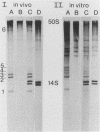
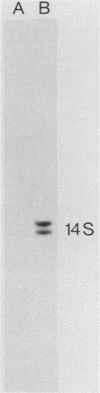
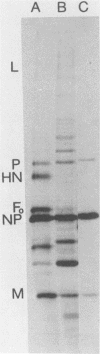
Abstract
A system for studying the in vitro replication of the genome RNAs of Sendai virus and its defective interfering particle DI-H has been developed. Cytoplasmic extracts of baby hamster kidney cells infected with wild-type Sendai virus or coinfected with wild-type Sendai virus plus DI-H were prepared after lysolecithin treatment at 12 h postinfection. The extracts supported the transcription of six viral mRNAs as well as the replication of the Sendai virus 50S (wild-type) and 14S DI-H genome RNAs and their encapsidation into nucleocapsids in the absence of de novo protein synthesis. RNA replication in vitro represented more than 50% of total RNA synthesis, a relative level higher than that found in the infected cell. The proteins required for Sendai virus RNA replication were present in a soluble protein pool at the time of extract preparation. Depletion of the protein pool by prior treatment of infected cells with cycloheximide inhibited subsequent in vitro genome replication without affecting transcription. The cytoplasmic extract may be separated by high-speed centrifugation into two components: the Sendai virus wild-type and DI-H nucleocapsid templates containing the RNA and associated NP, L, and P proteins and the soluble protein fraction containing primarily the P, NP, and M viral proteins with trace amounts of the L, HN, Fo, and nonstructural C proteins. The isolated intracellular DI-H nucleocapsid template alone cannot replicate its RNA, but when recombined with the Sendai virus soluble protein fraction it catalyzes the replication and encapsidation of viral RNAs. The initiation of RNA replication in vitro can be demonstrated because detergent-disrupted purified DI-H virions replicate both positive- and negative-strand RNAs in the presence, but not in the absence, of the soluble protein fraction from an extract of infected cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. The nature of the RNA products synthesized in vitro by subviral components of visicular stomatitis virus. Virology. 1976 May;71(1):230–241. doi: 10.1016/0042-6822(76)90108-2. [DOI] [PubMed] [Google Scholar]
- Amesse L. S., Kingsbury D. W. Sendai virus gene sequences identified by oligonucleotide mapping. Virology. 1982 Apr 15;118(1):8–16. doi: 10.1016/0042-6822(82)90314-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Wertz G. W. Synthesis of vesicular stomatitis virus negative-strand RNA in vitro: dependence on viral protein synthesis. J Virol. 1982 Mar;41(3):821–832. doi: 10.1128/jvi.41.3.821-832.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Bratt M. A. Ribonucleic acid polymerase in virions of Newcastle disease virus: comparison with the vesicular stomatitis virus polymerase. J Virol. 1971 Mar;7(3):389–394. doi: 10.1128/jvi.7.3.389-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Rao D. D., Lanman G. Defective interfering particles of vesicular stomatitis virus: structure-function relationships. Ann N Y Acad Sci. 1980;354:238–250. doi: 10.1111/j.1749-6632.1980.tb27970.x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. The molecular biology of paramyxoviruses. Med Microbiol Immunol. 1974;160(2-3):73–83. doi: 10.1007/BF02121714. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Bruschi A. Antigenomes in Sendai virions and Sendai virus-infected cells. Virology. 1975 Jul;66(1):185–191. doi: 10.1016/0042-6822(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976 Aug;8(4):547–555. doi: 10.1016/0092-8674(76)90223-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. II. Intracellular distribution of polypeptides. Virology. 1977 Sep;81(2):371–381. doi: 10.1016/0042-6822(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Initiation and replication of vesicular stomatitis virus genome RNA in a cell-free system. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3198–3202. doi: 10.1073/pnas.80.11.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A. Synthesis of message and genome RNAs in vitro by Sendai virus-infected cell nucleocapsids. J Gen Virol. 1982 May;60(Pt 1):67–75. doi: 10.1099/0022-1317-60-1-67. [DOI] [PubMed] [Google Scholar]
- Re G. G., Gupta K. C., Kingsbury D. W. Genomic and copy-back 3' termini in Sendai virus defective interfering RNA species. J Virol. 1983 Feb;45(2):659–664. doi: 10.1128/jvi.45.2.659-664.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S. Sendai virus RNA synthesis and nucleocapsid formation in the presence of cycloheximide. Virology. 1971 Jun;44(3):494–502. doi: 10.1016/0042-6822(71)90362-x. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Holder J. W., Means A. R., O'Malley B. W. Preparation and preliminary characterization of purified ovalbumin messenger RNA from the hen oviduct. Biochemistry. 1975 Jan 14;14(1):69–78. doi: 10.1021/bi00672a012. [DOI] [PubMed] [Google Scholar]
- Roux L., Holland J. J. Role of defective interfering particles of Sendai virus in persistent infections. Virology. 1979 Feb;93(1):91–103. doi: 10.1016/0042-6822(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Rubio C., Kolakofsky C., Hill V. M., Summers D. F. Replication and assembly of VSV nucleocapsids: protein association with RNPs and the effects of cycloheximide on replication. Virology. 1980 Aug;105(1):123–135. doi: 10.1016/0042-6822(80)90161-0. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Stone H. O., Kingsbury D. W., Darlington R. W. Sendai virus-induced transcriptase from infected cells: polypeptides in the transcriptive complex. J Virol. 1972 Nov;10(5):1037–1043. doi: 10.1128/jvi.10.5.1037-1043.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]