Abstract
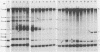
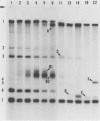
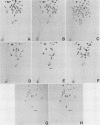
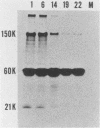
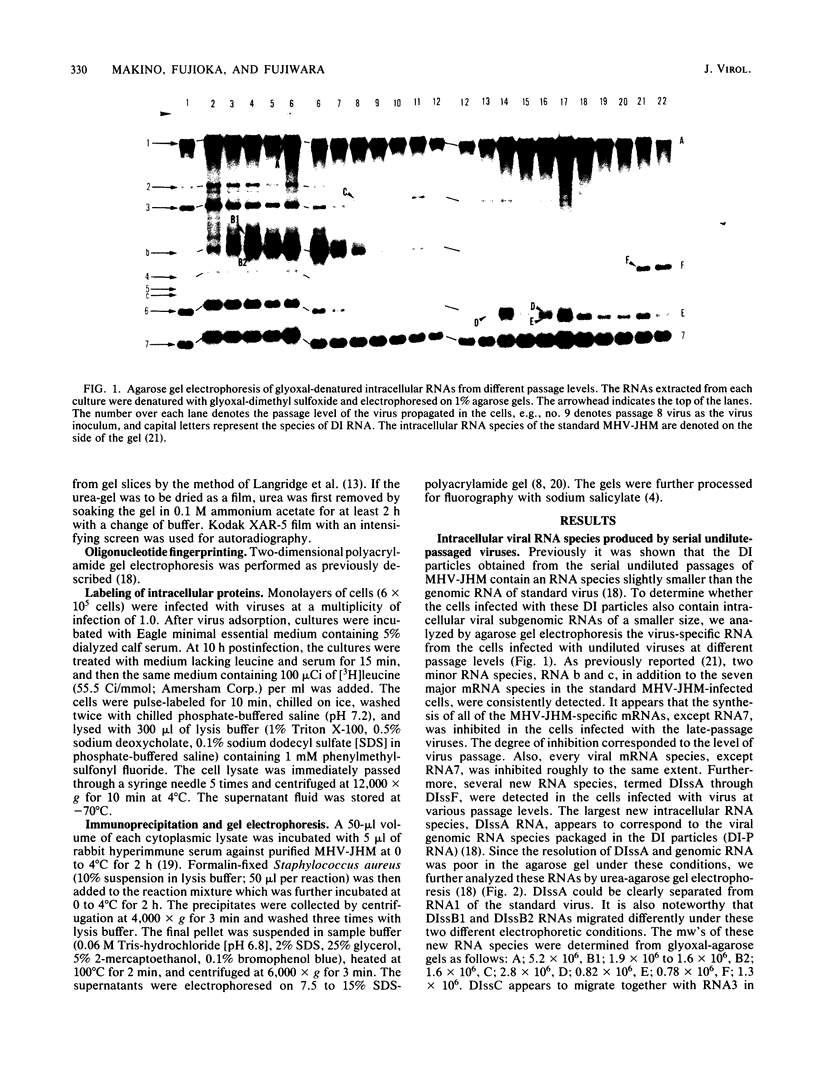
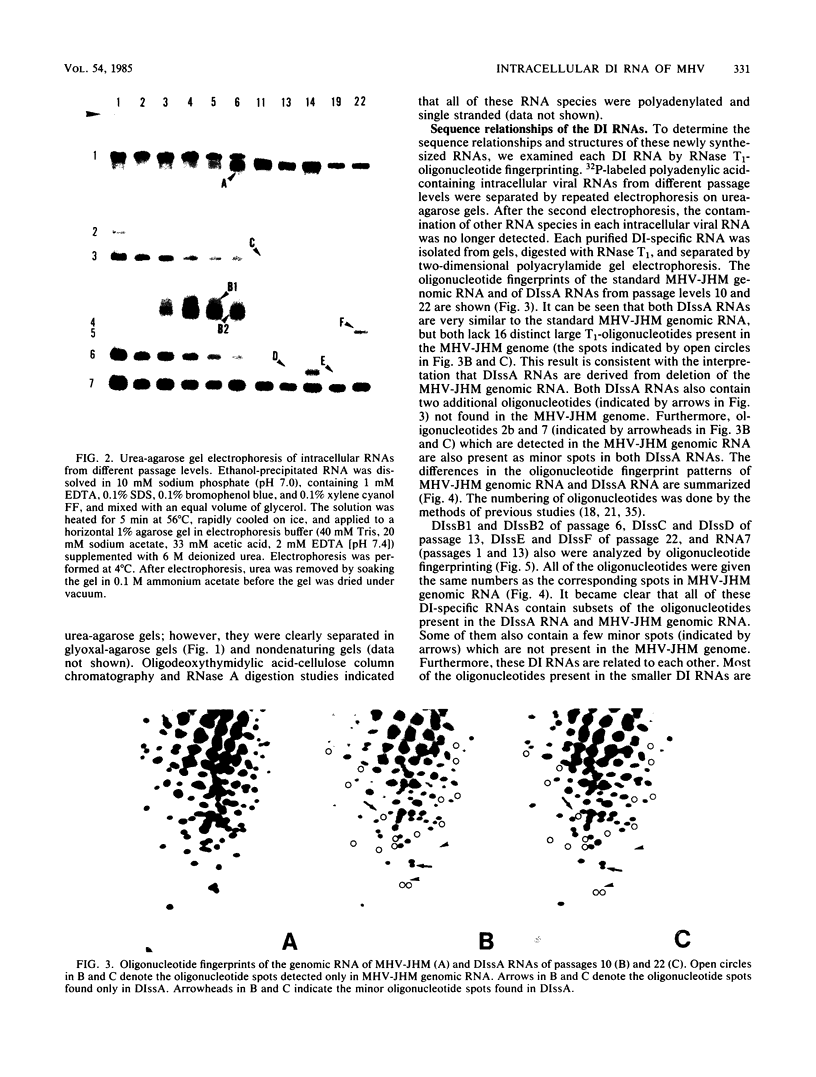
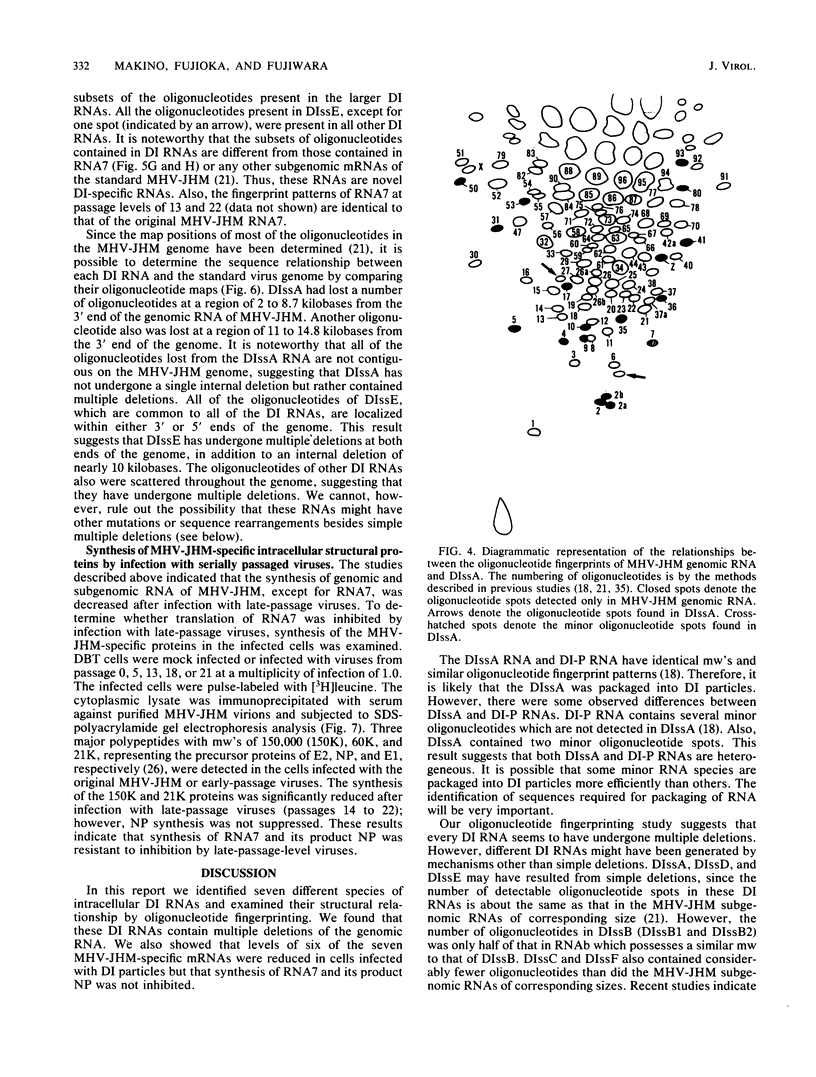
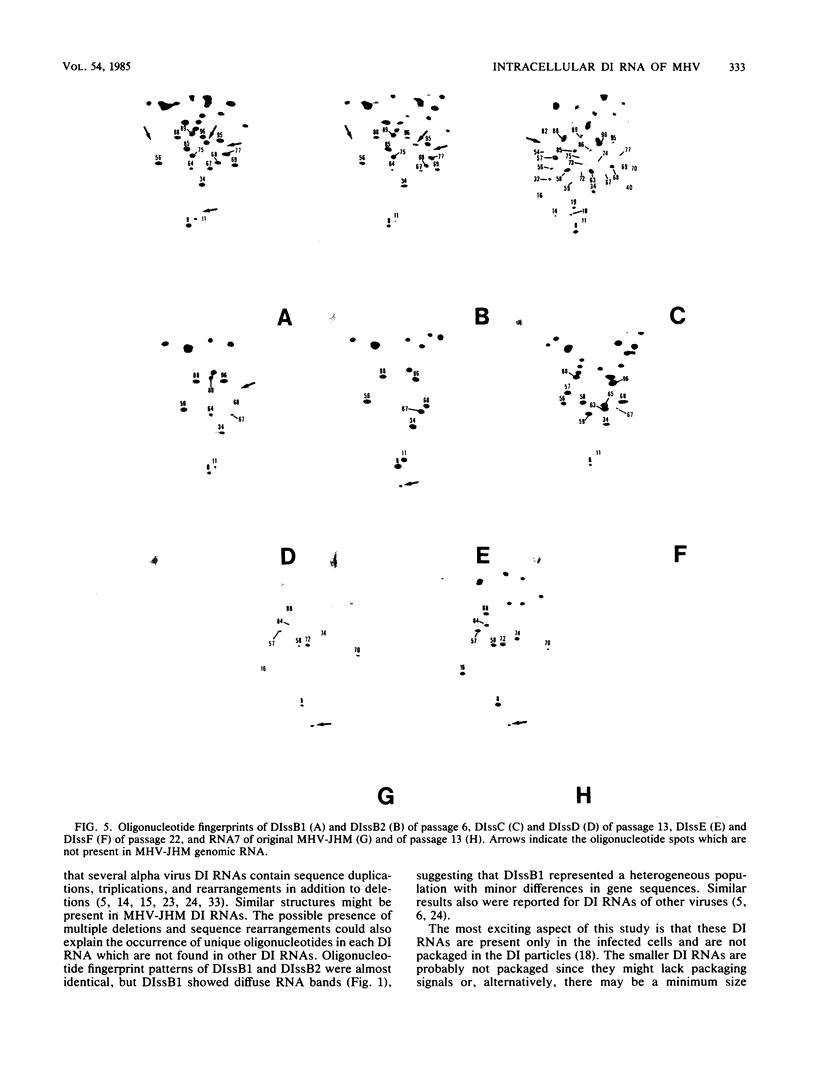
The intracellular defective RNAs generated during high-multiplicity serial passages of mouse hepatitis virus JHM strain on DBT cells were examined. Seven novel species of single-stranded polyadenylic acid-containing defective RNAs were identified from passages 3 through 22. The largest of these RNAs, DIssA (molecular weight [mw], 5.2 X 10(6)), is identical to the genomic RNA packaged in the defective interfering particles produced from these cells. Other RNA species, DIssB1 (mw, 1.9 X 10(6) to 1.6 X 10(6)), DIssB2 (mw, 1.6 X 10(6)), DIssC (mw, 2.8 X 10(6)) DIssD (mw, 0.82 X 10(6)), DIssE (mw, 0.78 X 10(6)), and DIssF (mw, 1.3 X 10(6)) were detected at different passage levels. RNase T1-resistant oligonucleotide fingerprinting demonstrated that all these RNAs were related and had multiple deletions of the genomic sequences. They contained different subsets of the genomic sequences from those of the standard intracellular mRNAs of nondefective mouse hepatitis virus JHM strain. Thus these novel intracellular viral RNAs were identified as defective interfering RNAs of mouse hepatitis virus JHM strain. The synthesis of six of the seven normal mRNA species specific to mouse hepatitis virus JHM strain was completely inhibited when cells were infected with viruses of late-passage levels. However, the synthesis of RNA7 and its product, viral nucleoprotein, was not significantly altered in late passages. The possible mechanism for the generation of defective interfering RNAs was discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baric R. S., Stohlman S. A., Lai M. M. Characterization of replicative intermediate RNA of mouse hepatitis virus: presence of leader RNA sequences on nascent chains. J Virol. 1983 Dec;48(3):633–640. doi: 10.1128/jvi.48.3.633-640.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton P. R., Lai M. M., Patton C. D., Stohlman S. A. Characterization of two RNA polymerase activities induced by mouse hepatitis virus. J Virol. 1982 Jun;42(3):847–853. doi: 10.1128/jvi.42.3.847-853.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayton P. R., Stohlman S. A., Lai M. M. Further characterization of mouse hepatitis virus RNA-dependent RNA polymerases. Virology. 1984 Feb;133(1):197–201. doi: 10.1016/0042-6822(84)90439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Dohner D., Monroe S., Weiss B., Schlesinger S. Oligonucleotide mapping studies of standard and defective Sindbis virus RNA. J Virol. 1979 Feb;29(2):794–798. doi: 10.1128/jvi.29.2.794-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Bruton C. J., Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: nature of the defective virion RNA. J Virol. 1976 Sep;19(3):1034–1043. doi: 10.1128/jvi.19.3.1034-1043.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanishi T. Brain tumors induced with Rous sarcoma virus, Schmidt-Ruppin strain. I. Induction of brain tumors in adult mice with Rous chicken sarcoma cells. Jpn J Exp Med. 1967 Oct;37(5):461–474. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Baric R. S., Brayton P. R., Stohlman S. A. Characterization of leader RNA sequences on the virion and mRNAs of mouse hepatitis virus, a cytoplasmic RNA virus. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3626–3630. doi: 10.1073/pnas.81.12.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Brayton P. R., Armen R. C., Patton C. D., Pugh C., Stohlman S. A. Mouse hepatitis virus A59: mRNA structure and genetic localization of the sequence divergence from hepatotropic strain MHV-3. J Virol. 1981 Sep;39(3):823–834. doi: 10.1128/jvi.39.3.823-834.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Patton C. D., Baric R. S., Stohlman S. A. Presence of leader sequences in the mRNA of mouse hepatitis virus. J Virol. 1983 Jun;46(3):1027–1033. doi: 10.1128/jvi.46.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Patton C. D., Stohlman S. A. Replication of mouse hepatitis virus: negative-stranded RNA and replicative form RNA are of genome length. J Virol. 1982 Nov;44(2):487–492. doi: 10.1128/jvi.44.2.487-492.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Söderlund H., Keränen S., Pettersson R. F., Käriäinen L. 18S defective interfering RNA of Semliki Forest virus contains a triplicated linear repeat. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5353–5357. doi: 10.1073/pnas.78.9.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtovaara P., Söderlund H., Keränen S., Pettersson R. F., Käriäinen L. Extreme ends of the genome are conserved and rearranged in the defective interfering RNAs of Semliki Forest virus. J Mol Biol. 1982 Apr 25;156(4):731–748. doi: 10.1016/0022-2836(82)90139-5. [DOI] [PubMed] [Google Scholar]
- Leibowitz J. L., Weiss S. R., Paavola E., Bond C. W. Cell-free translation of murine coronavirus RNA. J Virol. 1982 Sep;43(3):905–913. doi: 10.1128/jvi.43.3.905-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B. W., Siddell S., Wege H., ter Meulen V. RNA-dependent RNA polymerase activity in murine coronavirus-infected cells. J Gen Virol. 1983 Jan;64(Pt 1):103–111. doi: 10.1099/0022-1317-64-1-103. [DOI] [PubMed] [Google Scholar]
- Makino S., Taguchi F., Fujiwara K. Defective interfering particles of mouse hepatitis virus. Virology. 1984 Feb;133(1):9–17. doi: 10.1016/0042-6822(84)90420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Taguchi F., Fujiwara K., Hayami M. Heterologous response of antiserum-treated cell clones from a persistently infected DBT cell line to mouse hepatitis virus. Jpn J Exp Med. 1982 Dec;52(6):297–302. [PubMed] [Google Scholar]
- Makino S., Taguchi F., Hayami M., Fujiwara K. Characterization of small plaque mutants of mouse hepatitis virus, JHM strain. Microbiol Immunol. 1983;27(5):445–454. doi: 10.1111/j.1348-0421.1983.tb00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Taguchi F., Hirano N., Fujiwara K. Analysis of genomic and intracellular viral RNAs of small plaque mutants of mouse hepatitis virus, JHM strain. Virology. 1984 Nov;139(1):138–151. doi: 10.1016/0042-6822(84)90335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. S., Ou J. H., Rice C. M., Schlesinger S., Strauss E. G., Strauss J. H. Sequence analysis of cDNA's derived from the RNA of Sindbis virions and of defective interfering particles. J Virol. 1982 Jan;41(1):153–162. doi: 10.1128/jvi.41.1.153-162.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. S., Schlesinger S. Common and distinct regions of defective-interfering RNAs of Sindbis virus. J Virol. 1984 Mar;49(3):865–872. doi: 10.1128/jvi.49.3.865-872.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981 Apr;38(1):20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Barthel A., ter Meulen V. Coronavirus JHM: a virion-associated protein kinase. J Gen Virol. 1981 Feb;52(Pt 2):235–243. doi: 10.1099/0022-1317-52-2-235. [DOI] [PubMed] [Google Scholar]
- Siddell S. G. Coronavirus JHM: tryptic peptide fingerprinting of virion proteins and intracellular polypeptides. J Gen Virol. 1982 Oct;62(Pt 2):259–269. doi: 10.1099/0022-1317-62-2-259. [DOI] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. Coronavirus JHM: coding assignments of subgenomic mRNAs. J Gen Virol. 1983 Jan;64(Pt 1):113–125. doi: 10.1099/0022-1317-64-1-113. [DOI] [PubMed] [Google Scholar]
- Siddell S., Wege H., Ter Meulen V. The biology of coronaviruses. J Gen Virol. 1983 Apr;64(Pt 4):761–776. doi: 10.1099/0022-1317-64-4-761. [DOI] [PubMed] [Google Scholar]
- Siddell S., Wege H., ter Meulen V. The structure and replication of coronaviruses. Curr Top Microbiol Immunol. 1982;99:131–163. doi: 10.1007/978-3-642-68528-6_4. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Siddell S. G. Coronavirus JHM: nucleotide sequence of the mRNA that encodes nucleocapsid protein. Nucleic Acids Res. 1983 Aug 11;11(15):5045–5054. doi: 10.1093/nar/11.15.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Brayton P. R., Fleming J. O., Weiner L. P., Lai M. M. Murine coronaviruses: isolation and characterization of two plaque morphology variants of the JHM neurotropic strain. J Gen Virol. 1982 Dec;63(2):265–275. doi: 10.1099/0022-1317-63-2-265. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Fleming J. O., Patton C. D., Lai M. M. Synthesis and subcellular localization of the murine coronavirus nucleocapsid protein. Virology. 1983 Oct 30;130(2):527–532. doi: 10.1016/0042-6822(83)90106-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Lai M. M. Phosphoproteins of murine hepatitis viruses. J Virol. 1979 Nov;32(2):672–675. doi: 10.1128/jvi.32.2.672-675.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H., Keränen S., Lehtovaara P., Palva I., Pettersson R. F., Käriäinen L. Structural complexity of defective-interfering RNAs of Semliki Forest virus as revealed by analysis of complementary DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3403–3417. doi: 10.1093/nar/9.14.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]