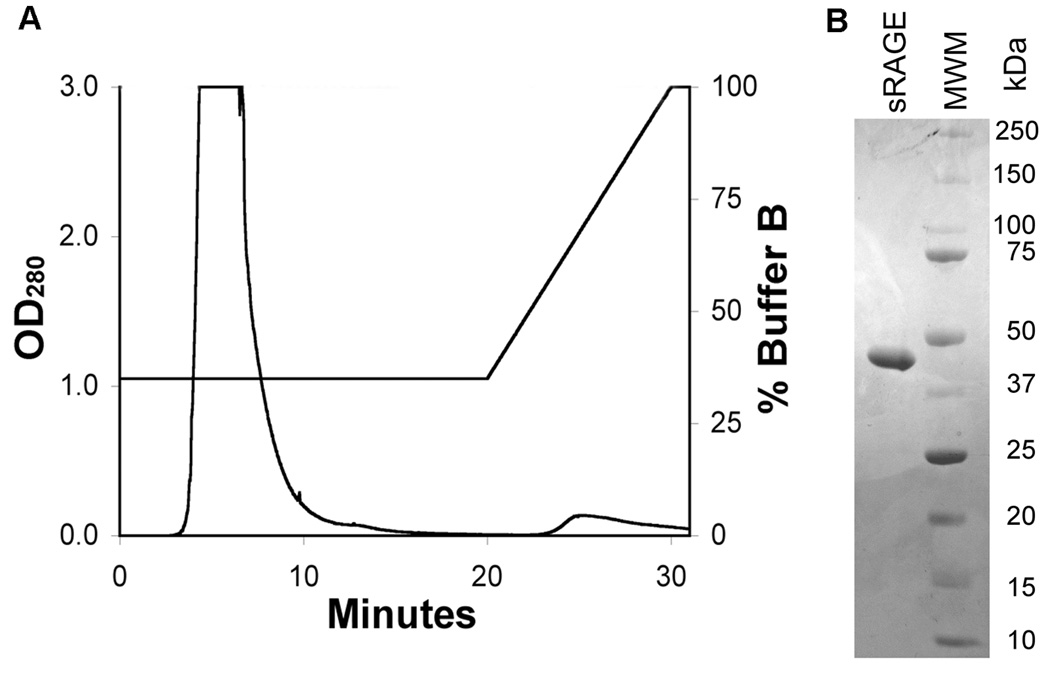
(A) The protein was completely eluted with an isocratic elution of 35% Buffer B between 3 and 20 minutes. No additional protein eluted with a subsequent gradient of 35–100% Buffer B. (B) SDS-PAGE of the final product confirmed a single band of ~ 45 kDa. Fractions from 3–20 minutes were pooled.
Endotoxin Removal
Endotoxin was removed using a 1-mL Detoxi-Gel Column (Pierce) according to the manufacturer’s protocol. Endotoxin was assayed using the Pyrotell® LAL Single Test Vial (Associates of Cape Cod, Inc., East Falmouth, MA) and found to be < 0.12 EU/mL.
Protein identification by MALDI-MS/MS analysis
An aliquot of the final pool was subjected to reducing SDS-PAGE followed by coomassie blue stain. The gel band of interest (~45 kDa) was excised and digested at 37 °C for 18 hours using sequencing-grade modified trypsin (Promega). The resulting tryptic peptides were isolated (ZipTip tip µ−C18, Millipore Corporation) and spotted onto a MALDI sample target using 1 µl matrix solution containing 70% (v/v) acetonitrile, 0.03% (v/v) trifluoroacetic acid (Protein Sequencer Grade), and 0.4% (w/v) recrystallized α–cyano-4-hydroxy-cinnamic acid (Sigma). The sample was analyzed by MALDI-MS peptide mass fingerprinting and MALDI-MS/MS of selected ions using a Q-Tof UltimaTM Global mass spectrometer (Waters, Micromass, Manchester, UK) operated under MassLynx 4.0. The spectra were combined, background subtracted, deisotoped (MaxEnt 3) and exported as a Mascot-searchable SEQUEST file. The files were merged and used to query all mouse Swiss-Prot and MSDB entries using the Mascot software package (Matrix Sciences, London, U.K).
Generation of anti-RAGE antibodies
A mouse RAGE specific polyclonal antibody was produced against residues 38–51 (Genbank accession #Q62151) by GenScript Coporation (Scotch Plains, NJ). Rabbits were immunized with a KLH conjugated mouse RAGE peptide and antiserums containing the RAGE antibodies were collected at sacrifice. Titers against the peptide were performed to confirm immunogenicity. Antibody specificity for membrane and soluble RAGE was confirmed by the detection of only those two proteins on Western Blot analysis of lung homogenate.
sRAGE ELISA
The concentration of sRAGE at each step of the purification process was measured by direct ELISA. In brief, the samples and sRAGE standards were diluted in TBS and incubated in high-binding polystyrene 96-well plates (R&D Systems, Minneapolis, MN) overnight at 4 °C. The wells were blocked with 3% BSA in TBS-T at 37 °C for 1.5 hrs. The wells were washed 3 times with TBS-T. A mouse RAGE specific antibody was diluted 1:1000 in 1% BSA/TBS-T and 100 µL was added to each well and incubated at room temperature for 2 hrs. The plate was washed again and 100 µl of biotin conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted 1:10,000 in 1% BSA/TBS-T was added to each well and incubated for 1 hr at room temperature. The plate was once again washed and streptavidin-alkaline phosphatase (Jackson ImmunoResearch Laboratories) was diluted 1:1000 in 1% BSA/TBS-T and 100 uL was added to each well for 1 hr at room temperature. The plate was washed and then developed with an alkaline phosphatase substrate kit (Bio-Rad, Hercules, CA) for 5 min. The plate was read at 405 nm on a SpectraMax 96-well plate reader (Molecular Devices, Sunnyvale, CA). The concentrations were calculated from the linear regression of the standard curve using SoftMax Pro software (Molecular Devices).
RESULTS AND DISCUSSION
A single band was identified in the final product with a molecular weight of ~ 45 kDa as previously reported for sRAGE.[10]. The band was also confirmed to be mouse sRAGE by mass spectral analyses. No other proteins were detectable by coomassie blue staining in the final product indicating very high purity, an improvement over previous studies (Figure 2). The protocol resulted in a 46% yield with the greatest loss of protein occurring during the Heparin Sepharose step (Table 1). The final product contained less than 0.12 EU/mL of endotoxin after passage over the Detoxi-Gel column. The amounts of tissue and column volumes were chosen to prevent oversaturation and loss of sRAGE in the column washes. Minimal amounts of sRAGE were detected by western blot in the Heparin Sepharose and HiTrap Q washes.
Table 1.
Summary of the results of each step in the purification.
| sRAGE Concentration (µg/mL) | Pool Volume (mL) | Total sRAGE (mg) | % Yield | |
|---|---|---|---|---|
| 1. Lung Homogenate | 36.2 | 625 | 22.6 | 100 |
| 2. Concanavalin A | 21.1 | 1000 | 21.1 | 93 |
| 3. Heparin Sepharose | 97.5 | 148.5 | 14.5 | 64 |
| 4. HiTrap Q | 762.7 | 18 | 13.7 | 61 |
| 5. Detoxi-Gel | 604.8 | 17 | 10.3 | 46 |
In addition to providing a greater yield and purity, this protocol has several advantages over a previously published protocol.[10] 1)The Concanavalin A eluate no longer needs to be concentrated and dialyzed before being applied to the Heparin Sepharose column. Direct application at the higher salt concentration actually reduces the number of contaminates in the heparin elution and increases the column’s binding capacity for sRAGE. 2) A faster gradient is used for the heparin elution shortening the duration of this step without reducing the separation. 3) A much less expensive HiTrapQ column was used to replace the costly MonoQ column in the anion exchange chromatography step. 4) sRAGE was eluted from the HiTrapQ column with an isocratic elution, which increased the protein concentration of the final product and reduced the length of time for this step. 5) Lastly, an endotoxin removal step was added so that the purified protein could be administered to animals as a therapeutic.
This enhanced protocol will greatly improve investigators ability to more easily purify large quantities of sRAGE for in vitro and in vivo investigations. Additionally, this protocol yielded nearly identical results with bovine lung and likely provides a method to isolate sRAGE from the lung tissue of other animals as well.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R21ES013986) to T.D.O.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- 2.Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. The Journal of biological chemistry. 1997;272:16498–16506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circulation research. 1999;84:489–497. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- 4.Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. The American journal of pathology. 2004;164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, Schulz JB, Heuss D, Neundorfer B, Dierl S, Huber J, Tritschler H, Schmidt AM, Schwaninger M, Haering HU, Schleicher E, Kasper M, Stern DM, Arnold B, Nawroth PP. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. The Journal of clinical investigation. 2004;114:1741–1751. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nature medicine. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 7.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, Ramsgaard L, Richards TJ, Loutaev I, Nawroth PP, Kasper M, Bierhaus A, Oury TD. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. The American journal of pathology. 2008;172:583–591. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dattilo BM, Fritz G, Leclerc E, Kooi CW, Heizmann CW, Chazin WJ. The extracellular region of the receptor for advanced glycation end products is composed of two independent structural units. Biochemistry. 2007;46:6957–6970. doi: 10.1021/bi7003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostendorp T, Weibel M, Leclerc E, Kleinert P, Kroneck PM, Heizmann CW, Fritz G. Expression and purification of the soluble isoform of human receptor for advanced glycation end products (sRAGE) from Pichia pastoris. Biochemical and biophysical research communications. 2006;347:4–11. doi: 10.1016/j.bbrc.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 10.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, Oury TD. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE) The Journal of biological chemistry. 2004;279:50019–50024. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]


