Abstract
Exposure of A431 squamous and MDA-MB-231 mammary carcinoma cells to ionizing radiation has been associated with short transient increases in epidermal growth factor receptor (EGFR) tyrosine phosphorylation and activation of the mitogen-activated protein kinase (MAPK) and c-Jun NH2-terminal kinase (JNK) pathways. Irradiation (2 Gy) of A431 and MDA-MB-231 cells caused immediate primary activations (0–10 min) of the EGFR and the MAPK and JNK pathways, which were surprisingly followed by later prolonged secondary activations (90–240 min). Primary and secondary activation of the EGFR was abolished by molecular inhibition of EGFR function. The primary and secondary activation of the MAPK pathway was abolished by molecular inhibition of either EGFR or Ras function. In contrast, molecular inhibition of EGFR function abolished the secondary but not the primary activation of the JNK pathway. Inhibition of tumor necrosis factor α receptor function by use of neutralizing monoclonal antibodies blunted primary activation of the JNK pathway. Addition of a neutralizing monoclonal antibody versus transforming growth factor α (TGFα) had no effect on the primary activation of either the EGFR or the MAPK and JNK pathways after irradiation but abolished the secondary activation of EGFR, MAPK, and JNK. Irradiation of cells increased pro-TGFα cleavage 120–180 min after exposure. In agreement with radiation-induced release of a soluble factor, activation of the EGFR and the MAPK and JNK pathways could be induced in nonirradiated cells by the transfer of media from irradiated cells 120 min after irradiation. The ability of the transferred media to cause MAPK and JNK activation was blocked when media were incubated with a neutralizing antibody to TGFα. Thus radiation causes primary and secondary activation of the EGFR and the MAPK and JNK pathways in autocrine-regulated carcinoma cells. Secondary activation of the EGFR and the MAPK and JNK pathways is dependent on radiation-induced cleavage and autocrine action of TGFα. Neutralization of TGFα function by an anti-TGFα antibody or inhibition of MAPK function by MEK1/2 inhibitors (PD98059 and U0126) radiosensitized A431 and MDA-MB-231 cells after irradiation in apoptosis, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and clonogenic assays. These data demonstrate that disruption of the TGFα–EGFR–MAPK signaling module represents a strategy to decrease carcinoma cell growth and survival after irradiation.
INTRODUCTION
Ionizing radiation has been shown to activate multiple signaling pathways within cells in vitro, which can lead to either increased cell death or increased proliferation depending on the cell type, the radiation dose, and the culture conditions (Xia et al., 1995; Rosette and Karin, 1996; Santana et al., 1996; Chmura et al., 1997; Schmidt-Ullrich et al., 1997; Carter et al., 1998; Haimovitz-Friedman, 1998; Kavanagh et al., 1998). Recently, a novel cellular target for ionizing radiation has been shown to be the epidermal growth factor receptor (EGFR, also called ErbB1), which is activated in response to irradiation of A431 squamous and MDA-MB-231 and MCF-7 mammary carcinoma cells (Schmidt-Ullrich et al., 1997; Carter et al., 1998; Kavanagh et al., 1998). Radiation exposure, via the EGFR, can activate the mitogen-activated protein kinase (MAPK) pathway to a level similar to that observed by physiological (∼0.1 nM) EGF concentrations (Schmidt-Ullrich et al., 1997; Kavanagh et al., 1998). Increased signaling by EGFR and the MAPK pathway has been suggested in previous studies from our laboratory to be protective against both ionizing radiation and drug treatments, although the precise mechanism(s) by which this occurs are unclear (Balaban et al., 1996; Gokhale et al., 1997; Goldkorn et al., 1997; Schmidt-Ullrich et al., 1997; Carter et al., 1998; Kavanagh et al., 1998).
Currently, the ability of the MAPK cascade to regulate differentiation and proliferative responses of cells is the focus of intense research (Sewing et al., 1997; Woods et al., 1997; Auer et al., 1998b; Dent et al., 1998; Tombes et al., 1998). The ability of MAPK signaling to regulate proliferation versus differentiation appears to depend on the cell type examined as well as on the amplitude and duration of MAPK activation. A short activation of the MAPK cascade by growth factors has been correlated with increased proliferation, via both increased cyclin D1 expression and an increased ability to progress through the G2–M transition. In contrast, prolonged elevation of MAPK activity has been demonstrated to inhibit DNA synthesis, via induction of the cyclin-dependent kinase inhibitor protein p21Cip-1/WAF1 (Auer et al., 1998b; Tombes et al., 1998). In addition to a role for MAPK signaling during G1–S phase, we and others have also argued that MAPK signaling is involved in the ability of cells to progress through G2–M, particularly in cells after irradiation (Warenius et al., 1996; Gokhale et al., 1997; Abbott and Holt, 1999; Vrana et al., 1999). Recent studies have suggested that one mechanism by which radiation and signaling by ErbB1–4 family receptors can transiently increase p21Cip-1/WAF1 protein levels is via activation of the MAPK pathway (Carter et al., 1998; Fiddes et al., 1998). These data argue that radiation-induced MAPK signaling may play dual positive and negative roles in the regulation of cell cycle progression after irradiation of carcinoma cells.
Exposure of cells to ionizing radiation may also induce apoptosis and lead to loss of clonogenic potential. Several groups have shown that radiation-induced activation of acidic sphingomyelinase leads to the generation of ceramide and the activation of the c-Jun NH2-terminal kinase (JNK) pathway, playing a major role in the initiation of apoptosis in various leukemic cell lines (Santana et al., 1996; Chmura et al., 1997). It has been suggested that the mechanism for JNK activation after irradiation in leukemic cells is dependent on ceramide generation (Santana et al., 1996; Chmura et al., 1997; Haimovitz-Friedman, 1998). However, it is not currently well understood how the MAPK and JNK pathways might act in concert or in a dynamic balance in carcinoma cells to regulate cell proliferation and cell death after irradiation, particularly in the case of low radiation doses.
The proliferation of many squamous and mammary carcinoma cell lines in vitro is in part regulated by the synthesis and autocrine action of transforming growth factor α (TGFα) (Fernandez-Pol et al., 1989; Levenson et al., 1998). When exposed to increasing exogenous concentrations of EGF or TGFα, autocrine growth-regulated carcinoma cells such as A431 and MDA-MB-231 exhibit biphasic growth kinetics. Low concentrations of growth factor promote proliferation, whereas elevated concentrations of the growth factor cause growth arrest and eventually lead to cell death (Veber et al., 1994; Jakus and Yeudall, 1996). EGF exposure of A431 cells has also been shown to cause cleavage of pro-TGFα in the plasma membrane (Baselga et al., 1996), and irradiation of MCF-7 mammary carcinoma cells can increase transcription of TGFα mRNA and to enhance the proliferative rate of surviving cells (Schmidt-Ullrich et al., 1992). These data argue that radiation may also have a self-limiting effect on its toxicity, via increased expression and action of TGFα. Increased expression of TGFα will lead to increased proliferation and survival of irradiated cells, potentially via increased activation of the EGFR and associated downstream signaling pathways such as the MAPK pathway.
To investigate a possible relationship among low-dose ionizing radiation, TGFα function, signaling by the EGFR to the MAPK and JNK pathways, and an ability to proliferate and survive exposure to radiation, we examined radiation-induced alterations in TGFα function and EGFR, MAPK, and JNK activation in A431 squamous carcinoma and MDA-MB-231 mammary carcinoma cells.
MATERIALS AND METHODS
Materials
Antip42MAPK (sc-154AC), and anti-JNK1 (sc-571AC) were from Santa Cruz Biotechnology (Santa Cruz, CA) (Carter et al., 1998). For immunoprecipitation of the EGFR a monoclonal antibody (Ab-5) from Oncogene Science (Cambridge, MA) was used. For immunoblotting of the EGFR, an anti-phosphotyrosine monoclonal antibody (Ab-2) from Oncogene Science and an anti-EGFR monoclonal antibody (E120020) from Transduction Laboratories (Lexington, KY) were used (Schmidt-Ullrich et al., 1997; Kavanagh et al., 1998). Neutralizing monoclonal antibody that recognizes an epitope in both pro-TGFα (∼21.5 kDa) and cleaved TGFα (∼5 kDa) (Ab-3) and control antibody to TFIID (Ab-2) were from Calbiochem (San Diego, CA). Neutralizing monoclonal antibodies that recognize epitopes in both p55 tumor necrosis factor α (TNF-α) receptor (TNFR; MAB225) and p75 TNFR (MAB226) were from R & D systems (Minneapolis, MN). Radiolabeled [γ-32P]ATP was from New England Nuclear (Boston, MA). The EGFR inhibitory tyrphostin AG1478 was used as described (Schmidt-Ullrich et al., 1997; Carter et al., 1998; Kavanagh et al., 1998). The novel MEK1/2 inhibitor U0126 was a kind gift from DuPont (Wilmington, DE) (Favata et al., 1998). Western immunoblotting was performed using the Amersham (Bucks, England) Enhanced Chemiluminescence system. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; thiazolyl blue M5655) was from Sigma (St. Louis, MO). GST-c-Jun (aa 1–169) was synthesized in Escherichia coli and purified on glutathione-Sepharose. Preparations of other reagents were as described (Schmidt-Ullrich et al., 1997; Auer et al., 1998b; Carter et al., 1998).
Methods
Generation of A431-TR25-EGFR-Antisense and MDA-TR15-EGFR-CD533 Cells.
Mammary carcinoma cell line MDA-TR15-EGFR-CD533 used in this study was previously developed in our laboratories from the parental MDA-MB-231 cell line (Contessa et al., 1999; Reardon et al., 1999). CD533 is the wild-type EGFR with the COOH-terminal 533 amino acids deleted, previously shown to be a dominant negative EGFR molecule, inhibiting EGFR function. In the text, these cells are referred to as EGFR-CD533 cells. Squamous vulval carcinoma cell line A431-TR25-EGFR-antisense was generated as described for MDA-TR15-EGFR-CD533 (Contessa et al., 1999; Reardon et al., 1999) using the CD533 construct in the antisense orientation. In the text, these cells are referred to as EGFR-antisense cells. Treatment of EGFR-CD533 cells with 1 μg/ml doxycycline for 24–48 h induces expression of EGFR-CD533 (Figure 1A). Treatment of EGFR-antisense cells with 1 μg/ml doxycycline for 48 h induces antisense EGFR and reduces expression of full-length wild-type EGFR protein by >100-fold (Figure 1B).
Figure 1.
Treatment of EGFR-CD533 and EGFR-antisense cells with doxycycline increases expression of EGFR-CD533 and reduces expression of EGFR, respectively. EGFR-CD533 (A) and EGFR-antisense (B) cells were cultured as in MATERIALS AND METHODS and were treated with 1 μg/ml doxycycline for 48 h before experimentation. After 48 h, cells were lysed, and equal total protein amounts (100 μg) were immunoprecipitated, followed by immunoblotting with the same antibody to determine the expression of EGFR and EGFR-CD533. Exposure of A was for 2 min; exposure of B was for 60 s. Representative experiments for EGFR-CD533 (n = 5) and EGFR-antisense (n = 3) cells are shown.
Culture of EGFR-Antisense and EGFR-CD533 Cells.
Cells were cultured in RPMI-1640 medium supplemented with 5% (vol/vol) FCS at 37°C in 95% (vol/vol) air/5% (vol/vol) CO2 (Carter et al., 1998; Contessa et al., 1999; Reardon et al., 1999). Cells were plated at the following densities for each plate size: 100 mm, 2.5 × 106 cells per plate, 5 ml media; 60 mm, 0.9 × 106 cells per plate, 2 ml media; and 24-well plate, 4 × 103 cells per well, 0.5 ml media. For radiation-induced activation of protein kinases, cells were cultured for 4 d in these media and for 2 h before irradiation were cultured in serum-reduced RPMI medium (0.5% [vol/vol] FCS).
Recombinant Adenoviral Vectors: Generation and Infection In Vitro.
The adenovirus to express dominant negative Ras N17 was prepared as described by Valerie and Singhal (1995). EGFR-CD533 and EGFR-antisense cells were infected with dominant negative Ras N17 adenovirus in vitro (multiplicity of infection, 100) and incubated at 37°C for an additional 24 h. To assess expression, we performed Western immunoblots 24 h after infection.
Treatment of Cells with Drugs, Neutralizing Antibody, Ionizing Radiation, and Cell Lysis.
Cells were cultured as above. AG1478 and U0126 treatment were from 100 mM stock solutions (2 μM and 100 nM final, respectively) and the maximal concentration of vehicle (DMSO) in media was 0.02% (vol/vol). In the indicated experiments, anti-TGFα antibody (1 μg antibody/ml media) was added 60 min before irradiation. For media transfer assays, anti-TGFα antibody (2 μg) was added to recovered media (2 ml) 120 min after irradiation and media incubated with antibody for 60 min before further use. A control antibody (1 μg/ml) to the transcriptional regulator TFIID corresponding to the same monoclonal antibody subtype (IgG2) as the anti-TGFα antibody was used as a control.
Cells were irradiated using a 60Co source at dose rate of 1.1 Gy/min (Carter et al., 1998). Cells were maintained at 37°C throughout the experiment except during the irradiation itself. Zero time is designated as the time point at which exposure to radiation ceased. After irradiation, cells were incubated for specified times, followed by aspiration of media and snap freezing at −70°C on dry ice. Cells were lysed in 1 ml ice-cold buffer A (25 mM HEPES, pH 7.4 at 4°C, 5 mM EDTA, 5 mM EGTA, 5 mM benzamidine, 1 mM phenylmethylsulfonylfluoride, 1 mg/ml soybean trypsin inhibitor, 40 μg/ml pepstatin A, 1 μM microcystin-LR, 0.5 mM sodium orthovanadate, 0.5 mM sodium pyrophosphate, 0.05% [wt/vol] sodium deoxycholate, 1% [vol/vol] Triton X100, and 0.1% [vol/vol] 2-mercaptoethanol), with trituration using a P1000 pipette. Lysates were stored on ice before clarification by centrifugation (4°C).
Immunoprecipitations from Lysates.
Fifty microliters of protein A-agarose slurry (25 μl bead volume) was washed twice with 1 ml PBS containing 0.1% (vol/vol) Tween 20 and resuspended in 0.1 ml of the same buffer. Antibodies (2 μg, 20 μl) and serum (20 μl) were added to each tube and incubated (3 h, 4°C). For preconjugated antibodies, 10 μl of slurry (4 μg antibody) was used. Clarified equal aliquots of EGFR-CD533 and EGFR-antisense cell lysates (0.25 ml, ∼100 μg total protein) were mixed with agarose-conjugated antibodies in duplicate using gentle agitation (2.5 h, 4°C). Agarose–antibody–antigen complexes were recovered by centrifugation, the supernatant was discarded, and the complexes were washed (10 min) sequentially with 0.5 ml buffer A (twice), PBS, and buffer B (25 mM HEPES, pH 7.4, 15 mM MgCl2, 0.1 mM Na3VO4, and 0.1% [vol/vol] 2-mercaptoethanol).
Assay of MAPK Activity.
Immunoprecipitates were incubated (final volume, 50 μl) with 50 μl of buffer B containing 0.2 mM [γ-32P]ATP (5000 cpm/pmol), 1 μM microcystin-LR, and 0.5 mg/ml myelin basic protein (MBP), which initiated reactions. After 20 min, 40 μl of the reaction mixtures were spotted onto a 2-cm circle of P81 paper (Whatman, Maidstone, England) and immediately placed into 180 mM phosphoric acid. Papers were washed four times (10 min each) with phosphoric acid and once with acetone, and 32P-incorporation into MBP was quantified by liquid scintillation spectroscopy. Preimmune controls were performed to ensure that MBP phosphorylation was dependent on specific immunoprecipitation of MAPK.
Assay of JNK Activity.
Immunoprecipitates were incubated (final volume, 100 μl) with 2 μl (10 μg) GST-c-Jun (aa 1–169), and reactions were initiated with 98 μl of buffer B containing 0.2 mM [γ-32P]ATP (5000 cpm/pmol) and 1 μM microcystin-LR. After 30 min, reactions were terminated with sample buffer and prepared for SDS-PAGE (10% gel) to quantify 32P incorporation into excised, Coomassie blue–stained GST-c-Jun (aa 1–169) bands by liquid scintillation spectroscopy. Preimmune control assays were performed to ensure GST-c-Jun (aa 1–169) phosphorylation was dependent on specific immunoprecipitation of JNK1 in the assay.
SDS-PAGE) and Western Blotting.
Cells were irradiated, and at specified time points and treatments media were aspirated, and the plates were snap frozen. Cells were lysed with homogenization buffer and subjected to immunoprecipitation. Immunoprecipitates were solubilized with 100 μl 5× SDS-PAGE sample buffer, diluted to 250 μl with distilled water, and placed in a 100°C dry bath for 15 min. One hundred-microliter aliquots of each time point were subjected to SDS-PAGE on 8% (vol/vol) gels (for EGFR blots) and on 10% (vol/vol) gels (for JNK activity). Gels were transferred to nitrocellulose and Western blotting using specific antibodies performed as indicated. Blots were developed using Enhanced Chemiluminescence (Amersham).
MTT Assay for Cell Growth.
Cells were grown in 24-well plates and 2 d after plating pretreated for 30 min with either 2 μg TGFα-neutralizing antibody or 2 μg control antibody before irradiation. In parallel, cells 2 d after plating were pretreated for 30 min with either 2 μM U0126 or an equivalent volume of vehicle control (DMSO) before irradiation. Cells were irradiated every day over a 3-d period (3 × 2 Gy), with further identical additions of antibodies 30 min before each radiation exposure, followed by culture for a further 6 d. U0126-containing media were also changed 30 min before each radiation exposure, followed by culture for an additional 4 d. At days 1, 3, and 6 after cessation of irradiation, cells were prepared for MTT assay (Carmichael et al., 1987). A 5 mg/ml stock solution of MTT reagent was prepared in Dulbecco’s modified Eagle’s medium. The MTT stock solution was diluted 1:10 in fresh media (RPMI medium without serum), and 300 μl of this solution were added to each aspirated well of a 24-well plate. Cells were incubated for a further 3 h at 37°C. After 3 h, media were aspirated, and cells were lysed with 400 μl DMSO. Cells were incubated for a further 10 min at 37°C with gentle shaking. Absorbance readings at 540 nM were determined using a computer-controlled microplate analyzer.
Clonogenic Assays.
Cells were irradiated (2 Gy) over 3 d (total 6 Gy). Twenty-four hours after the final irradiation and drug treatment, cells were isolated by trypsinization, and live cell number was determined by hemocytometer. Cells were washed three times in drug-free medium. Their ability to form colonies was determined by a previously described technique (Schmidt-Ullrich et al., 1992). Cells were plated at either 500 or 2500 cells per well. Colonies were counted 14 d after plating, when they contained ≥50 cells.
Data Analysis.
Comparison of the effects of various treatments was done using one-way analysis of variance and a two-tailed t test. Differences with p < 0.05 were considered statistically significant. Experiments shown are the means of multiple individual points from multiple separate experiments ± SEM.
RESULTS
Generation of A431-TR25-EGFR-Antisense and MDA-TR15-EGFR-CD533 Carcinoma Cells
The mammary carcinoma cell line MDA-TR15-EGFR-CD533 used in this study was previously developed in our laboratories from the parental MDA-MB-231 cell line (Contessa et al., 1999; Reardon et al., 1999). CD533 is the wild-type EGFR with the COOH-terminal 533 amino acids deleted, previously shown to be a dominant negative EGFR molecule, inhibiting EGFR function. In the text, these cells are hereafter referred to as EGFR-CD533 cells. The squamous vulval carcinoma cell line A431-TR25-EGFR-antisense was generated as described for MDA-TR15-EGFR-CD533 (Contessa et al., 1999; Reardon et al., 1999) using the CD533 construct in the antisense orientation. In the text, these cells are hereafter referred to as EGFR-antisense cells. Treatment of EGFR-CD533 cells with 1 μg/ml doxycycline for 24–48 h induces expression of EGFR-CD533 (Figure 1A). Treatment of EGFR-antisense cells with 1 μg/ml doxycycline for 24–48 h induces antisense EGFR and reduces expression of full-length wild-type EGFR protein by >100-fold (Figure 1B).
Radiation Induces Immediate Primary and Secondary Activations of the EGFR and the MAPK Pathway in EGFR-Antisense and EGFR-CD533 Carcinoma Cells.
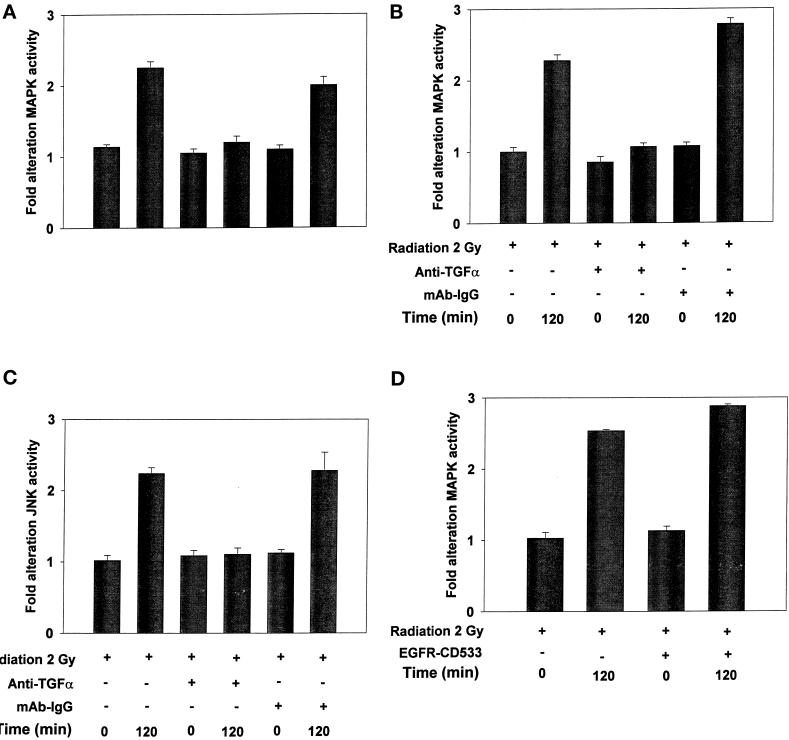
The ability of radiation to modulate EGFR and MAPK activity was investigated in EGFR-antisense cells and EGFR-CD533 cells. Radiation caused immediate primary activation of the EGFR and the MAPK pathway (0–10 min) followed by a later secondary activation (90–240 min) in EGFR-antisense cells before antisense induction. Inhibition of EGFR function by induction of antisense EGFR mRNA reduced EGFR protein levels and abolished activation of the EGFR (Figure 2). Furthermore, inhibition of EGFR function by induction of antisense EGFR mRNA also completely blocked the ability of radiation to activate MAPK (Figure 3A). Identical data were obtained when EGFR function was inhibited in EGFR-CD533 cells by expression of EGFR-CD533 (our unpublished results).
Figure 2.
Radiation-induced activation of EGFR in EGFR-antisense cells is blocked by expression of antisense EGFR mRNA. EGFR-antisense cells were cultured and were treated with doxycycline or neutralizing antibody or infected with adenovirus as described in MATERIALS AND METHODS. Cells were irradiated (2 Gy), and the tyrosine phosphorylation of the EGFR was determined over 0–300 min as in MATERIALS AND METHODS. Cells were lysed, and portions (∼100 μg) from each plate were used to immunoprecipitate EGFR, followed by SDS-PAGE and immunoblotting versus either EGFR or phosphotyrosine (active) EGFR (EGFR-phosphotyrosine); a representative experiment is shown; n = 4. Exposure time, 30 s.
Figure 3.
Radiation-induced activation of MAPK in EGFR-antisense cells is blocked by expression of antisense EGFR mRNA, tyrphostin AG1478, dominant negative Ras N17, or incubation of cells with a neutralizing antibody to TGFα. EGFR-antisense cells were cultured and were treated with doxycycline or neutralizing antibody or infected with adenovirus as described in MATERIALS AND METHODS. Cells were irradiated (2 Gy), and MAPK activity was determined over 0–300 min as in MATERIALS AND METHODS. Cells were lysed, and portions (∼100 μg) from each plate were used to immunoprecipitate MAPK followed by immune complex kinase assays as in MATERIALS AND METHODS. MAPK activity data are shown as fold increases in 32P incorporation into MBP substrate and are normalized to activity at time = 0 from the means ± SEM of four independent experiments.
The ability of growth factors, via EGFR, to activate MAPK is known to be dependent on signaling through the Ras proto-oncogene; however, a direct role for Ras has not been definitively proven for radiation (Kasid et al., 1996; Suy et al., 1997; Auer et al., 1998a). Expression of dominant negative Ras N17 blocked activation of MAPK by radiation in EGFR-antisense cells (Figure 3A), and in EGFR-CD533 cells (our unpublished results). Incubation of cells with a specific inhibitor of MEK1/2, U0126, also blunted the ability of radiation to activate MAPK. These data demonstrate that radiation increased MAPK activity in carcinoma cells via an EGFR- and Ras-dependent mechanism.
Because the novel second phase of EGFR and MAPK activation occurred several hours after radiation exposure, and both EGFR-antisense and EGFR-CD533 cell growth may be partially regulated in an autocrine manner by TGFα, we investigated whether the second phase of EGFR and MAPK activation was dependent on the function of TGFα. Preincubation of EGFR-antisense cells with a neutralizing antibody to TGFα had no effect on the ability of radiation to cause immediate primary activation of the EGFR (Figure 2). However, addition of neutralizing antibody to TGFα abolished the secondary activation of EGFR (Figure 2). In agreement with a pivotal role for EGFR signaling in radiation-induced MAPK activation, neutralizing antibody to TGFα had no effect on the immediate primary activation of MAPK but abolished the secondary activation of this pathway (Figure 3B). Similarly, chemical inhibition of EGFR by AG1478 also abolished radiation-induced MAPK activation. Identical responses were also observed using EGFR-CD533 cells (our unpublished results). These data demonstrate that ionizing radiation stimulates secondary activation of EGFR, and thus the MAPK cascade, in carcinoma cells via a mechanism requiring the function of TGFα.
Radiation-induced Primary and Secondary Activation of JNK Is Dependent on the Actions of the TNF-α Receptor and TGFα/EGFR, Respectively
Radiation has recently been shown to cause activation of EGFR and TNFR and to activate the JNK pathway (Xia et al., 1995; Santana et al., 1996; Chmura et al., 1997; Carter et al., 1998; Sheikh et al., 1998). We next examined whether inhibition of EGFR function, TNFR function, TGFα function, or Ras function blocked radiation-induced JNK activation in carcinoma cells.
Irradiation of EGFR-antisense cells before antisense induction caused an immediate primary activation of the JNK pathway (0–10 min) followed by a later secondary activation (90–300 min) (Figure 4A). Expression of either antisense EGFR mRNA or incubation of these cells with neutralizing antibody to TGFα had no effect on the ability of radiation to cause immediate primary activation of the JNK pathway. In a manner similar to what was observed for the MAPK pathway, expression of Ras N17 completely blocked radiation-induced JNK activation, in agreement with data in other cell systems using growth factors (Trent et al., 1996; Auer et al., 1998a; Deng et al., 1998). Furthermore, expression of antisense EGFR mRNA, addition of neutralizing TGFα antibody, or expression of Ras N17 abolished the secondary activation of JNK (Figure 4). Thus in carcinoma cells, the secondary activation of both the MAPK and JNK pathways is dependent on the functions of EGFR, TGFα, and Ras.
Figure 4.
Radiation-induced activation of JNK1 is dependent on the functions of TNFR, EGFR, TGFα, and Ras. EGFR-antisense cells were cultured and were treated with doxycycline or neutralizing antibody or infected with adenovirus (A) or treated with neutralizing antibody (B), as described in MATERIALS AND METHODS and the legend to Figure 2. Cells were irradiated (2 Gy), and JNK activity was determined via immunoprecipitation over 0–300 min as in MATERIALS AND METHODS and the legend to Figure 2. JNK activity data are shown as fold increases in 32P-incorporation into GST-c-Jun substrate and are normalized to activity at time = 0 from the means ± SEM of three independent experiments.
Radiation has been shown to cause clustering of several plasma membrane receptors, including the EGFR and TNFR, which is indicative of their activation (Rosette and Karin, 1996; Sheikh et al., 1998). Because the primary activation of JNK was EGFR independent, we examined whether TNFR signaling played a role in this process. Cells were incubated with neutralizing monoclonal antibodies raised against two forms of the human TNFR, followed by irradiation. Inhibition of TNFR function blunted the primary JNK activation by 90%, whereas these antibodies had little or no effect on radiation-induced secondary activation of JNK (Figure 4B). Inhibition of TNFR function did not alter radiation-induced MAPK activation (our unpublished results). These data argue that the primary activation of the JNK pathway in carcinoma cells is dependent on radiation-induced TNFR signaling.
Radiation Induces a Dose-dependent Increase in Secondary EGFR Tyrosine Phosphorylation and in the Secondary Activations of the MAPK and JNK Pathways
We next examined whether increasing doses of radiation differentially modulate the immediate primary and secondary EGFR–MAPK–JNK activations in EGFR-antisense cells. Increasing doses of radiation resulted in slightly reduced immediate primary activation of EGFR (Figure 5, A and B). In a similar manner, increasing doses of radiation also resulted in slightly reduced immediate primary activation of the MAPK pathway (Figure 6A). However, increasing doses of radiation did not significantly alter the ability of radiation to activate the JNK pathway at these times (Figure 6B). In contrast, increasing doses of radiation caused dose-dependent increases in secondary activation of EGFR (Figure 5C). Similarly, the secondary activation of the MAPK and JNK pathways were also enhanced in a dose-dependent manner by increasing doses of radiation (Figure 6, A and B). The dose-dependent increases in secondary EGFR, MAPK, and JNK activity were abolished when cells were incubated with neutralizing TGFα antibody (Figures 5C and 6, C and D). These data argue that increasing doses of radiation caused increased secondary activation of EGFR, MAPK, and JNK pathways via a TGFα-dependent mechanism.
Figure 5.
Radiation-induced secondary activation of EGFR is dose dependent and is mediated by TGFα. EGFR-antisense cells were cultured and were treated 30 min before irradiation with either control antibody or neutralizing antibody as described in MATERIALS AND METHODS. Cells were exposed to increasing doses of radiation (2–20 Gy). (A and B) After radiation exposure, at the indicated times media were aspirated, and plates were snap frozen; (C) after radiation exposure (180 min), media were aspirated, and plates were snap frozen. Cells were lysed, and identical portions (∼100 μg) from each plate were used to immunoprecipitate EGFR, followed by SDS-PAGE and immunoblotting versus either total EGFR protein or phosphotyrosine-containing (active) EGFR (EGFR-phosphotyrosine) as indicated. A representative experiment is shown (n = 3). Exposure time, 40 s.
Figure 6.
Radiation-induced secondary activation of MAPK and JNK is dose dependent and is mediated by TGFα. EGFR-antisense cells were treated 30 min before irradiation with either control antibody (A, MAPK; B, JNK) or anti-TGFα-neutralizing antibody (C, MAPK; D, JNK) as described in MATERIALS AND METHODS. Cells were exposed to increasing doses of radiation (1–20 Gy). At the indicated times after radiation exposure, plates were snap frozen. Cells were lysed, and identical portions (∼100 μg) from each plate were used to immunoprecipitate either MAPK (A and C) or JNK (B and D) for activity assessment as in MATERIALS AND METHODS. Data are shown as fold increases in 32P incorporation into MBP (MAPK) or GST-c-Jun (JNK) substrates and are normalized to activity at time = 0. Data are from a representative of three independent experiments.
Radiation Causes Release of TGFα from EGFR-Antisense and EGFR-CD533 Cells, Which Is Responsible for the Secondary Activation of EGFR and the MAPK and JNK Pathways
In Figures 2–6 we demonstrated that the secondary activation of EGFR, MAPK, and JNK is inhibited by direct incubation of cells with a neutralizing antibody to TGFα. To further examine whether this neutralizing effect was due to inhibition of a soluble active form of TGFα, at various times after irradiation, media were taken and incubated with either neutralizing TGFα antibody or a nonspecific antibody followed by addition to plates of unirradiated cells for assessment of MAPK and JNK activity (Figure 7). Media from irradiated EGFR-antisense or EGFR-CD533 cells (0–60 min) did not stimulate MAPK activity in unirradiated cells (our unpublished results). However, media from irradiated cells, 120 min after irradiation, were capable of stimulating MAPK activity when transferred to culture dishes of unirradiated cells (Figure 7, A and B). Preincubation of this media with neutralizing TGFα antibody, but not with control antibody, abolished the ability of the media to activate MAPK (Figure 7, A and B). In a similar manner, media from irradiated EGFR-antisense cells 120 min after irradiation were capable of stimulating JNK activity when transferred to cell culture dishes containing unirradiated cells (Figure 7C). Preincubation of these media with a neutralizing TGFα antibody abolished the ability of the media to activate JNK.
Figure 7.
Media from irradiated cells contains a soluble factor, which can activate both MAPK and JNK in nonirradiated cells, which is blocked by a neutralizing anti-TGFα antibody. EGFR-CD533 and EGFR-antisense cells (as indicated) were cultured as described in MATERIALS AND METHODS. In D, cells were treated with doxycycline to induce EGFR-CD533 as in MATERIALS AND METHODS. Cells were irradiated (2 Gy), and 0 and 120 min after irradiation the media were removed from these cells. The media were incubated with either neutralizing anti-TGFα antibody or control antibody as in MATERIALS AND METHODS. Antibody-incubated media were added to nonirradiated plates, and 5 min after addition the plates were aspirated and snap frozen. (A) MAPK activity in EGFR-CD533 cells was determined after immunoprecipitation as in MATERIALS AND METHODS; (B) MAPK activity in EGFR-antisense cells was determined after immunoprecipitation as in MATERIALS AND METHODS. (C) JNK activity in EGFR-antisense cells was determined after immunoprecipitation as in MATERIALS AND METHODS. (D) MAPK activity in EGFR-CD533 cells expressing EGFR-CD533 was determined after immunoprecipitation as in MATERIALS AND METHODS. MAPK activity data are shown as fold increases in 32P incorporation into MBP substrate and are normalized to activity at time = 0 from the means ± SEM of three independent experiments. JNK activity data are shown as fold increases in 32P incorporation into GST-c-Jun substrate and are normalized to activity at time = 0 from the means ± SEM of four independent experiments.
Baselga et al. (1996) have argued that activation of the EGFR can stimulate the proteolytic release of TGFα. Because radiation activates EGFR, which is followed by release of TGFα, we determined whether the radiation-induced TGFα release was EGFR-dependent. Inhibition of EGFR function by expression of EGFR-CD533 in EGFR-CD533 cells did not alter the ability of radiation to stimulate MAPK activity when transferred to culture dishes of unirradiated cells (Figure 7D). Similar data were obtained in EGFR-antisense cells (our unpublished results). These data argue that radiation-induced TGFα release is EGFR independent, in contrast to reports using the growth factor EGF in these cells (Baselga et al., 1996; Nutt and Lunec, 1996).
To further examine these phenomena, we immunoprecipitated TGFα from EGFR-antisense cells 0–300 min after irradiation using the neutralizing TGFα antibody, which recognizes an epitope found in pro-TGFα (∼21.5 kDa) and proteolytically cleaved TGFα (∼5 kDa). After immunoprecipitation we determined the relative amounts of intact pro-TGFα and cleaved TGFα associated with the cells (Figure 8). Irradiation of EGFR-antisense cells transiently increased the amount of 5-kDa proteolytically cleaved TGFα associated with the cells 120–180 min after exposure. Little or no 5-kDa TGFα fragment could be detected in unirradiated cells. The data in Figures 7 and 8 demonstrate that radiation causes an EGFR-independent proteolytic cleavage of TGFα, and that this soluble active TGFα induces the secondary activation of EGFR, MAPK, and JNK.
Figure 8.
Radiation-induced cleavage of 21-kDa pro-TGFα into a 5-kDa active TGFα fragment. EGFR-antisense cells were cultured as described in MATERIALS AND METHODS. Cells were irradiated (2 Gy), and 0, 30, 120, 180, and 300 min after irradiation (as indicated) the media were removed from these cells. Cells were not washed. Cells were immediately lysed in homogenization buffer, followed by immunoprecipitation using an antibody that recognizes both full-length pro-TGFα (∼21.5 kDa) and cleaved active TGFα (∼5 kDa) but not residual proteolytically cleaved TGFα forms (∼17–12 kDa). Proteins were resolved from immunoprecipitates by SDS-PAGE followed by immunoblotting using the same immunoprecipitating antibody, which recognizes pro-TGFα (∼21.5 kDa) and cleaved active TGFα (∼5 kDa), as indicated. A representative experiment is shown (n = 3). Exposure time, 4 min.
Neutralization of TGFα or Inhibition of MAPK Function Radiosensitizes Mammary and Squamous Carcinoma Cells
Several studies have suggested that MAPK signaling may exert a radio- and chemoprotective role in transformed cells. To determine whether inhibition of TGFα function, EGFR function, or MAPK signaling altered the ability of radiation to cause cell death (apoptosis), EGFR-antisense cells were irradiated (2 Gy) in the presence of either neutralizing anti-TGFα antibody, induction of antisense EGFR, U0126, or U0126, and neutralizing anti-TGFα antibody, and cell viability was determined 24 h after exposure by terminal uridyl-nucleotide end labeling of DNA. Radiation exposure increased the level of apoptosis from 4 to 6% without any additional treatment. Treatment of cells with either neutralizing anti-TGFα antibody, induction of antisense EGFR, U0126, or U0126, and neutralizing anti-TGFα antibody increased apoptosis from 4 to 7, 6, 6, and 7%, respectively. However, combined irradiation with either neutralizing anti-TGFα antibody, induction of antisense EGFR, U0126, or U0126, and neutralizing anti-TGFα antibody significantly increased apoptosis above either radiation-alone or treatment-alone values to 12, 13, 12, and 13%, respectively (all p < 0.05). Anti-TGFα antibody did not augment the ability of U0126 to potentiate radiation-induced apoptosis, which suggests that both treatments act via the same mechanism to increase apoptosis. Our data argues that functional inhibition of the TGFα–EGFR–MAPK pathway enhances the ability of radiation to kill carcinoma cells.
In the clinic, patients undergoing radiotherapy are exposed to multiple doses of radiation over several weeks. To mimic the clinical prescription of radiation, we devised a novel proliferation assay in which cells in vitro were irradiated daily with 2 Gy, over 3 d. To determine whether radiation-induced TGFα signaling, via the MAPK pathway, exerts a radioprotective effect on EGFR-CD533 cells, we irradiated cells in the presence of either neutralizing TGFα antibody or control antibody and examined their growth potential in MTT assays over the following 4 d. In parallel experiments, we incubated cells in the presence of either a specific MEK1/2 inhibitor, U0126 (2 μM), or DMSO control.
The proliferative potential of irradiated cells or cells incubated in the presence of neutralizing TGFα antibody was reduced in comparison with control cells (Figure 9A). However, an even greater reduction in the proliferative capacity of cells treated with neutralizing TGFα antibody and radiation was observed. In a similar manner, the proliferative potential of irradiated cells or cells incubated in the presence of U0126 was reduced in comparison with control cells (Figure 9B). Inhibition of MAPK signaling combined with irradiation caused a much larger reduction in growth potential. Data identical to those described for EGFR-CD533 cells were obtained in EGFR-antisense cells using either an anti-TGFα-neutralizing antibody or the MEK1/2 inhibitor U0126 (our unpublished results). Of particular note, when radiation-induced activation of the MAPK pathway was blunted by U0126, the measured MTT absorbance decreased 50% below the time = 0 control absorbance levels, which is indicative of enhanced cell killing by multiple irradiations rather than an increase in the numbers of growth-arrested cells. Collectively, our data argue that after irradiation, the ability of EGFR-CD533 and EGFR-antisense cells to proliferate becomes MAPK dependent.
Figure 9.
Neutralization of TGFα or MAPK function abolishes proliferation after irradiation of EGFR-CD533 cells in a cellular proliferation assay. Decreased proliferative rate of EGFR-CD533 cells in MTT assays over 1–4 d wherein cells were treated with either neutralizing anti-TGFα antibody (2 μg/ml) (A) or the MEK1/2 inhibitor U0126 (2 μM) (B). Cells were cultured in 24-well plates as in MATERIALS AND METHODS. Cells were pretreated with the indicated concentrations of anti-TGFα antibody or U0126 30 min before radiation exposure. Cells were exposed or not exposed to radiation and cultured for 1–7 d in the presence of drug or antibody. MTT assays were performed on each day as described in MATERIALS AND METHODS, and growth curves were plotted to determine the growth potential of treated cells. Data are shown from the means of 12 data points ± SEM from a representative experiment (n = 2).
In general agreement with the data presented in Figure 9, either expression of EGFR-CD533 in EGFR-CD533 cells or induction of antisense EGFR mRNA in EGFR-antisense cells combined with irradiation also caused a large reduction in the growth potential of cells (our unpublished results; in general agreement with data by Carter et al., 1998; Contessa et al., 1999; Reardon et al., 1999). These data argue that radiation-induced TGFα–EGFR–MAPK activation plays an important growth-promoting and cytoprotective role after irradiation of autocrine-regulated carcinoma cells.
Inhibition of the MAPK Pathway Reduces Clonogenic Survival of EGFR-Antisense Cells and EGFR-CD533 Cells after Irradiation and U0126 Treatment
To determine whether the increased cell death and loss of growth potential of cells correlated with diminished long-term proliferative potential, cells from each condition were subjected to clonogenic survival assays (Figure 10). Cells were treated with either vehicle control (DMSO) or with drug (U0126, 2 μM) and were then either unirradiated or irradiated (3 × 2 Gy over 3 d). Twenty-four hours after the final irradiation, live cells from each condition were counted and plated at two cell densities as described in MATERIALS AND METHODS for clonogenic assays. Fourteen days later, the clonogenic potential of each condition was determined. U0126-treated carcinoma cells exhibited slightly lower levels of clonogenicity compared with control cells (∼90%) (Figure 10). In contrast, irradiation of cells caused a much greater reduction in clonogenic survival (∼40–50%). However, irradiated cells cotreated with U0126 exhibited markedly reduced clonogenic survival compared with control-treated, U0126-treated, or irradiated cells alone (∼2–10% survival). Identical data were obtained in these cells using the chemically dissimilar MEK1/2 inhibitor PD98059 (our unpublished results). These data argue that inhibition of MAPK activity in combination with radiation exposure increases apoptosis and leads to a large reduction of clonogenic growth potential in carcinoma cells.
Figure 10.
Inhibition of the MAPK pathway reduces the clonogenic survival of EGFR-antisense cells and EGFR-CD533 cells after irradiation and U0126 treatment. Cells were irradiated (2 Gy) over 3 d (total 6 Gy) in the presence or absence of 2 μM U0126 and DMSO vehicle control. Twenty-four hours after the final irradiation and drug treatment, cells were isolated by trypsinization, and live cell number was determined by hemocytometer. Cells were washed three times in drug-free medium. Their ability to form colonies was determined by a previously described technique. Cells were plated at either 500 or 2500 cells per well. Colonies were counted 14 d after plating, when they contained ≥50 cells. Data are the means of eight separate experiments ± SEM.
DISCUSSION
This study was initiated to examine the effects of ionizing radiation on the activities of the EGFR and downstream signaling pathways in autocrine-regulated carcinoma cells. We demonstrated that radiation causes short immediate primary (0–5 min) and prolonged secondary activation (90–240 min) of the EGFR. Radiation also caused primary and secondary activation of the MAPK pathway, which were both dependent on EGFR function, as judged by the ability of either antisense EGFR mRNA or dominant negative EGFR-CD533 to block activation of both EGFR and MAPK. In contrast, although radiation caused primary and secondary activation of the JNK pathway, only the secondary activation was dependent on EGFR function. Expression of dominant negative Ras N17 blocked the ability of radiation to alter either MAPK or JNK pathway activity, which argues that radiation uses similar mechanisms to stimulate these signaling pathways as do natural ligands of EGFR.
The secondary activation of EGFR, MAPK and JNK was dependent on the function of TGFα as judged by several criteria. Addition of a neutralizing TGFα antibody to culture media inhibited radiation-induced secondary activation of the EGFR. Furthermore, neutralizing TGFα antibody also abolished the secondary activation of the MAPK and JNK pathways, in agreement with our data demonstrating an essential role for EGFR signaling in pathway activation at this time. Radiation induced the generation of a soluble EGFR–MAPK–JNK activator, and the function of this activator could be abolished by incubation of media with a neutralizing TGFα antibody. Furthermore, radiation was shown to induce proteolytic cleavage of pro-TGFα at times corresponding to the secondary increases in EGFR, MAPK, and JNK activity. These data strongly argue that the ability of radiation to cause activation of EGFR and the MAPK and JNK pathways at times distant to the initial radiation exposure is dependent on the proteolytic cleavage and functional activation of the autocrine growth factor TGFα.
Recent studies from our laboratories have suggested that activation of the MAPK pathway represents a cytoprotective signal (Carter et al., 1998; Contessa et al., 1999). In agreement with these findings and with data herein showing that TGFα function is responsible for the prolonged secondary EGFR activation, neutralization of TGFα function reduced the growth potential of cells after repeated radiation exposures. Similarly, inhibition of MAPK function by the MEK1/2 inhibitor U0126 during irradiation reduced cell numbers to below unirradiated control values and abolished the growth potential of these cells. These data suggest that ionizing radiation may exert a self-limiting effect on its ability to kill and to reduce the proliferation of autocrine-regulated tumor cells, potentially by increasing both the rates of transcription (Schmidt-Ullrich et al., 1992) and proteolytic cleavage and activation of TGFα. Increased expression and cleavage of TGFα will lead to increased EGFR and MAPK activity, which in turn will lead to both increased proliferation of tumor cells as well as other enhanced cytoprotective responses. A reduction in TGFα–EGFR–MAPK function is therefore one target to improve the efficacy of radiotherapy.
The ability of radiation to activate the JNK pathway has been solely ascribed to the function of acidic sphingomyelinase and ceramide, and cells that do not express this enzyme were shown to be incapable of JNK activation after irradiation (Santana et al., 1996; Chmura et al., 1997; Haimovitz-Friedman, 1998). However, these investigators examined radiation-induced JNK activation in hematopoietic cells and used doses of radiation substantially greater than used in this study. In contrast, we surprisingly found that the radiation-induced secondary activation of the JNK pathway in autocrine-regulated carcinoma cells was dependent on the function of TGFα and the ability of TGFα to activate EGFR. However, the immediate primary activation of the JNK pathway was not significantly inhibited by reduced EGFR function yet was still dependent on the function of Ras. The ability of TNF-α and growth factors to activate JNK via Ras is dependent on the system examined and may also be effected by the degree to which receptors are activated by agonist and radiation (Minden et al., 1994; Gardner and Johnson, 1996; Trent et al., 1996; Aktas et al., 1997; Auer et al., 1998a; Deng et al., 1998).
Studies using neutralizing antibodies toward TNF-α receptors demonstrated that the primary JNK activation by ionizing radiation is dependent on TNF-α receptor function. This is in general agreement with recent findings using high doses of UV radiation in lymphoma cells (Sheikh et al., 1998). Collectively, the data suggest that multiple mechanisms exist by which ionizing radiation may mediate the activation of signaling pathways in a cell type–specific manner. In addition, our findings argue that Ras molecules exist in separate pools within cells. Signaling via the EGFR and Ras can contribute to both the primary and secondary activation of MAPK and to the secondary activation of JNK. In contrast, the primary activation of JNK is dependent on TNFR and Ras function but is independent of increased EGFR signaling. Further studies will be needed to determine the precise mechanism(s) by which ionizing radiation induces the immediate primary activation of JNK in carcinoma cells.
We found that increasing doses of radiation correlated with increasing secondary MAPK and JNK pathway activation. The dose-dependent enhancement in the activities of these protein kinases were dependent on the function of TGFα. The regulation of TGFα function at both the transcriptional and posttranscriptional levels has been of interest in our laboratory. We recently demonstrated that repeated irradiation of MCF-7 mammary carcinoma cells increases the transcription of TGFα mRNA (Schmidt-Ullrich et al., 1992). UV irradiation of cells has been shown to activate a metalloprotease in the plasma membrane of cells, which can catalyze the cleavage of pro-TGFα (21.5 kDa) into an active soluble TGFα (5 kDa) (Piva et al., 1997). Others have suggested that growth factor–mediated activation of the EGFR can stimulate proteolytic cleavage of pro-TGFα, leading to the generation of a self-stimulating autocrine loop (Baselga et al., 1996). Increased signaling by the EGFR may also play a role in the increased expression of TGFα-ase enzymes over prolonged periods (Huang et al., 1996; Nutt and Lunec, 1996). In contrast to these findings using natural ligands of the EGFR, we found that loss of EGFR function did not alter the ability of radiation to stimulate TGFα cleavage, suggesting that ionizing radiation stimulates TGFα cleavage via an EGFR-independent mechanism. The mechanism by which ionizing radiation stimulates proteolytic cleavage of TGFα in carcinoma cells thus remains to be determined.
Radiation was shown to cause a prolonged secondary activation of the MAPK pathway in both A431 and MDA-MB-231 cells. Increased MAPK signaling has been reported to induce the cyclin kinase inhibitor protein p21Cip-1/WAF1, which plays an important role in the formation of active cyclin-dependent kinase complexes and cell cycle progression through G1 phase and into S phase (Weber et al., 1997; Fiddes et al., 1998). In contrast, other studies have suggested that prolonged signaling by the MAPK pathway plays a growth-inhibitory role, via a more potent induction of the cyclin kinase inhibitor protein p21Cip-1/WAF1 (Wang et al., 1997; Auer et al., 1998b). We recently demonstrated that low doses of radiation, equivalent to mitogenic concentrations of EGF, could increase p21Cip-1/WAF1 expression in A431 cells and that this increased expression was in part dependent on MAPK signaling (Carter et al., 1998). In contrast, others have recently shown that higher EGF concentrations, which are antiproliferative and cytotoxic, can increase p21Cip-1/WAF1 in an MAPK-independent manner (Bromberg et al., 1998; Silvy et al., 1998; Toyoda et al., 1998). This suggests that the signaling mechanisms recruited by low levels of EGFR activation (low-dose radiation) are different from those recruited by high levels of EGFR activation (high-dose radiation). Further studies will be required to understand how radiation, in a time- and dose-dependent manner, can alter the signaling pathways recruited by activated EGFR.
A role for MAPK signaling and p21Cip-1/WAF1 in the regulation of G2–M progression has also been documented (Deng et al., 1995; Macloed et al., 1995; Reed et al., 1998; Xu et al., 1998). We and others have demonstrated that inhibition of MAPK signaling prolongs radiation-induced G2–M growth arrest, leading to an increase in apoptosis and loss of both growth and clonogenic potential (Warenius et al., 1996; Carter et al., 1998; Vrana et al., 1999). Before irradiation, carcinoma cell growth was only partially reduced by MAPK inhibition. In contrast, after irradiation, proliferation and cell survival became totally dependent on an intact MAPK pathway. In addition, other investigators have shown that high levels of Raf-1 expression, the upstream activator of MAPK, can also increase radiosensitivity by abrogating the radiation-induced G2–M arrest, which leads to mitosis with damaged DNA, and loss of clonogenic potential (Warenius et al., 1996). Collectively, these data argue that gross positive or negative modulations of MAPK pathway activity may radiosensitize tumor cells.
The expression and regulation of EGFR and the ErbB family of receptors in human cancer is currently under intense investigation (Mendelson and Fan, 1997; Petit et al., 1997; Baselga et al., 1998; Giani et al., 1998; Miyaguchi et al., 1998; Pegram et al., 1998; Huang et al., 1999). In general agreement with a cytoprotective role for EGFR signaling in response to ionizing radiation, neutralization of TGFα function reduced the total amount of EGFR activation after irradiation and caused a partial reduction in proliferative potential. However, combined irradiation and inhibition of TGFα function resulted in a pronounced growth-inhibitory effect. When radiation-induced activation of the MAPK pathway was blunted by a specific MEK1/2 inhibitor, U0126, an even greater decrease in proliferative potential was observed. Of note, when radiation-induced activation of the MAPK pathway was blunted by U0126, the MTT absorbance values decreased below control absorbance levels. The decrease in proliferative potential as found using the MTT assay was corroborated by our finding a similar decrease in the clonogenic potential of these cells. Collectively, our data are indicative of enhanced cell killing by radiation when MAPK activity is inhibited.
In conclusion, we suggest that a reduced ability of carcinoma cells to activate EGFR after radiation is detrimental to cell proliferation, and that EGFR-mediated cell proliferation after irradiation is, in part, mediated by increased activation of the MAPK pathway. Ongoing studies in our laboratory are continuing to explore the downstream mechanisms by which TGFα, EGFR, and MAPK signaling protects cells from death after exposure to ionizing radiation.
ACKNOWLEDGMENTS
We thank Julie Farnsworth for the expansion of the Ras N17 adenovirus. We also thank Drs. J. Sebolt-Leopold (Parke-Davis/Warner Lambert Pharmaceuticals) and J. Trzaskos (DuPont Pharmaceuticals) for providing PD98059 and U0126, respectively. This work was funded a fellowship from the V-foundation (to P.D.), Jeffres Research Fund grant J-464 (to P.D.), Department of Defense grant BC98-0148 (to P.D.), and US Public Health Service grants P01CA72955 and R01CA65896 (to R.S.U).
Abbreviations used:
- EGFR
epidermal growth factor receptor
- JNK
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- MEK
mitogen activated/extracellular-regulated kinase
- TGFα
transforming growth factor α
- TNF
tumor necrosis factor
- TNFR
TNF-α receptor
REFERENCES
- Abbott DW, Holt J. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem. 1999;274:2732–2742. doi: 10.1074/jbc.274.5.2732. [DOI] [PubMed] [Google Scholar]
- Aktas H, Cai H, Cooper GM. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer K, et al. Stimulation of DNA synthesis in primary cultures of rat hepatocytes via a Ras/Rac1/Cdc42/SEK/JNK/c-Jun dependent mechanism. Mol Biol Cell. 1998a;9:561–573. doi: 10.1091/mbc.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer KL, Ishac E, Seth P, Coffey RJ, DePinho R, Fisher PB, Dent P. Prolonged activation of the mitogen activated protein (MAP) kinase pathway promotes DNA synthesis in primary hepatocytes from p21Cip-1/WAF1 knock out mice, but not in hepatocytes from p16INK4a knock out mice. Biochem J. 1998b;336:551–560. doi: 10.1042/bj3360551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban N, Moni J, Shannon M, Dang L, Murphy E, Goldkorn T. The effect of ionizing radiation on signal transduction: antibodies to EGF receptor sensitize A431 cells to radiation. Biochim Biophys Acta. 1996;1314:147–156. doi: 10.1016/s0167-4889(96)00068-7. [DOI] [PubMed] [Google Scholar]
- Baselga J, Mendelson J, Kim Y-M, Pandiella A. Autocrine regulation of membrane transforming growth factor-α cleavage. J Biol Chem. 1996;271:3279–3284. doi: 10.1074/jbc.271.6.3279. [DOI] [PubMed] [Google Scholar]
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized antiHER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–2831. [PubMed] [Google Scholar]
- Bromberg JF, Fan Z, Brown C, Mendelsohn J, Darnell JE., Jr Epidermal growth factor-induced growth inhibition requires Stat1 activation. Cell Growth Differ. 1998;9:505–512. [PubMed] [Google Scholar]
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]
- Carter S, Auer KL, Birrer M, Fisher PB, Scmidtt-Ullrich R, Valerie K, Mikkelsen R, Dent P. Potentiation of ionizing radiation induced cell killing by inhibition of the mitogen activated protein (MAP) kinase cascade in A431 human squamous carcinoma cells. Oncogene. 1998;16:2787–2796. doi: 10.1038/sj.onc.1201802. [DOI] [PubMed] [Google Scholar]
- Chmura SJ, Mauceri HJ, Advani S, Heimann R, Nodzenski E, Quintans J, Kufe DW, Weichselbaum RR. Decreasing the apoptotic threshold of tumor cells through protein kinase C inhibition and sphingomyelinase activation increases tumor cell killing by ionizing radiation. Cancer Res. 1997;57:4340–4347. [PubMed] [Google Scholar]
- Contessa JN, Reardon DB, Todd D, Dent P, Mikkelsen RB, Valerie K, Bower GD, Schmidt-Ullrich RK. The inducible expression of dominant negative EGFR-CD533 results in radiosensitization of human mammary carcinoma cells. Clin Cancer Res. 1999;5:405–411. [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Deng J, Kawakami Y, Hartman SE, Satoh T, Kawakami T. Involvement of Ras in Bruton’s tyrosine kinase-mediated JNK activation. J Biol Chem. 1998;273:16787–16791. doi: 10.1074/jbc.273.27.16787. [DOI] [PubMed] [Google Scholar]
- Dent P, Jarvis WD, Birrer MJ, Fisher PB, Schmidt-Ullrich RK, Grant S. The roles of signaling by the p42/44 mitogen-activated protein (MAP) kinase pathway: a potential route to radio- and chemosensitization of tumor cells. Leukemia. 1998;12:1843–1850. doi: 10.1038/sj.leu.2401222. [DOI] [PubMed] [Google Scholar]
- Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pol JA, Klos DJ, Hamilton PD. Modulation of transforming growth factor α-dependent expression of epidermal growth factor receptor gene by transforming growth factor β, triiodothyronine, and retinoic acid. J Cell Biochem. 1989;41:159–170. doi: 10.1002/jcb.240410306. [DOI] [PubMed] [Google Scholar]
- Fiddes RJ, Janes PW, Sivertsen SP, Sutherland RL, Musgrove EA, Daly RJ. Inhibition of the MAP kinase cascade blocks heregulin-induced cell cycle progression in T-47D human breast cancer cells. Oncogene. 1998;16:2803–2813. doi: 10.1038/sj.onc.1201815. [DOI] [PubMed] [Google Scholar]
- Gardner AM, Johnson GL. Fibroblast growth factor-2 suppression of tumor necrosis factor α-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- Giani C, Casalini P, Pupa SM, De Vecchi R, Ardini E, Colnaghi MI, Giordano A, Menard S. Increased expression of c-erbB-2 in hormone-dependent breast cancer cells inhibits cell growth and induces differentiation. Oncogene. 1998;30:425–432. doi: 10.1038/sj.onc.1201954. [DOI] [PubMed] [Google Scholar]
- Gokhale PC, Soldatenkov V, Wang FH, Rahman A, Dritschilo A, Kasid U. Antisense raf oligodeoxyribonucleotide is protected by liposomal encapsulation and inhibits Raf-1 protein expression in vitro and in vivo: implication for gene therapy of radioresistant cancer. Gene Ther. 1997;12:1289–1299. doi: 10.1038/sj.gt.3300543. [DOI] [PubMed] [Google Scholar]
- Goldkorn T, Balaban N, Shannon M, Matsukuma K. EGF receptor phosphorylation is affected by ionizing radiation. Biochim Biophys Acta. 1997;1358:289–299. doi: 10.1016/s0167-4889(97)00063-3. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A. Radiation-induced signal transduction and stress response. Radiat Res. 1998;150:S102–S108. [PubMed] [Google Scholar]
- Huang RP, Wu JX, Fan Y, Adamson ED. UV activates growth factor receptors via reactive oxygen intermediates. J Cell Biol. 1996;133:211–220. doi: 10.1083/jcb.133.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- Jakus, J., and Yeudall, W.A. Growth inhibitory concentrations of EGF induce p21 (WAF1/Cip1) and alter cell cycle control in squamous carcinoma cells. Oncogene 12, 2369–2376. [PubMed]
- Kasid U, Suy S, Dent P, Whiteside T, Sturgill TW. Membrane recruitment followed by tyrosine phosphorylation and activation of Raf-1 kinase marks the ionizing radiation-responsive signaling pathway. Nature. 1996;382:316–318. doi: 10.1038/382813a0. [DOI] [PubMed] [Google Scholar]
- Kavanagh BD, Dent P, Schmidt-Ullrich RK, Chen P, Mikkelsen RB. Ca 2+-dependent stimulation of mitogen activated protein kinase activity in A431 cells by low dose ionizing radiation. Radiat Res. 1998;149:579–587. [PubMed] [Google Scholar]
- Levenson AS, Tonetti DA, Jordan VC. The estrogen-like effect of 4-hydroxytamoxifen on induction of transfroming growth factor α mRNA in MDA-MB-231 breast cancer cells stably expressing the estrogen receptor“ Br. J Cancer. 1998;77:1812–1819. doi: 10.1038/bjc.1998.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macloed KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes & Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Fan Z. Epidermal growth factor receptor family and chemosensitization. J Natl Cancer Inst. 1997;89:341–343. doi: 10.1093/jnci/89.5.341. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- Miyaguchi M, Takeuchi T, Morimoto K, Kubo T. Correlation of epidermal growth factor receptor and radiosensitivity in human maxillary carcinoma cell lines. Acta Otolaryngol. 1998;118:428–431. doi: 10.1080/00016489850183566. [DOI] [PubMed] [Google Scholar]
- Nutt JE, Lunec J. Induction of metalloproteinase (MMP1) expression by epidermal growth factor (EGF) receptor stimulation and serum deprivation in human breast tumor cells. Eur J Cancer. 1996;32:2127–2135. doi: 10.1016/s0959-8049(96)00261-4. [DOI] [PubMed] [Google Scholar]
- Pegram MD, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized antip185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- Piva TJ, Krause DR, Ellem KO. UVC activation of the HeLa cell membrane “TGF α-ase,” a metalloenzyme. J Cell Biochem. 1997;64:353–368. doi: 10.1002/(sici)1097-4644(19970301)64:3<353::aid-jcb2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Reardon, D.B., Contessa, J.N., Mikkelsen, R.B., Valerie, K., Amir, C., Dent, P., and Schmidt-Ullrich, R.K. (1999). Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene (in press). [DOI] [PubMed]
- Reed MF, Liu VF, Ladha MH, Ando K, Griffin JD, Weaver DT, Ewen ME. Enforced CDK4 expression in a hematopoietic cell line confers resistance to the G1 arrest induced by ionizing radiation. Oncogene. 1998;17:2961–2971. doi: 10.1038/sj.onc.1202450. [DOI] [PubMed] [Google Scholar]
- Rosette C, Karin M. UV light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnik R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation induced proliferation of human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Valerie K, Chan W, Wazer DE, Lin PS. Expression of oestrogen receptor and transforming growth factor-α in MCF-7 cells after exposure to fractionated irradiation. Int J Radiat Biol. 1992;61:405–415. doi: 10.1080/09553009214551101. [DOI] [PubMed] [Google Scholar]
- Sewing A, Wiseman B, Lloyd AC, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh MS, Antimore MJ, Huang Y, Fornace A. UV-irradiation-induced apoptosis is mediated via ligand independent activation of tumor necrosis factor receptor 1. Oncogene. 1998;17:2555–2563. doi: 10.1038/sj.onc.1202292. [DOI] [PubMed] [Google Scholar]
- Silvy M, Martin PM, Chajry N, Berthois Y. Differential dose-dependent effects of epidermal growth factor on gene expression in A431 cells: evidence for a signal transduction pathway that can bypass Raf-1 activation. Endocrinology. 1998;139:2382–2391. doi: 10.1210/endo.139.5.5981. [DOI] [PubMed] [Google Scholar]
- Suy S, Anderson WB, Dent P, Chang E, Kasid U. Association of Grb2 with Sos and Ras with Raf-1 upon gamma irradiation of breast cancer cells. Oncogene. 1997;15:53–61. doi: 10.1038/sj.onc.1201165. [DOI] [PubMed] [Google Scholar]
- Tombes R, Auer KL, Brenz-Verca S, Marshall CJ, McMahon M, Mikkelsen RS, Valerie K, Wymann MP, Dent P. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem J. 1998;330:1451–1460. doi: 10.1042/bj3301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda M, Gotoh N, Handa H, Shibuya M. Involvement of MAP kinase-independent protein kinase C signaling pathway in the EGF-induced p21 (WAF1/Cip1) expression and growth inhibition of A431 cells. Biochem Biophys Res Commun. 1998;250:430–435. doi: 10.1006/bbrc.1998.9332. [DOI] [PubMed] [Google Scholar]
- Trent JC, McConkey DJ, Loughlin SM, Harbison MT, Fernandez A, Ananthaswamy HN. Ras signaling in tumor necrosis factor-induced apoptosis. EMBO J. 1996;15:4497–4505. [PMC free article] [PubMed] [Google Scholar]
- Valerie K, Singhal A. Host-cell reactivation of reporter genes introduced into cells by adenovirus as a convenient way to measure cellular DNA repair. Mutat Res. 1995;336:91–100. doi: 10.1016/0921-8777(94)00046-9. [DOI] [PubMed] [Google Scholar]
- Veber N, Prevost G, Planchon P, Starzec A. Evidence for a growth effect of epidermal growth factor on MDA-MB-231 breast cancer cells. Eur J Cancer. 1994;30:1352–1359. doi: 10.1016/0959-8049(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Vrana J, Grant S, Dent P. Inhibition of the MAPK pathway abrogates Bcl-2-mediated leukemic cell survival after exposure of HL60 cells to low dose ionizing radiation. Radiat Res. 1999;151:559–569. [PubMed] [Google Scholar]
- Wang YA, Elson A, Leder P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci USA. 1997;94:14590–14595. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenius HM, Jones MD, Thompson CC. Exit from G2 phase after 2 Gy gamma irradiation is faster in radiosensitive human cells with high expression of the RAF1 proto-oncogene. Radiat Res. 1996;146:485–493. [PubMed] [Google Scholar]
- Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xu Y, Yang EM, Brugarolas J, Jacks T, Baltimore D. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm−/− mice. Mol Cell Biol. 1998;18:4385–4390. doi: 10.1128/mcb.18.7.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]