Abstract
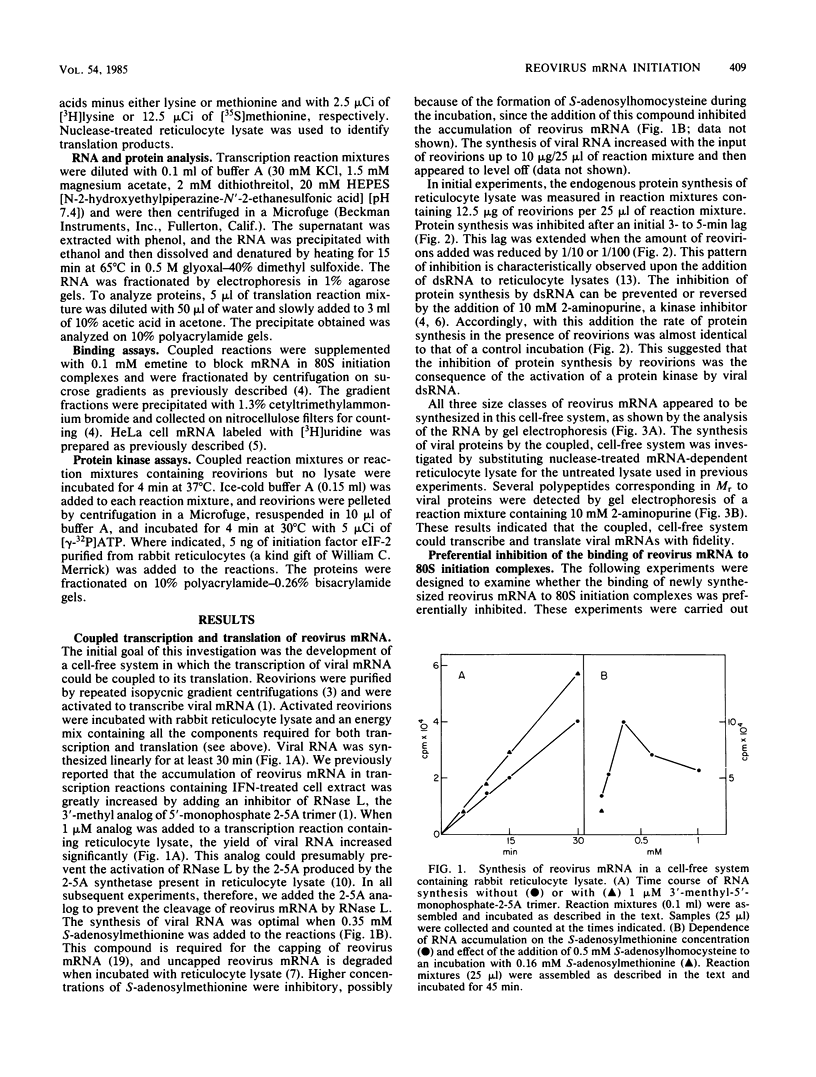
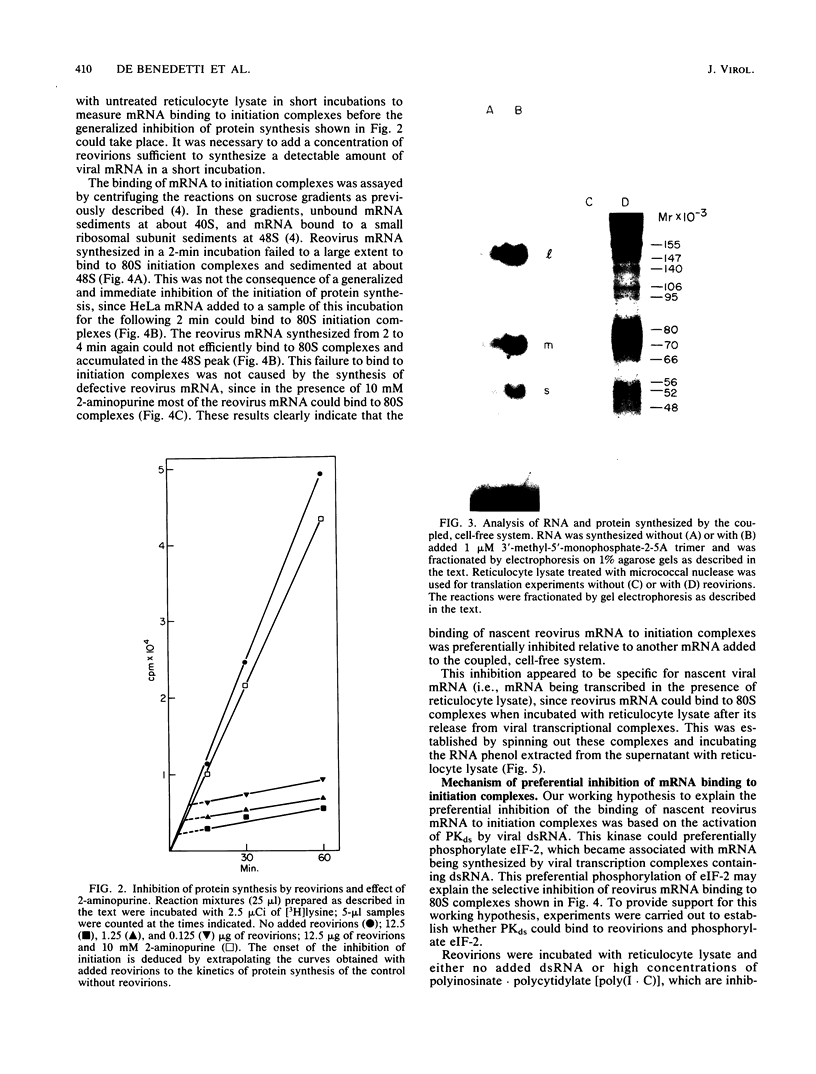
A coupled, cell-free system for the transcription and translation of reovirus mRNA was developed. Activated reovirions were incubated with reticulocyte lysate and an appropriate energy mix. Active transcription was obtained, but protein synthesis was inhibited after a short lag even by low concentrations of reovirions. This inhibition was abolished by the addition of the kinase inhibitor 2-aminopurine. With this addition, the synthesis of viral proteins could be detected in reaction mixtures containing nuclease-treated reticulocyte lysate. The binding of nascent reovirus mRNA to 80S initiation complexes measured after 2 min of incubation was greatly inhibited, whereas the binding of cellular mRNA added to the same reaction mixtures for the next 2 min was not inhibited. The inhibition of reovirus mRNA binding could not be explained by the synthesis of defective templates, since most of the mRNA could be bound to 80S complexes after the addition of 2-aminopurine. These results indicate that the binding of nascent reovirus mRNA was preferentially inhibited by a protein kinase. Reovirions preincubated with reticulocyte lysate could phosphorylate initiation factor eIF-2. This phosphorylation was inhibited by the addition of high concentrations of double-stranded RNA, which are inhibitory for the eIF-2 kinase present in elevated levels in reticulocyte lysate and in interferon-treated cells. These results indicate that the translation of viral mRNA may be preferentially inhibited in interferon-treated cells by the eIF-2 kinase activated by viral transcriptional complexes containing double-stranded RNA.
Full text
PDF
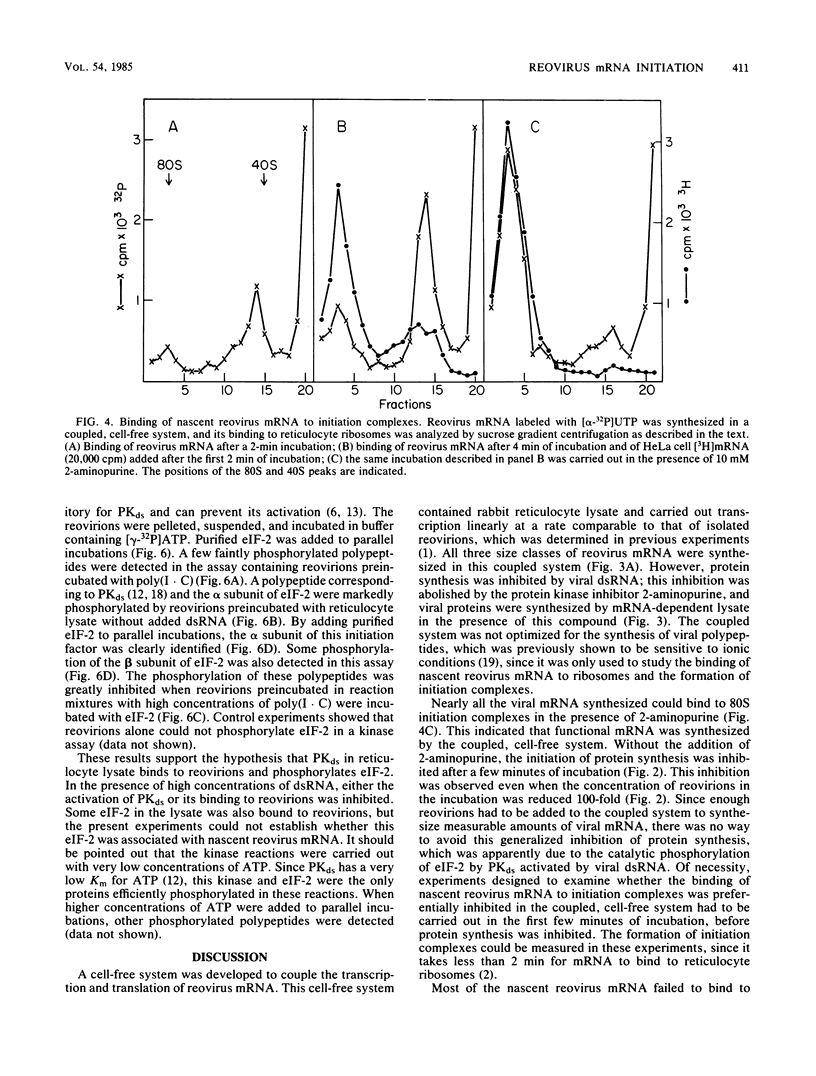
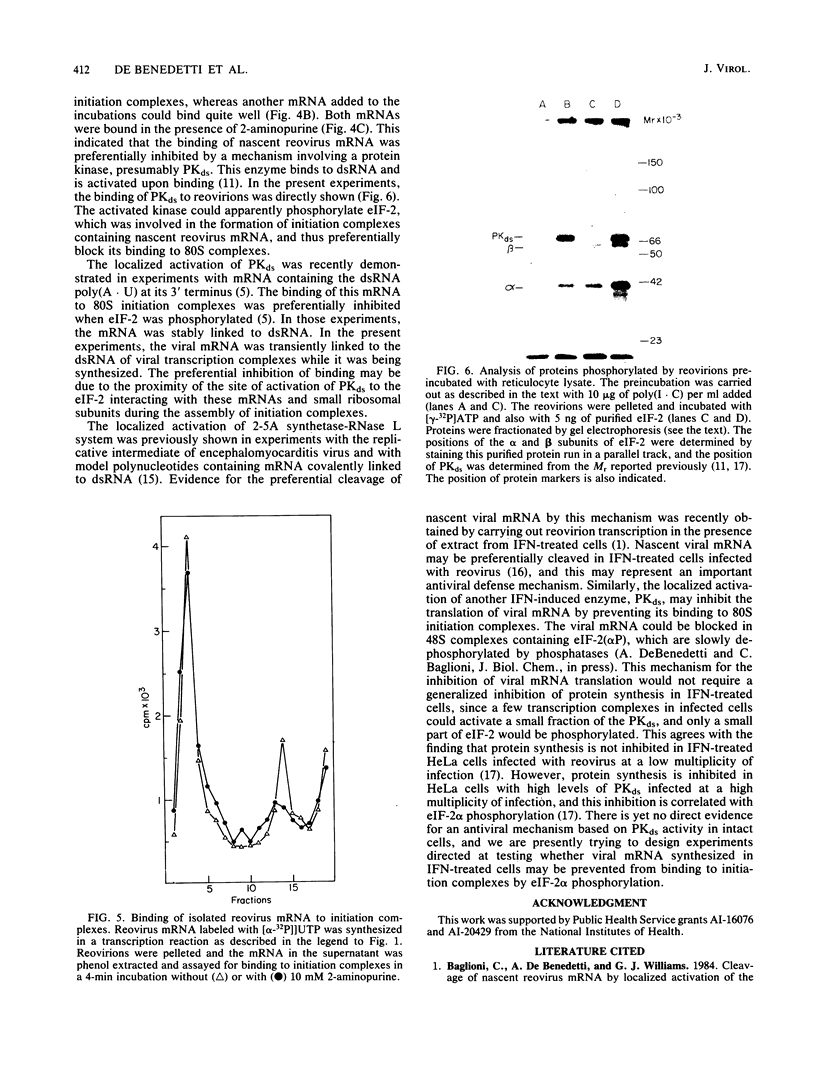

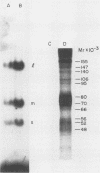
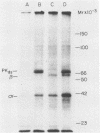
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., De Benedetti A., Williams G. J. Cleavage of nascent reovirus mRNA by localized activation of the 2'-5'-oligoadenylate-dependent endoribonuclease. J Virol. 1984 Dec;52(3):865–871. doi: 10.1128/jvi.52.3.865-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C., Simili M., Shafritz D. A. Initiation activity of EMC virus RNA, binding to initiation factor eIF-4B and shut-off of host cell protein synthesis. Nature. 1978 Sep 21;275(5677):240–243. doi: 10.1038/275240a0. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Shatkin A. J. Transcription in vitro by reovirus-associated ribonucleic acid-dependent polymerase. J Virol. 1970 Jul;6(1):1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Inhibition of mRNA binding to ribosomes by localized activation of dsRNA-dependent protein kinase. Nature. 1984 Sep 6;311(5981):79–81. doi: 10.1038/311079a0. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Phosphorylation of initiation factor eIF-2 alpha, binding of mRNA to 48 S complexes, and its reutilization in initiation of protein synthesis. J Biol Chem. 1983 Dec 10;258(23):14556–14562. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Grace M., Ralston R. O., Banerjee A. C., Gupta N. K. Protein synthesis in rabbit reticulocytes: characteristics of the protein factor RF that reverses inhibition of protein synthesis in heme-deficient reticulocyte lysates. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6517–6521. doi: 10.1073/pnas.79.21.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. Control of protein synthesis by hemin. Evidence that the hemin-controlled translational repressor inhibits formation of 80 S initiation complexes from 48 S intermediate initiation complexes. J Biol Chem. 1979 Apr 10;254(7):2370–2377. [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. The (2'-5') oligoadenylate (pppA2'-5'A2'-5'A) synthetase and protein kinase(s) from interferon-treated cells. Eur J Biochem. 1979 Feb 1;93(3):515–526. doi: 10.1111/j.1432-1033.1979.tb12850.x. [DOI] [PubMed] [Google Scholar]
- Krust B., Galabru J., Hovanessian A. G. Further characterization of the protein kinase activity mediated by interferon in mouse and human cells. J Biol Chem. 1984 Jul 10;259(13):8494–8498. [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L., London I. M. The regulation of initiation of protein synthesis by phosphorylation of eIF-2(alpha) and the role of reversing factor in the recycling of eIF-2. J Biol Chem. 1984 Jun 10;259(11):6708–6711. [PubMed] [Google Scholar]
- Nilsen T. W., Baglioni C. Mechanism for discrimination between viral and host mRNA in interferon-treated cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2600–2604. doi: 10.1073/pnas.76.6.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Inhibition of protein synthesis in reovirus-infected HeLa cells with elevated levels of interferon-induced protein kinase activity. J Biol Chem. 1982 Dec 25;257(24):14593–14596. [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Synthesis of (2'-5')oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J Virol. 1982 Jun;42(3):1039–1045. doi: 10.1128/jvi.42.3.1039-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the double stranded RNA activated protein kinase. Biochem Biophys Res Commun. 1980 Nov 17;97(1):252–262. doi: 10.1016/s0006-291x(80)80162-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Both G. W. Reovirus mRNA: transcription and translation. Cell. 1976 Mar;7(3):305–313. doi: 10.1016/0092-8674(76)90159-8. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Manne V., Ochoa S. Mechanism of translational control by partial phosphorylation of the alpha subunit of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1984 Jan;81(2):352–356. doi: 10.1073/pnas.81.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mauser L., Ochoa S. Mechanism of polypeptide chain initiation in eukaryotes and its control by phosphorylation of the alpha subunit of initiation factor 2. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2537–2540. doi: 10.1073/pnas.79.8.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]