Abstract
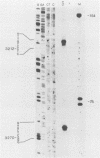
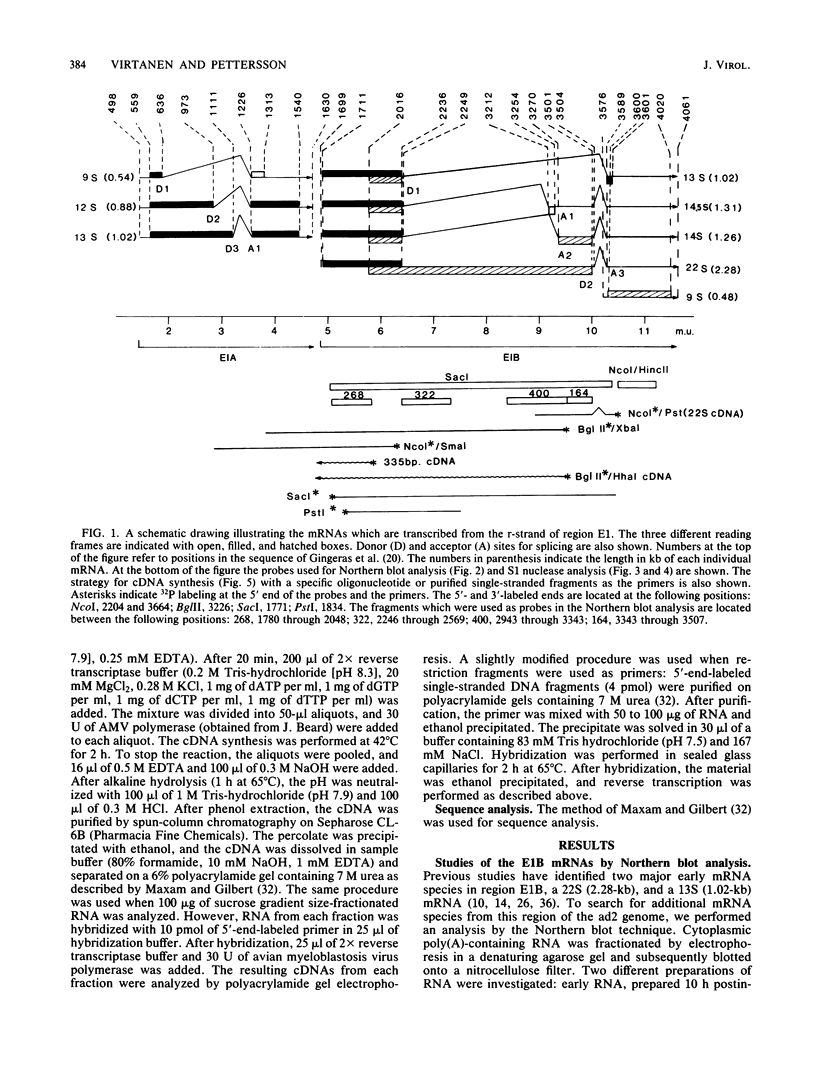
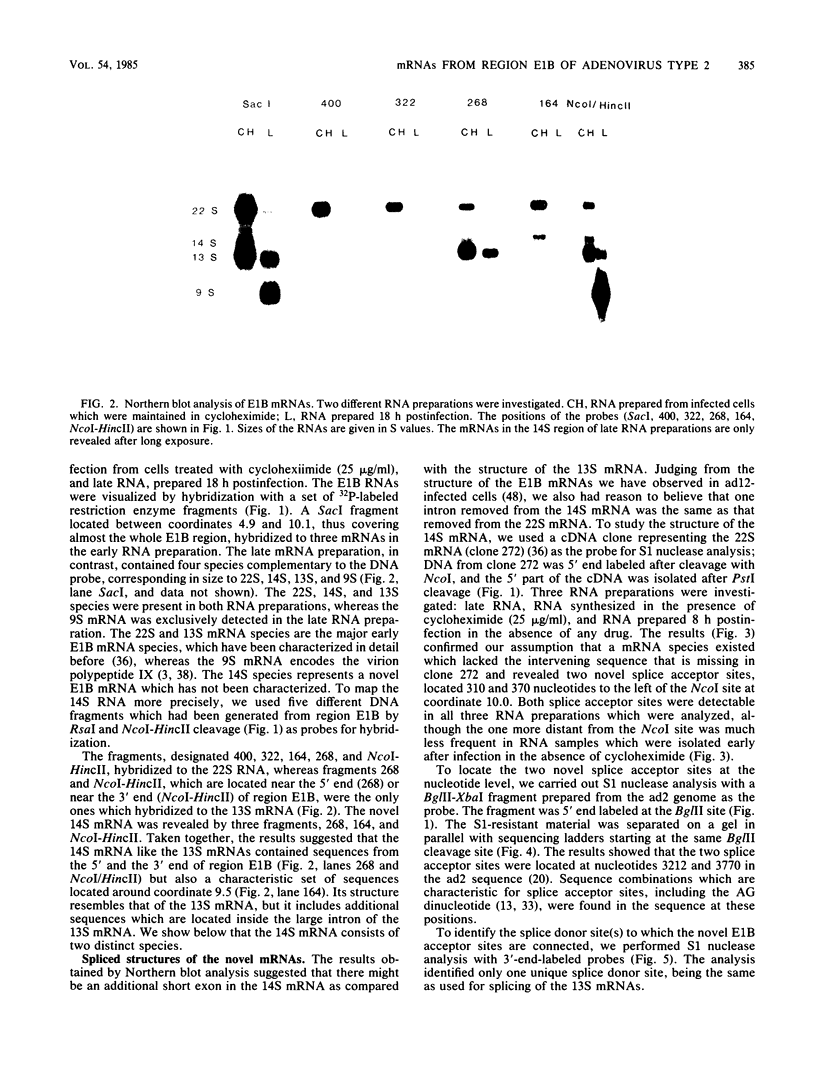
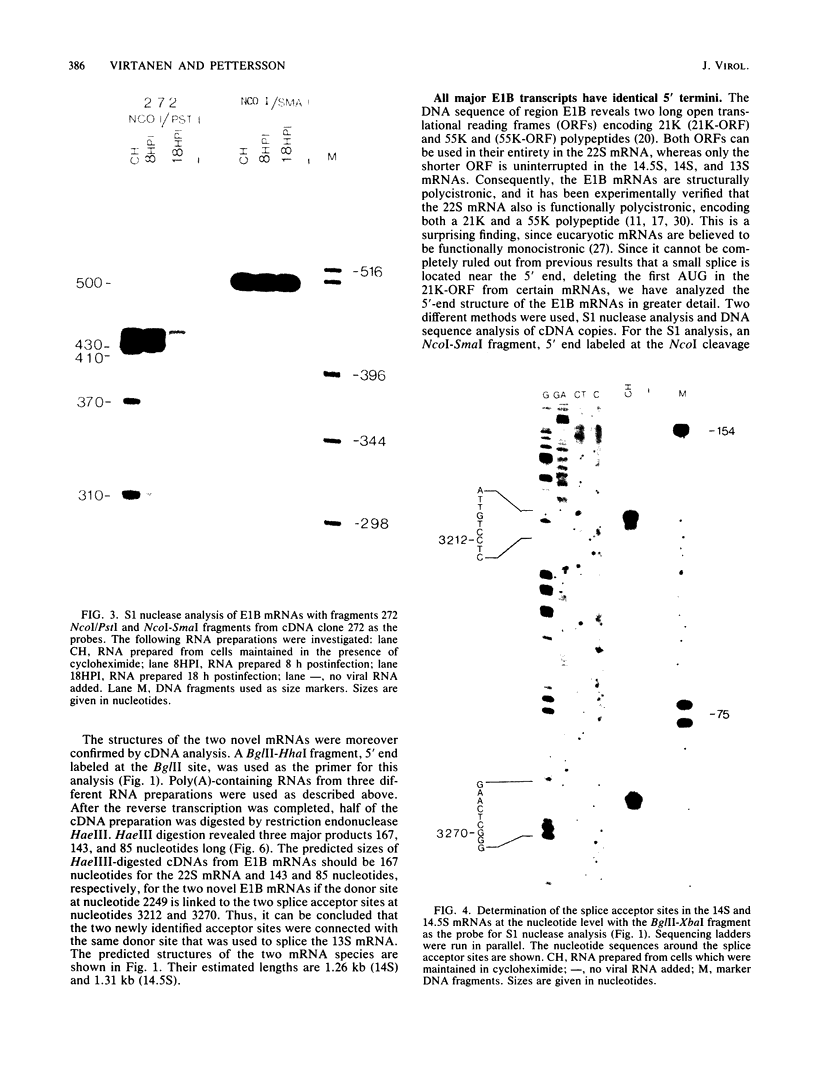
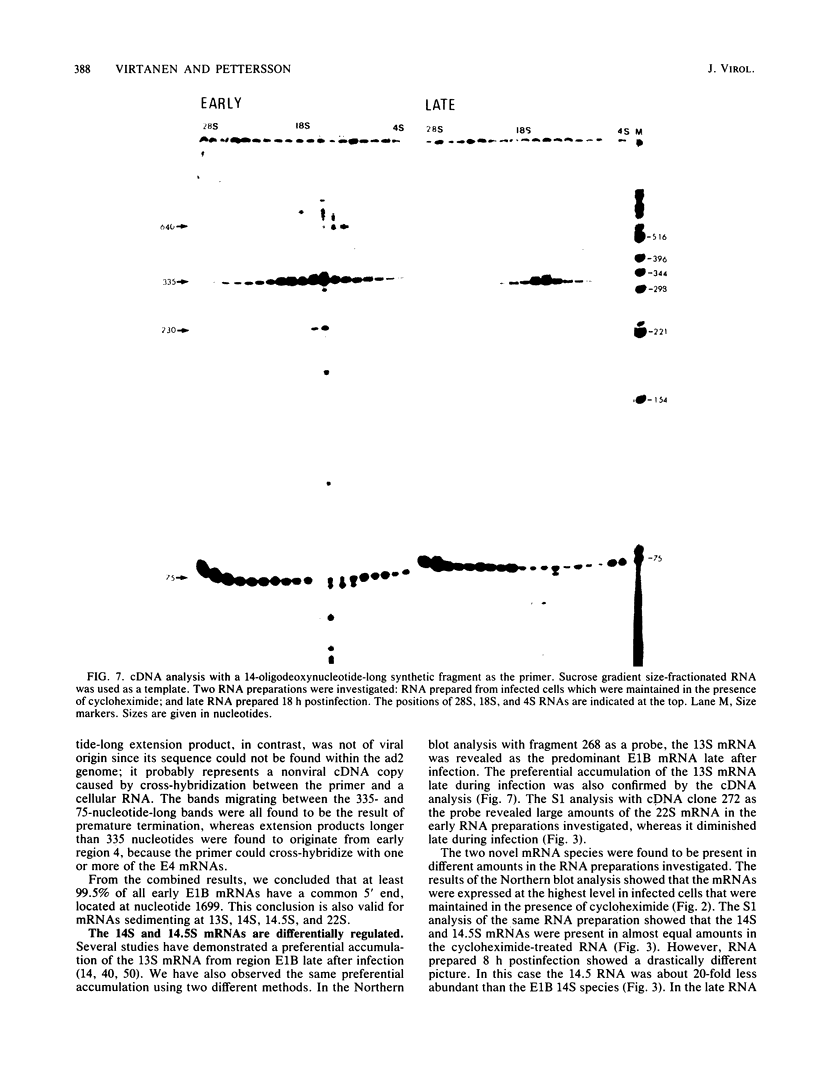
The mRNAs from early region 1B of adenovirus type 2 have been studied by Northern blot, S1 nuclease, and cDNA analysis. Two novel mRNAs, designated 14S and 14.5S, have been observed in addition to the previously identified 9S, 13S, and 22S mRNAs. They are 1.26 and 1.31 kilobases long and differ from the 13S and 22S mRNAs in being composed of three exons instead of two. Their two terminal exons are the same as those present in the 13S mRNA, whereas the middle exon is unique to each of the two novel mRNA species. The structures of the 14S and 14.5S mRNAs allow the prediction of their coding capacities: both mRNA species, like the 22S and 13S mRNAs, contain an uninterrupted translational reading frame encoding a 21,000-molecular-weight (21K) polypeptide. The 14S mRNA can, in addition, encode a 16.5K polypeptide which shares N-terminal and C-terminal sequences with the 55K polypeptide, known to be encoded by the 22S mRNA. The 14.5S mRNA species encodes a hypothetical 9.2K polypeptide which has the same N terminus as the 55K polypeptide but a unique C terminus. The two mRNAs differ in their kinetics of appearance; the 14.5S mRNA is preferentially expressed late after infection in contrast to the 14S mRNA, which is present in approximately equal amounts early and late after infection. Taken together with previously published information the results suggest that early region 1B of adenovirus type 2 encodes five proteins in addition to virion polypeptide IX. These have predicted molecular weights of 55,000, 21,000, 16,500, 9,200, and 8,100.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Brunstedt J., Noyes B. E. A general method for detection and characterization of an mRNA using an oligonucleotide probe. J Biol Chem. 1981 Jan 25;256(2):1023–1028. [PubMed] [Google Scholar]
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Aleström P., Akusjärvi G., Perricaudet M., Mathews M. B., Klessig D. F., Pettersson U. The gene for polypeptide IX of adenovirus type 2 and its unspliced messenger RNA. Cell. 1980 Mar;19(3):671–681. doi: 10.1016/s0092-8674(80)80044-4. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Schmitt R. C., Smart J. E., Lewis J. B. Early region 1B of adenovirus 2 encodes two coterminal proteins of 495 and 155 amino acid residues. J Virol. 1984 May;50(2):387–396. doi: 10.1128/jvi.50.2.387-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich A., Nevins J. R. The stability of early adenovirus mRNA is controlled by the viral 72 kd DNA-binding protein. Cell. 1981 Nov;26(3 Pt 1):371–379. doi: 10.1016/0092-8674(81)90206-3. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Ziff E. B. Promoters and heterogeneous 5' termini of the messenger RNAs of adenovirus serotype 2. J Mol Biol. 1981 Jun 25;149(2):189–221. doi: 10.1016/0022-2836(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Air G. M., Hutchison C. A., 3rd Overlapping genes in bacteriophage phiX174. Nature. 1976 Nov 4;264(5581):34–41. doi: 10.1038/264034a0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M., van Ormondt H. Gene organization of the transforming region of adenovirus type 7 DNA. Gene. 1982 May;18(2):143–156. doi: 10.1016/0378-1119(82)90112-3. [DOI] [PubMed] [Google Scholar]
- Eggerding F., Raskas H. J. Regulation of an early RNA transcribed from the transforming segment of the adenovirus 2 genome. Virology. 1978 Dec;91(2):312–320. doi: 10.1016/0042-6822(78)90379-3. [DOI] [PubMed] [Google Scholar]
- Esche H., Mathews M. B., Lewis J. B. Proteins and messenger RNAs of the transforming region of wild-type and mutant adenoviruses. J Mol Biol. 1980 Sep 25;142(3):399–417. doi: 10.1016/0022-2836(80)90279-x. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K. H., Cartas M. A., Matsuo T. Identification and purification of a protein encoded by the human adenovirus type 2 transforming region. J Virol. 1982 Apr;42(1):30–41. doi: 10.1128/jvi.42.1.30-41.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Wold W. S., Brackmann K. H., Green M. Nucleotide sequences of 5' termini of adenovirus 2 early transforming region E1a and E1b messenger ribonucleic acids. Biochemistry. 1981 Nov 10;20(23):6640–6647. doi: 10.1021/bi00526a019. [DOI] [PubMed] [Google Scholar]
- Kimura T., Sawada Y., Shinawawa M., Shimizu Y., Shiroki K., Shimojo H., Sugisaki H., Takanami M., Uemizu Y., Fujinaga K. Nucleotide sequence of the transforming early region E1b of adenovirus type 12 DNA: structure and gene organization, and comparison with those of adenovirus type 5 DNA. Nucleic Acids Res. 1981 Dec 11;9(23):6571–6589. doi: 10.1093/nar/9.23.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchingman G. R., Westphal H. The structure of adenovirus 2 early nuclear and cytoplasmic RNAs. J Mol Biol. 1980 Feb 15;137(1):23–48. doi: 10.1016/0022-2836(80)90155-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lucher L. A., Brackmann K. H., Symington J. S., Green M. Antibody directed to a synthetic peptide encoding the NH2-terminal 16 amino acids of the adenovirus type 2 E1B-53K tumor antigen recognizes the E1B-20K tumor antigen. Virology. 1984 Jan 15;132(1):217–221. doi: 10.1016/0042-6822(84)90106-5. [DOI] [PubMed] [Google Scholar]
- Lupker J. H., Davis A., Jochemsen H., van der Eb A. J. In vitro synthesis of adenovirus type 5 T antigens. I. Translation of early region 1-specific rna from lytically infected cells. J Virol. 1981 Jan;37(1):524–529. doi: 10.1128/jvi.37.1.524-529.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., Le Moullec J. M., Pettersson U. Predicted structure of two adenovirus tumor antigens. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3778–3782. doi: 10.1073/pnas.77.7.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Pettersson U., Mathews M. B. Synthesis of a structural adenovirus polypeptide in the absence of viral DNA replication. Virology. 1978 Oct 1;90(1):67–79. doi: 10.1016/0042-6822(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mathews M. B. The gene and messenger RNA for adenovirus polypeptide IX. Cell. 1977 Nov;12(3):741–750. doi: 10.1016/0092-8674(77)90274-4. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Tibbetts C., Philipson L. Hybridization maps of early and late messenger RNA sequences on the adenovirus type 2 genome. J Mol Biol. 1976 Mar 15;101(4):479–501. doi: 10.1016/0022-2836(76)90241-2. [DOI] [PubMed] [Google Scholar]
- Spector D. J., McGrogan M., Raskas H. J. Regulation of the appearance of cytoplasmic RNAs from region 1 of the adenovirus 2 genome. J Mol Biol. 1978 Dec 15;126(3):395–414. doi: 10.1016/0022-2836(78)90048-7. [DOI] [PubMed] [Google Scholar]
- Sugisaki H., Sugimoto K., Takanami M., Shiroki K., Saito I., Shimojo H., Sawada Y., Uemizu Y., Uesugi S., Fujinaga K. Structure and gene organization in the transformed Hind III-G fragment of Ad12. Cell. 1980 Jul;20(3):777–786. doi: 10.1016/0092-8674(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Virtanen A., Pettersson U., Le Moullec J. M., Tiollais P., Perricaudet M. Different mRNAs from the transforming region of highly oncogenic and non-oncogenic human adenoviruses. Nature. 1982 Feb 25;295(5851):705–707. doi: 10.1038/295705a0. [DOI] [PubMed] [Google Scholar]
- Virtanen A., Pettersson U. The molecular structure of the 9S mRNA from early region 1A of adenovirus serotype 2. J Mol Biol. 1983 Apr 15;165(3):496–499. doi: 10.1016/s0022-2836(83)80215-0. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Darnell J. E., Jr Control of messenger RNA concentration by differential cytoplasmic half-life. Adenovirus messenger RNAs from transcription units 1A and 1B. J Mol Biol. 1981 May 25;148(3):231–251. doi: 10.1016/0022-2836(81)90537-4. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Fraser N. W., Darnell J. E., Jr Mapping of RNA initiation sites by high doses of uv irradiation: evidence for three independent promoters within the left 11% of the Ad-2 genome. Virology. 1979 Apr 15;94(1):175–184. doi: 10.1016/0042-6822(79)90447-1. [DOI] [PubMed] [Google Scholar]
- van Ormondt H., Maat J., van Beveren C. P. The nucleotide sequence of the transforming early region E1 of adenovirus type 5 DNA. Gene. 1980 Nov;11(3-4):299–309. doi: 10.1016/0378-1119(80)90070-0. [DOI] [PubMed] [Google Scholar]