Abstract
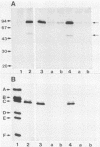
In productively infected cells, a fraction of large-tumor antigen (T antigen) is tightly bound to replicating simian virus 40 (SV40) minichromosomes and does not dissociate at salt concentrations of greater than 1 M NaCl. We present electronmicrograms demonstrating the presence of T antigen on the replicated sections of replicating SV40 minichromosomes. We also show that the fraction of tightly bound T antigen is recognized by antibodies from mouse tumor serum and, more specifically, by a particular T-antigen-specific monoclonal antibody, PAb 1630. A second T-antigen-specific monoclonal antibody, PAb 101, does not react with the T-antigen fraction remaining on replicating SV40 chromatin at high salt concentrations. We used an in vitro replication system which allows, via semiconservative DNA replication, the completion of in vivo-initiated replicative intermediate DNA molecules. We show that monoclonal antibody PAb 1630, but not monoclonal antibody PAb 101, inhibits viral DNA replication. We discuss the possibility that SV40 T antigen may play a role in chain elongation during SV40 chromatin replication.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. K., Siegl B., Quellhorst S., Brandner G., Braun D. G. Monoclonal antibodies against simian virus 40 nuclear large T tumour antigen: epitope mapping, papova virus cross-reaction and cell surface staining. EMBO J. 1984 Jul;3(7):1485–1491. doi: 10.1002/j.1460-2075.1984.tb02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Gurney E. G. Time-dependent maturation of the simian virus 40 large T antigen-p53 complex studied by using monoclonal antibodies. J Virol. 1982 Nov;44(2):565–573. doi: 10.1128/jvi.44.2.565-573.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Lane D. P., Tjian R. Use of monoclonal antibodies as probes of simian virus 40 T antigen ATPase activity. J Biol Chem. 1981 Nov 25;256(22):11854–11858. [PubMed] [Google Scholar]
- Deppert W., Gurney E. G., Harrison R. O. Monoclonal antibodies against simian virus 40 tumor antigens: analysis of antigenic binding sites, using adenovirus type 2-simian virus 40 hybrid viruses. J Virol. 1981 Jan;37(1):478–482. doi: 10.1128/jvi.37.1.478-482.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A poly(dT)-stimulated ATPase activity associated with simian virus 40 large T antigen. J Biol Chem. 1979 Sep 10;254(17):8113–8116. [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper F., Florentin Y., Puvion E. Localization of T-antigen on simian virus 40 minichromosomes by immunoelectron microscopy. EMBO J. 1984 Jun;3(6):1235–1241. doi: 10.1002/j.1460-2075.1984.tb01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Fanning E., Otto B., Knippers R. Maturation of newly replicated chromatin of simian virus 40 and its host cell. J Mol Biol. 1980 Feb 5;136(4):359–374. doi: 10.1016/0022-2836(80)90395-2. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Levine A. J. Transformation-associated tumor antigens. Adv Cancer Res. 1982;37:75–109. doi: 10.1016/s0065-230x(08)60882-9. [DOI] [PubMed] [Google Scholar]
- McKay R. D. Binding of a simian virus 40 T antigen-related protein to DNA. J Mol Biol. 1981 Jan 25;145(3):471–488. doi: 10.1016/0022-2836(81)90540-4. [DOI] [PubMed] [Google Scholar]
- Oren M., Winocour E., Prives C. Differential affinities of simian virus 40 large tumor antigen for DNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):220–224. doi: 10.1073/pnas.77.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Fanning E. DNA polymerase alpha is associated with replicating SV40 nucleoprotein complexes. Nucleic Acids Res. 1978 May;5(5):1715–1728. doi: 10.1093/nar/5.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J., Renart J., Crawford L. V., Stark G. R. Specific association of simian virus 40 tumor antigen with simian virus 40 chromatin. J Virol. 1980 Jan;33(1):78–87. doi: 10.1128/jvi.33.1.78-87.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Scheu R., Otto B. Replication of simian virus 40 chromatin in vitro depends on the amount of DNA polymerase alpha associated with replicating chromatin. Eur J Biochem. 1980 Aug;109(1):67–73. doi: 10.1111/j.1432-1033.1980.tb04768.x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. Transforming genes and gene products of polyoma and SV40. CRC Crit Rev Biochem. 1982;13(3):215–286. doi: 10.3109/10409238209114230. [DOI] [PubMed] [Google Scholar]
- Scheller A., Covey L., Barnet B., Prives C. A small subclass of SV40 T antigen binds to the viral origin of replication. Cell. 1982 Jun;29(2):375–383. doi: 10.1016/0092-8674(82)90154-4. [DOI] [PubMed] [Google Scholar]
- Segawa M., Sugano S., Yamaguchi N. Association of simian virus 40 T antigen with replicating nucleoprotein complexes of simian virus 40. J Virol. 1980 Aug;35(2):320–330. doi: 10.1128/jvi.35.2.320-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Salzman N. P. Late replicative intermediates are accumulated during simian virus 40 DNA replication in vivo and in vitro. J Virol. 1979 May;30(2):600–609. doi: 10.1128/jvi.30.2.600-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Mutants of simian virus 40 with base substitutions at the origin of DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):663–668. doi: 10.1101/sqb.1979.043.01.074. [DOI] [PubMed] [Google Scholar]
- Stahl H., Bauer M., Knippers R. The simian-virus-40 large-tumor antigen in replicating viral chromatin. A salt-resistant protein-DNA interaction. Eur J Biochem. 1983 Jul 15;134(1):55–61. doi: 10.1111/j.1432-1033.1983.tb07530.x. [DOI] [PubMed] [Google Scholar]
- Stahl H., Knippers R. Simian virus 40 large tumor antigen on replicating viral chromatin: tight binding and localization on the viral genome. J Virol. 1983 Jul;47(1):65–76. doi: 10.1128/jvi.47.1.65-76.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. Simian virus 40 DNA replication in isolated replicating viral chromosomes. J Virol. 1978 Oct;28(1):53–65. doi: 10.1128/jvi.28.1.53-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., DePamphilis M. L. Analysis of simian virus 40 chromosome-T-antigen complexes: T-antigen is preferentially associated with early replicating DNA intermediates. J Virol. 1983 Oct;48(1):281–295. doi: 10.1128/jvi.48.1.281-295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Andersen B., Shaw S. B., Wilson V. G. Alternative interactions of the SV40 A protein with DNA. Virology. 1981 Nov;115(1):75–87. doi: 10.1016/0042-6822(81)90090-8. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Trendelenburg M. F., Scheer U., Zentgraf H., Franke W. W. Heterogeneity of spacer lengths in circles of amplified ribosomal DNA of two insect species, Dytiscus marginalis and Acheta domesticus. J Mol Biol. 1976 Dec;108(2):453–470. doi: 10.1016/s0022-2836(76)80130-1. [DOI] [PubMed] [Google Scholar]
- Wilson V. G., Tevethia M. J., Lewton B. A., Tegtmeyer P. DNA binding properties of simian virus 40 temperature-sensitive A proteins. J Virol. 1982 Nov;44(2):458–466. doi: 10.1128/jvi.44.2.458-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Tevethia S. S. Transplantation immunity to simian virus 40-transformed cells in tumor-bearing mice. I. Development of cellular immunity to simian virus 40 tumor-specific transplantation antigens during tumorigenesis by transplanted cells. J Natl Cancer Inst. 1973 Jan;50(1):137–147. doi: 10.1093/jnci/50.1.137. [DOI] [PubMed] [Google Scholar]