Abstract
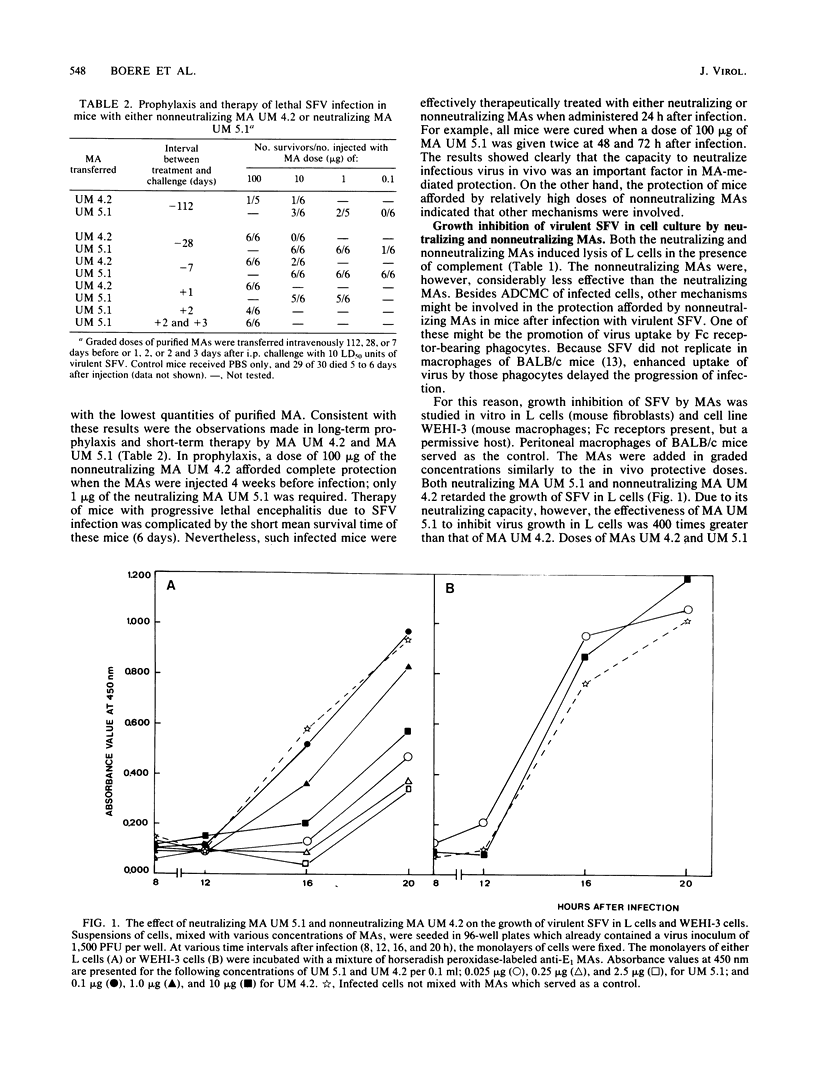
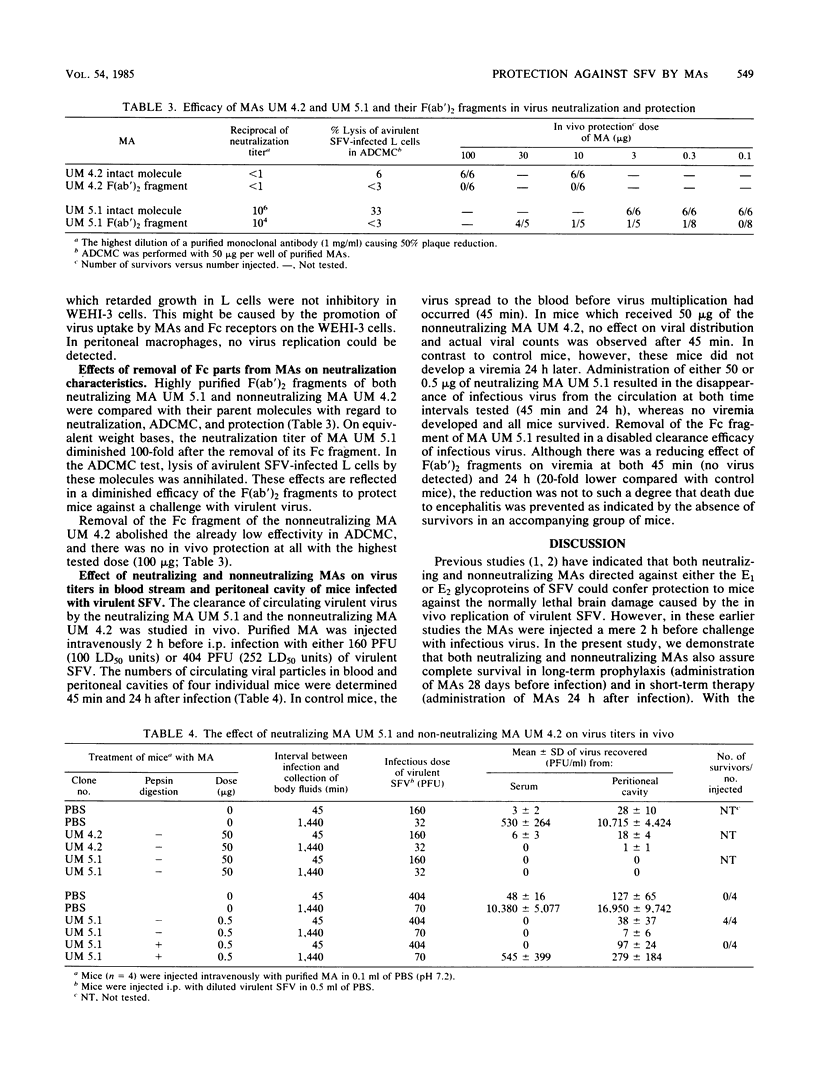
Both neutralizing and nonneutralizing immunoglobulin G2a monoclonal antibodies (MAs) directed against the E2 glycoprotein of Semliki Forest virus (SFV) protected mice prophylactically and therapeutically against virulent SFV infection. The neutralizing MAs, however, conferred protection to mice at lower doses than did nonneutralizing MAs. The antibody-dependent, complement-mediated cytolysis of SFV-infected L cells was effectuated by both kinds of antibodies, but again neutralizing MAs were more effective. Removal of the Fc part of the neutralizing MA UM 5.1 by pepsin digestion resulted in a 100-fold reduction of the neutralization titer (10(4) versus 10(6)) and a complete loss of its capacity to mediate antibody-dependent, complement-mediated cytolysis. Passive protection of infected mice occurred only after administration of relatively high doses of F(ab')2 of MA UM 5.1 (30.0 micrograms versus 0.1 microgram). F(ab')2 fragments prepared from the nonneutralizing MA UM 4.2 had lost their protective capacity completely. Surprisingly, the nonneutralizing MA UM 4.2 retarded virus growth in mouse fibroblasts (L cells), although inhibition was at much higher doses than with the neutralizing MA UM 5.1. Furthermore, both MAs promoted the uptake of virulent SFV in the Fc receptor-bearing WEHI-3 cells. The results suggest that nonneutralizing MAs protect mice not only by antibody-dependent, complement-mediated cytolysis but also by growth inhibition and enhanced uptake of SFV in the nonpermissive macrophages of BALB/c mice. This hypothesis is supported by the absence of viremia in recipients of nonneutralizing MA UM 4.2 at 24 h after infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boere W. A., Benaissa-Trouw B. J., Harmsen M., Kraaijeveld C. A., Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983 Jun;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- Boere W. A., Harmsen T., Vinjé J., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol. 1984 Nov;52(2):575–582. doi: 10.1128/jvi.52.2.575-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- Burstin S. J., Brandriss M. W., Schlesinger J. J. Infection of a macrophage-like cell line, P388D1 with reovirus; effects of immune ascitic fluids and monoclonal antibodies on neutralization and on enhancement of viral growth. J Immunol. 1983 Jun;130(6):2915–2919. [PubMed] [Google Scholar]
- Clegg J. C., Chanas A. C., Gould E. A. Conformational changes in Sindbis virus E1 glycoprotein induced by monoclonal antibody binding. J Gen Virol. 1983 May;64(Pt 5):1121–1126. doi: 10.1099/0022-1317-64-5-1121. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. Mechanisms of neutralization of animal viruses. J Gen Virol. 1984 Jun;65(Pt 6):1015–1022. doi: 10.1099/0022-1317-65-6-1015. [DOI] [PubMed] [Google Scholar]
- Evans D. M., Minor P. D., Schild G. S., Almond J. W. Critical role of an eight-amino acid sequence of VP1 in neutralization of poliovirus type 3. Nature. 1983 Aug 4;304(5925):459–462. doi: 10.1038/304459a0. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Bampton M., Gordon S. Macrophage activation selectively enhances expression of Fc receptors for IgG2a. J Exp Med. 1983 Feb 1;157(2):807–812. doi: 10.1084/jem.157.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., SELL S. THE IMMUNOGLOBULINS OF MICE. V. THE METABOLIC (CATABOLIC) PROPERTIES OF FIVE IMMUNOGLOBULIN CLASSES. J Exp Med. 1965 Jul 1;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B., Wust C. J., Brown A. Antibody-dependent, complement-mediated homologous and cross-cytolysis of togavirus-infected cells. J Immunol. 1977 Oct;119(4):1289–1292. [PubMed] [Google Scholar]
- Kraaijeveld C. A., Harmsen M., Khader Boutahar-Trouw B. Cellular immunity against Semliki Forest virus in mice. Infect Immun. 1979 Feb;23(2):213–218. doi: 10.1128/iai.23.2.213-218.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijeveld C. A., Harmsen M., Khader Boutahar-Trouw B. Delayed-type hypersensitivity against Semliki Forest virus in mice. Infect Immun. 1979 Feb;23(2):219–223. doi: 10.1128/iai.23.2.219-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel B. Neutralization of animal viruses. Adv Virus Res. 1978;23:205–268. doi: 10.1016/S0065-3527(08)60101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Topographical analysis of viral epitopes using monoclonal antibodies: mechanism of virus neutralization. Virology. 1981 Nov;115(1):20–32. doi: 10.1016/0042-6822(81)90085-4. [DOI] [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J Immunol. 1982 Dec;129(6):2763–2767. [PubMed] [Google Scholar]
- Peiris J. S., Porterfield J. S., Roehrig J. T. Monoclonal antibodies against the flavivirus West Nile. J Gen Virol. 1982 Feb;58(Pt 2):283–289. doi: 10.1099/0022-1317-58-2-283. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Kelsoe G. H., Sachs D. H. Induction of immune responses with anti-idiotypic antibodies: implications for the induction of protective immunity. Springer Semin Immunopathol. 1983;6(1):79–97. doi: 10.1007/BF01857368. [DOI] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]
- van Tiel F. H., Boere W. A., Vinjé J., Harmsen T., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Detection of Semliki Forest virus in cell culture by use of an enzyme immunoassay with peroxidase-labeled monoclonal antibodies specific for glycoproteins E1 and E2. J Clin Microbiol. 1984 Sep;20(3):387–390. doi: 10.1128/jcm.20.3.387-390.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


