Abstract
ABCG2 is an ATP-binding cassette half-transporter conferring resistance to chemotherapeutic agents such as mitoxantrone, irinotecan, and flavopiridol. With its one transmembrane and one ATP-binding domain, ABCG2 is thought to homodimerize for function. One conserved region potentially involved in dimerization is a 3-amino acid sequence in transmembrane segment 5 (residues 552–554). Mutations in the corresponding residues in the Drosophila white protein (an orthologue of ABCG2) are thought to disrupt heterodimerization. We substituted glycine 553 with leucine (G553L) followed by stable transfection in HEK 293 cells. The mutant was not detectable on the cell surface and markedly reduced protein expression levels were observed by immunoblot. A deficiency in N-linked glycosylation was suggested by reduction in molecular weight compared to the 72-kDa wild type ABCG2. Similar results were observed with the G553E mutant. Confocal microscopy demonstrated mostly ER localization of the G553L mutant in HEK 293 cells, even when coexpressed with the wild type protein. Despite its altered localization, the G553L and G553E mutants were cross-linked using amine-reactive cross-linkers with multiple arm lengths, suggesting that the monomers are in close proximity but unable to complete normal trafficking. Interestingly, when expressed in Sf9 insect cells, G553L traffics to the cell membrane but is unable to hydrolyze ATP or transport the Hoechst dye. Still, when coexpressed, the mutant interferes with the Hoechst transport activity of the wild type protein. These data show that glycine 553 is important for protein trafficking and are consistent with, but do not yet prove, its involvement in ABCG2 homodimerization.
The ATP-binding cassette1 (ABC) transporter family, with 48 known human members classified in seven subgroups, constitutes one of the largest families of membrane transporters present in all species (1). These proteins are involved in the energy-dependent transport of a wide variety of substrates and play an important role in numerous genetic disorders (2), a well-characterized example of which is cystic fibrosis, caused by mutations of the cystic fibrosis transmembrane conductance regulator (CFTR/ABCC7) protein (3). ABC transporters are also thought be responsible for the so called multidrug resistance phenomenon in antimicrobial (4) and anticancer (5) therapy; the most extensively studied example of the latter is resistance attributed to P-glycoprotein (P-gp/ABCB1)(6). ABCG2, also called BCRP/MXR/ABCP, is a member of the G subfamily of human ABC transporters, and has been demonstrated to confer resistance to multiple cancer chemotherapeutic agents, such as mitoxantrone, flavopiridol, methotrexate, and the camptothecin derivatives SN-38 and topotecan.(7–10) In addition to a potential role in cancer chemotherapy resistance, ABCG2 is expressed in the placenta, liver, small intestine, colon, blood-brain barrier, and in stem cells, suggesting a physiologic role in protection against xenobiotics.(11)
The canonical structure of a functional ABC transporter, exemplified by P-gp and CFTR, consists of two transmembrane domains (TMDs), typically with twelve transmembrane α-helices, and two nucleotide-binding domains (NBDs) (12). In contrast, members of the G subfamily are comprised of only one transmembrane domain and one nucleotide-binding domain and are considered half-transporters thought to either homo- or heterodimerize to generate functional transporters. Interestingly, the domain organization of the G subfamily (ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8) is reversed compared to most other human ABC transporters, having an N-terminal NBD and C-terminal transmembrane helices. ABCG2 is presumed to form a homodimer to function, though the mechanism of this process is largely unknown. Chimeric fusion proteins containing two ABCG2 monomers either with or without a flexible linker peptide were shown to be functional (13) supporting the idea of homodimer formation. In addition, coimmunoprecipitation experiments using two different tags on the ABCG2 monomers also suggested homodimer formation (31). A disulfide bridge formed between cysteines 603 of the monomers in the predicted extracellular loop connecting TM5 and TM6 was described as non-essential for trafficking and transport (14). Unlike ABCG2, ABCG5 and ABCG8 are obligate heterodimers (15) in forming a sterol transporter, mutations of which result in sitosterolemia (16). There is evidence suggesting that ABCG1 and ABCG4 can form both homo- and heterodimers (17) and are also candidates for lipid/sterol transport (18). Examples of other ABC half-transporter homodimers include the bacterial transporters MsbA (19) and LmrA (20). MsbA gives an interesting insight into how a homodimer is formed, given that high resolution crystal structures from both Escherichia coli (19) and Vibrio cholerae (21) are available.
One of the most widely studied groups of non-human ABC half-transporters is the Drosophila white subfamily, an orthologue of the human ABCG subfamily. The Drosophila white and scarlet proteins heterodimerize to form a tryptophan transporter responsible for brown eye color, while the white and brown heterodimer transports guanine, the precursor of red eye pigment. Mutations in amino acids 588–590 in TM5 of the white protein were shown to significantly reduce red pigment levels, presumably by disrupting the white-brown heterodimer, with a lesser effect on brown pigments (22). In the present study, we report that mutating amino acid 553 in TM5 of ABCG2, a well-conserved residue corresponding to glycine 589 of the Drosophila white protein, disrupts function and trafficking, implying a similar role in the dimerization of the human transporter.
Experimental Procedures
Cell Culture
Human embryonic kidney (HEK) 293 cells (ATCC, Manassas, VA) were maintained in Minimal Essential Medium (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (Invitrogen), 2 mM glutamine (BioFluids, Rockville, MD), and 100 units/l penicillin/streptomycin (BioFluids) at 37° C in 5% CO2. Transfected cell lines were grown in 2 mg/ml G418 (Invitrogen).
Mutagenesis
The G553L and G553E mutants were generated by site-directed mutagenesis in the pcDNA3.1/Myc-HisA(-)vector (Invitrogen) as previously described(23). The mutations were confirmed by sequencing the vectors initially and then cDNA from HEK 293 cells stably transfected with the mutants was also sequenced for full length ABCG2.
Transfection
Stable transfectants were generated in HEK 293 cells as previously described. Transfections were performed using TransFast transfection reagent (Promega, Madison, WI). Colonies were selected in 2 mg/ml G418 with frequent removal of dead cells and were expanded prior to study. Cells previously transfected with wild type ABCG2 were used as controls (24)
Membrane preparation, PNGase F treatment
Microsomal membrane preparation was performed as described previously (23). Briefly, cells were disrupted by nitrogen cavitation (Parr Instrument, Moline, IL) in a hypotonic lysis buffer and membranes were obtained by ultracentrifugation at 40,000 RPM. For treatment with the PNGase F enzyme, the Glyko® N-Glycanase® kit was used (ProZyme, San Leandro, CA). 100 μg of membranes were incubated with 3 μl PNGase F overnight at 37° C followed by immunoblotting as described below.
Immunoblotting
Immunoblotting was performed as previously described (23). Briefly, microsomal membrane proteins were loaded onto precast 7.5% (w/v) SDS-polyacrylamide gels (BioRad, Hercules, CA), subjected to electrophoresis, and electrotransfered onto PVDF membranes (Millipore, Bedford, MA). Blots were probed with a 1:250 dilution of the monoclonal anti-ABCG2 antibody BXP-21 (Kamiya Biomedical, Seattle, WA) and visualized with ECL (Amersham). Membranes were stained with 0.1% Ponceau S (Sigma, St. Louis, MO) and checked for comparable loading.
Northern blotting
RNA was extracted from cells using RNA STAT-60 (Tel-Test Inc., Friendswood, TX) according to the manufacturer’s instructions. Northern blot analysis was performed by standard methods. Labeling of cDNAs was accomplished using Riboprobe in Vitro Transcription Systems (Promega). To compare the quality and quantities of RNA, 20 μg total RNA were electrophoretically separated in a 1% agarose, 6% formaldehyde gel and transferred onto a nitrocellulose membrane. Gels were stained with ethidium bromide and checked for comparable loading. Northern blot labeling was performed using a riboprobe generated from the first 662 bp of ABCG2 subcloned in a pCRII-TOPO vector (Invitrogen).
Cross-linking
Chemical cross-linking was performed in vivo on intact cells (25). After incubation at room temperature for 3 minutes with the cross-linking agents m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS), (Pierce, Rockford, IL) or disuccinimidyl glutarate (DSG), (Pierce) at 1 mM final concentration, the reaction was terminated by the addition of Tris–HCl (pH 8) to 20 mM and cells were immediately harvested as described above.
Flow cytometry
Flow cytometry with the anti-ABCG2 antibody, 5D3 (eBioscience, San Diego, CA) was performed as previously described (23). Briefly, cells were trypsinized and resuspended in DPBS with 2% bovine serum albumin (BSA) to which phycoerythrin-conjugated 5D3 or phycoerythrin-conjugated mouse IgG was added. The cells were incubated with antibody for 30 min at room temperature, washed twice with DPBS and kept in the dark until analyzed.
Immunofluorescence
Immunofluorescence studies were performed as previously described (23). Briefly, cells were cultured for 3 days followed by fixation with 4% paraformaldehyde and permeabilization with pre-chilled (−20 °C) methanol. After blocking in a buffer containing 2 mg/ml BSA, 0.1% Triton-X 100, and 5% goat serum, samples were incubated with a 1:100 dilution of the mouse monoclonal anti-ABCG2 antibody, BXP-21 (Kamiya Biomedical) and with a 1:1000 dilution of the rabbit polyclonal anti-calnexin antibody (Abcam, Cambridge, MA) for 1 h at room temperature.
Generation of GFP-tagged ABCG2
N-terminally GFP-tagged wild type (wt) ABCG2 and ABCG2-G553L constructs were generated by cloning the XhoI-BamHI fragment of pAcUW21-L/wtABCG2 or pAcUW21-L/ABCG2-G553L into the corresponding site of pEGFP vector (Clontech, Mountain View, CA). HEK 293 cells were transfected with the pEGFP-wtG2 or pEGFP-G553L vectors using the FuGene reagent (Roche, Indianapolis, IN). 72 hours after transfection, cells were either natively analyzed for GFP fluorescence using an Olympus IX70 Laser Scanning Microscope or immunostained with the BXP-21 antibody. Stable cell lines were generated by selecting the transfected cells in 0.5 mg/ml G418 (Invitrogen)
Generation of Sf9 cells expressing wild type, K86M or G553L mutants
Generation of transfer vectors containing wt ABCG2 or K86M were described earlier (26; 27). The transfer vector carrying the G553L mutant was generated by cloning the SacI fragment of the pcDNA 3.1/G553L into the corresponding site of the pAcUW21-L vector. Recombinant baculoviruses carrying the different human ABCG2 cDNAs were generated with the BaculoGold Transfection Kit (Pharmingen, San Diego, CA) according to the manufacturer’s instructions. Sf9 (Spodoptera frugiperda) cells were infected and cultured as described earlier (28)
Individual virus clones, expressing high levels of the different human ABCG2 variants, were obtained by end point dilution and subsequent amplification. ABCG2 protein expression was determined by immunoblotting and immunoflow cytometry.
Membrane Preparation and Immunodetection of ABCG2 in Sf9 cells
After 3 days of virus infection, Sf9 cells were harvested and membranes were isolated. Membrane protein concentrations were determined by the modified Lowry method as previously described (28). Immunoblotting was performed as described for the HEK 293 cells with a 1:2000 dilution of the monoclonal BXP-21 antibody.
Flow cytometry was performed by labeling the Sf9 cells at 37°C using a final concentration of 1 μg/ml of the anti-ABCG2 monoclonal antibody 5D3. Binding was visualized by the addition of a secondary phycoerythrin-conjugated anti-mouse IgG (Immunotech, Marseille, France) at a final concentration of 1 μg/ml. Flow cytometric determination of the antibody reaction was carried out using a FACSCalibur cytometer at 488-nm excitation and 585/42-nm emission wavelengths.
Hoechst 33342 Dye Accumulation Assay
To study the function of wt ABCG2 when coexpressed with the G553L mutant, 4 × 106 Sf9 cells were transfected with the combination of different volumes of recombinant baculoviruses (as indicated in Figure 9) containing wt ABCG2, G553L, K86M or β-galactosidase. After 40 hours of transfection, accumulation of Hoechst dye was measured in a fluorescence spectrophotometer at 350 nm (excitation)/460 nm (emission), by using 5× 105 Sf9 cells in 2 ml of HPMI (120 mM NaCl, 5 mM KCl, 400 _M MgCl2, 40 _M CaCl2, 10 mM HEPES, 10 mM NaHCO3, 10 mM glucose, and 5 mM Na2HPO4) solution. Cells were preincubated at 37°C in HPMI for 4 min and further incubated with 1 μM Hoechst dye for 10 min. Subsequently, the inhibitor Ko143 (1 μM) was added to the cells. The increase in cellular fluorescence was determined in the presence of Ko143 completely inhibiting the transport function of ABCG2 (F100) and in the absence of any inhibitor (F0). The dye transport activity of ABCG2 was calculated as follows: (F100 −F0)/F100 * 100.
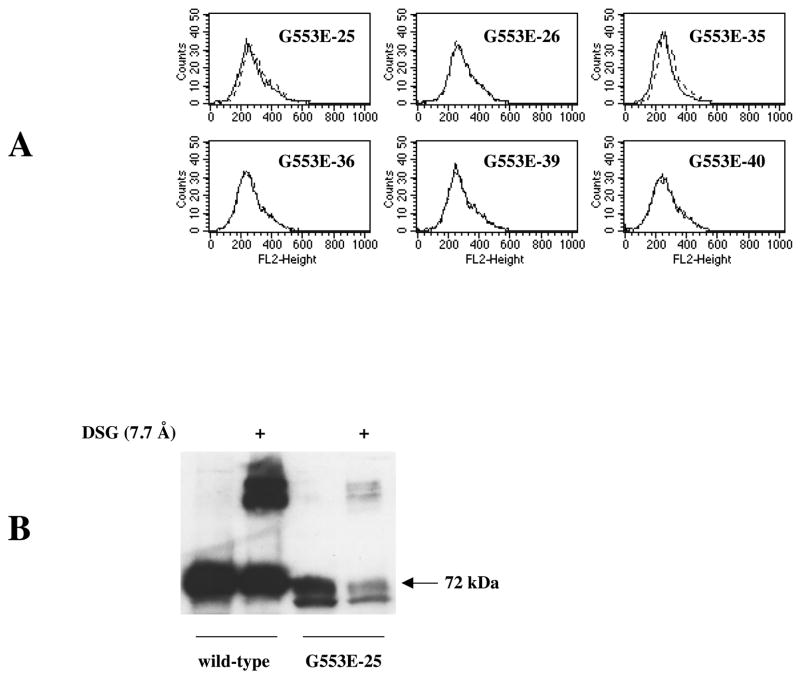
Figure 9. Surface expression of the G553E mutant in HEK 293 cells and cross-linking with DSG.
A: The G553E mutant (six clones are shown) is not detectable on the cell surface with the 5D3 antibody by flow cytometry performed as described in Figure 2, negative control antibody (solid line) or 5D3 antibody (dashed line). B: Cross-linking (as detailed in Figure 5) is observed following treatment of transfected HEK 293 cells with DSG in case of both the wild type (50 μg protein) and the G553E mutant (100 μg protein).
ATP hydrolysis
Sf9 membranes containing wild type ABCG2, G553L or K86M were harvested, membranes were isolated and stored at −80°C according to Sarkadi et al. (29). ATPase activity was measured as described previously by determining the liberation of inorganic phosphate from ATP with a colorimetric reaction (30).
Results
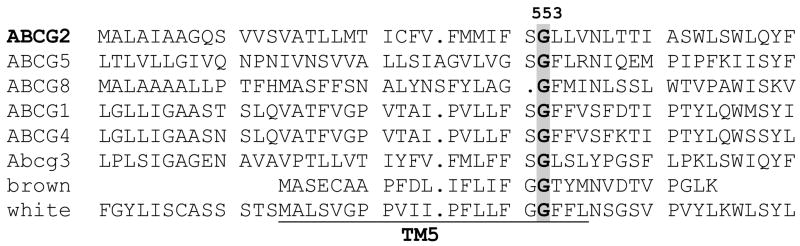
ABCG2 is a half-transporter associated with multidrug resistance that is widely thought to homodimerize for function. In the present study we examined the potential role of glycine 553 in TM5 of ABCG2 in the dimerization process, given that the corresponding residue in the Drosophila white protein is presumed to be important in the heterodimerization of this ABCG2 orthologue (22). Figure 1 represents an alignment for TM5 of the members of the human ABCG subfamily as well as for the Drosophila white and murine Abcg3 proteins with a well-conserved glycine at position 553 in ABCG2. In the Drosophila white protein mutations to the residues neighboring this glycine are also known to interfere with dimerization (22) and are highly conserved as demonstrated by this alignment.
Figure 1. Sequence alignment for members of the ABCG subfamily.
Glycine 553 in TM5 of ABCG2 is well conserved in the human G subfamily, the murine Abcg3, and the Drosophila white and brown proteins.
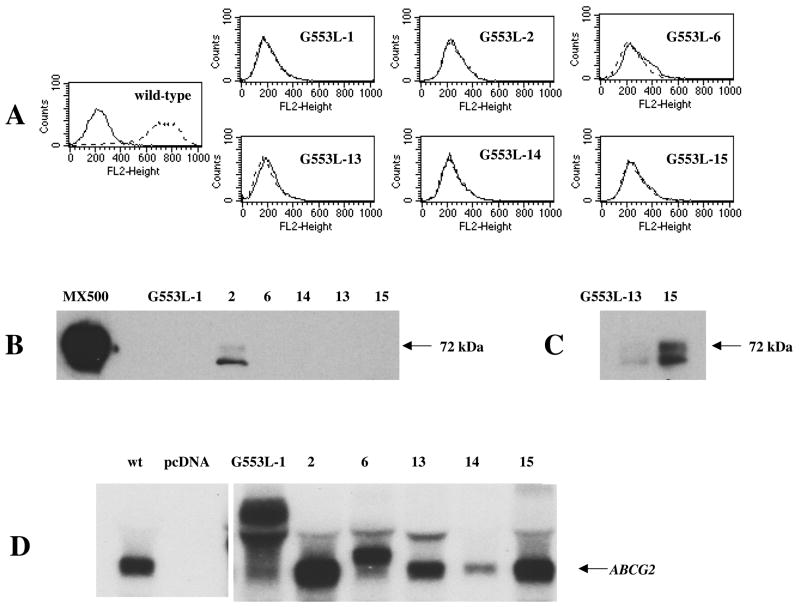
To begin characterization of the residue, HEK 293 cells were stably transfected with ABCG2 carrying a glycine to leucine substitution at amino acid 553 (G553L) using a pcDNA3 vector. We selected leucine because it is, like glycine, a neutral amino acid. As an initial screening step, flow cytometry with the 5D3 antibody recognizing an extracellular epitope of ABCG2 (31) was performed on 24 clones, which, unlike the wild type protein, revealed no surface expression for the G553L mutant (Figure 2A, 6 of the 24 clones shown). Next, immunoblotting with the monoclonal anti-ABCG2 antibody BXP-21 was performed, demonstrating a very low protein expression level in three of the clones (Figure 2B and 2C). As shown in Figure 2D, RNA levels on Northern blot for 5 of the 6 clones were equal to, or even considerably higher than RNA levels of the wild type transfectant. For clones #1 and #6 higher than expected molecular weight RNA was seen, most probably due to alternate insertion sites.
Figure 2. Surface expression, protein and RNA levels of the G553L mutant transfected to HEK 293 cells.
A: Flow cytometry with the 5D3 surface antibody for six of the G553L clones. Stably transfected HEK 293 cells were incubated for 30 min in phycoerythrin-labeled negative control antibody (solid line) or 5D3 antibody (dashed line). The G553L mutant is not detectable on the cell surface while the wild type protein is localized to the surface. B: Membrane proteins from the same G553L clones (25 μg/lane) were separated by SDS/PAGE, transferred onto a PVDF membrane, and probed with the monoclonal anti-ABCG2 antibody BXP-21. Membranes from the mitoxantrone selected SF295/MX500 cell line with high levels of ABCG2 expression used as a positive control (4 μg/lane). (10 min exposure) C: Overnight exposure of the blot shown in part B. D: Northern blot showing RNA levels of the same clones compared to wild type (wt) and empty vector transfected cells (pcDNA). Total RNA (20 μg/lane) from each transfectant was electrophoresed and transferred to a nitrocellulose membrane. The membrane was hybridized with a riboprobe generated from the first 662 bp of ABCG2.
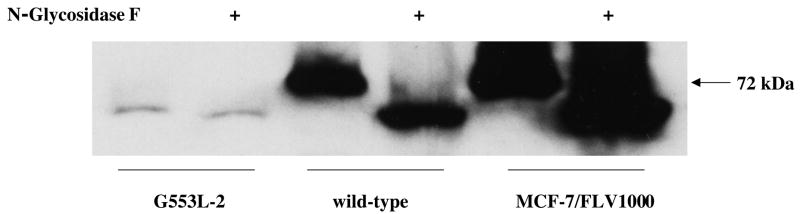
As illustrated in Figure 2B and 2C, the G553L mutant was represented by a double band on immunoblot with the majority of the protein running lower than the normal 72 kDa molecular weight. In order to investigate whether this lower molecular weight band was representative of the non-glycosylated protein, isolated membranes from cells bearing wild type or mutant vectors were treated with the PNGase F enzyme to remove N-linked glycans. In case of the G553L mutant, no significant shift in molecular weight was observed after PNGase F treatment (Figure 3) indicating that, indeed, the G553L mutant is underglycosylated, while a clear shift to a lower molecular weight band was seen in membranes extracted from HEK 293 cells transfected with wild type ABCG2 or from flavopiridol-selected MCF-7 cells used as controls (25).
Figure 3. N-Glycosydase F treatment.
100 μg of membranes were incubated with 3 μl PNGase F overnight at 37 ° C followed by SDS/PAGE separation and immunoblotting with the BXP-21 monoclonal anti-ABCG2 antibody, resulting in a clear shift to a smaller molecular weight band in the wild type ABCG2 transfected and the flavopiridol selected MCF-7 control cell lines (MCF-7/FLV1000), while there is now significant shift in case of the G553L mutant.
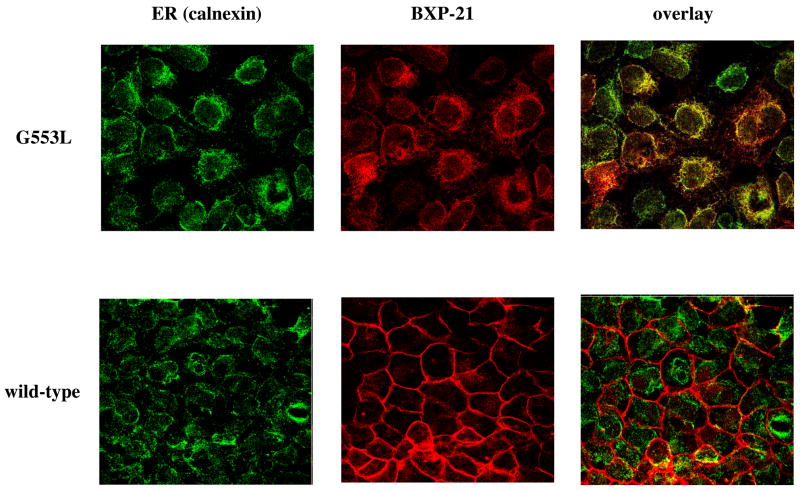
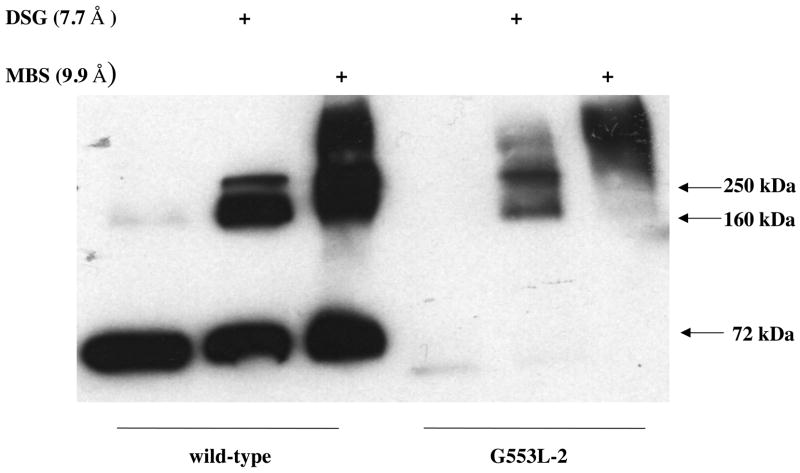
Since the G553L mutant did not demonstrate normal surface localization by flow cytometry with the 5D3 antibody, imunofluorescence studies were carried out with the BXP-21 antibody on the clone with the highest expression level (clone #2 in Figure 2B). Figure 4 demonstrates that wild type ABCG2 localizes to the cell membrane, while the G553L mutant is predominantly intracellular. Parallel staining with the anti-calnexin monoclonal antibody revealed that the G553L mutant colocalizes with the endoplasmic reticulum (ER) chaperone, calnexin. These results suggest that the G553L mutant is not able to complete normal folding or processing to traffic to the cell surface in mammalian cells. These findings correspond to data obtained with the ABCG5 and ABCG8 half-transporters; these proteins have been shown to require heterodimerization in order to leave the endoplasmic reticulum and traffic to the cell surface (32). To investigate whether mutating glycine 553 in ABCG2 leads to retention in the ER due to interference with homodimerization, cross-linking experiments serving as indirect measures of dimerization were performed. Unexpectedly, using the homobifunctional amine-reactive cross-linkers, MBS (9.9 Å arm length) and DSG (7.7 Å arm length) on intact cells the G553L mutant could be cross-linked as indicated by the appearance of higher molecular weight bands corresponding to dimers and higher order multimers of the 72 kDa ABCG2 monomer (Figure 5). The same results were achieved with DSS (11.4 Å) and under non-reducing conditions (data not shown). In the cross-linking experiments presented in this manuscript (Figure 5 and Figure 9/B) a stronger signal was observed on immunoblot in the samples treated with the cross-linking agents compared to the non-treated ones. This phenomenon has not been explained, although, it has been reported by other investigators and in our previous studies as well (21) (33)
Figure 4. Localization of the G553L mutant in HEK 293 cells.
Confocal microscopy of stably transfected HEK 293 reveals that the G553L mutant colocalizes with the ER marker calnexin, while the wild type protein localizes to the cell surface. Cells were cultured for 3 days followed by fixation with paraformaldehyde and permeabilization with methanol. Staining was performed for 1 hour at room temperature with the BXP-21 monoclonal anti-ABCG2 antibody (red) and the anti-calnexin polyclonal antibody (green).
Figure 5. Cross-linking studies.
Cross-linking was performed on intact cells at room temperature for 3 minutes with the cross-linking agents m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS), or disuccinimidyl glutarate (DSG), followed by membrane preparation, SDS/PAGE separation and immunoblotting with the BXP-21 anti-ABCG2 antibody. Cross-linking is observed with both amine-reactive cross-linkers in HEK 293 cells transfected with either wild type ABCG2 or the G553L mutant as suggested by dimers and higher order multimers. The molecular weight of monomeric wild type ABCG2 is 72 kDa.
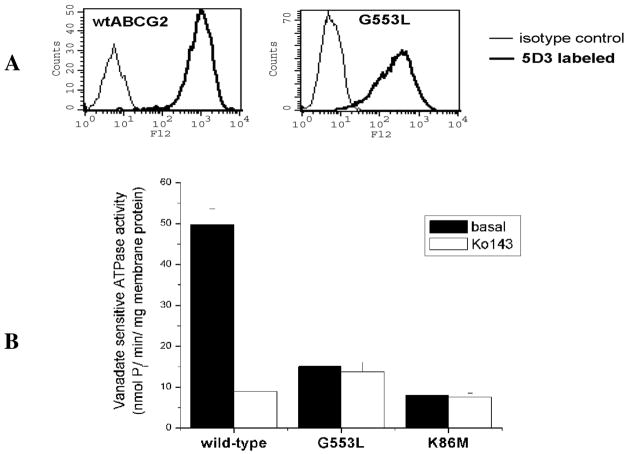
As the lower protein expression levels seen on immunoblot and the lack of surface expression might be the result of abnormal folding and/or dimerization, using the baculovirus heterologous expression system, we expressed the G553L mutant in Sf9 insect cells (Spodoptera frugiperda ovarian cells), which might be more tolerant of an aberrant protein and typically yields higher levels of protein expression (34). Interestingly, we found that the G553L mutant migrates to the cell surface in the insect cells as demonstrated by flow cytometry with the 5D3 antibody (Figure 6A). Despite its normal localization in Sf9 cells, the mutant exhibited no significant ability to hydrolyze ATP. The ATPase activity comparable to that of the nonfunctional K86M mutant (26) (Figure 6B).
Figure 6. The G553L mutant in Sf9 insect cells.
A: Wild type ABCG2 and the G553L mutant are detectable on the surface of Sf9 cells with the 5D3 monoclonal anti-ABCG2 antibody on flow cytometry (as detailed in Figure 2). B: The G553L mutant displays similar basal ATPase activity as the non-functional K86M mutant in Sf9 membranes. ATPase activity was measured determining the liberation of inorganic phosphate from ATP with a colorimetric reaction, Ko143 is a specific inhibitor of ABCG2.
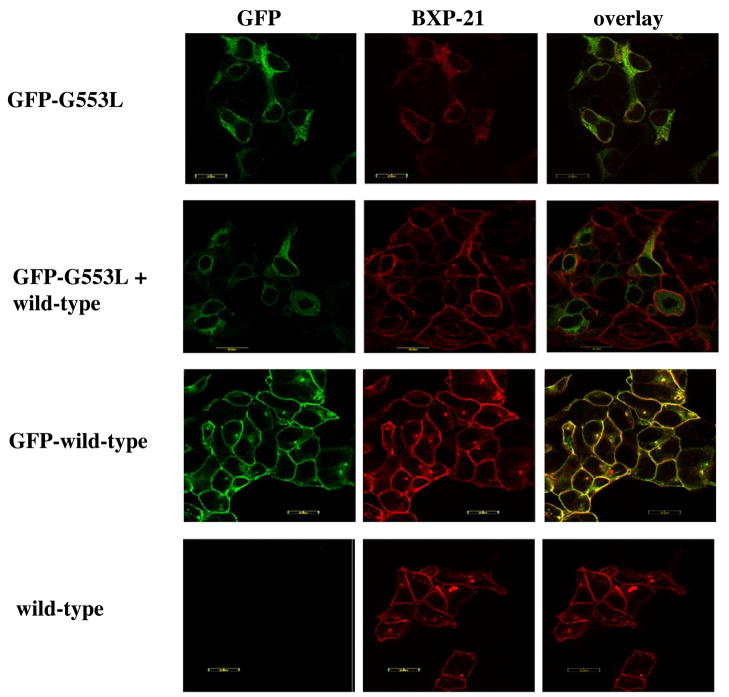
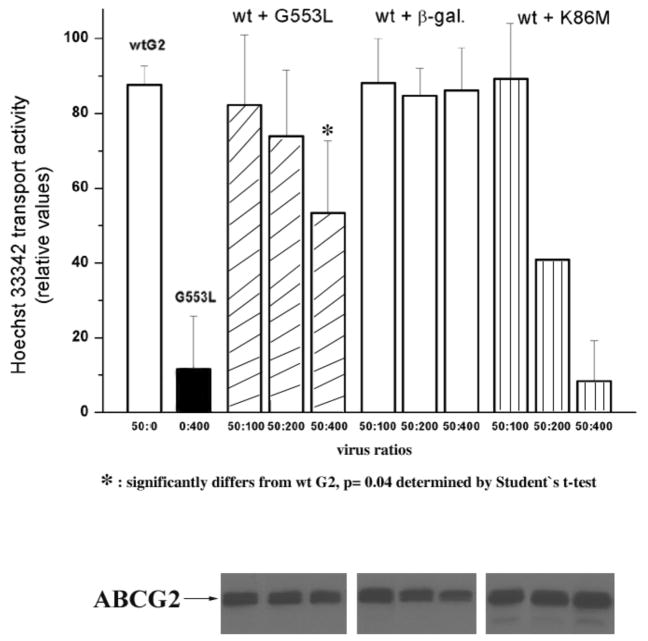
To further investigate whether the G553L mutation interferes with dimerization, we transiently expressed a GFP-tagged G553L mutant together with the wild type protein in HEK 293 cells and performed confocal microscopy. We reasoned that if the 553 mutation interfered with function rather then dimerization, the wild type protein could “rescue” the mutant and bring it to the cell surface. As shown in Figure 7, the GFP-tagged G553L mutant is localized to the ER as before and the non-tagged wild type protein trafficked to surface, suggesting that the mutant was unable to form competent dimers with the wild type protein. Coexpression studies were also performed in Sf9 cells. Figure 8 represents Hoechst 33342 transport activities for Sf9 cells coinfected with a constant amount of recombinant baculovirus carrying wild type ABCG2 together with varying amounts of the G553L and K86M mutants. In case of the 50:400 ratio, which represents approximately equal protein expression levels on immunoblot for the wild type and the G553L mutant (data not shown), a roughly 35% decrease in transport activity is seen and suggests some interaction between the wild type and mutant proteins. However, the impact is much less than observed with K86M, a mutation in Walker A that has been reported to retain dimerization with a functional dominant negative effect. (35)
Figure 7. Coexpression of wild type ABCG2 and the G553L mutant in HEK 293 cells.
Confocal microscopy of HEK 293 cells transiently transfected with either the GFP-tagged G553L mutant (first row) or the GFP-tagged wild type (third row) or cotransfected with the GFP-tagged mutant and non-tagged wild type (second row) is presented. 72 hours after transfection, cells were either natively analyzed for GFP fluorescence or immunostained with the BXP-21 antibody (shown in red). The cotransfection images (second row) show that despite the presence of the wild type protein the G553L mutant is retained intracellularly, while the wild type protein localizes to the cell surface. A control transfection with non-tagged wild type ABCG2 is also shown (bottom row).
Figure 8. Hoechst transport assay in Sf9 cells.
Sf9 cells were infected with a combination of the indicated volumes of recombinant baculoviruses containing wild type ABCG2, G553L, K86M or β-galactosidase. Hoechst fluorescence was measured in a fluorescence spectrophotometer 40 hours post transfection. The increase in cellular fluorescence was determined in the presence of the ABCG2 inhibitor Ko143 completely inhibiting the transport function of ABCG2 (F100) and in the absence of any inhibitor (F0). The dye transport activity of ABCG2 was calculated as follows: (F100 −F0)/F100 * 100. Each column represents the average of 3 measurements (except for the 50:200 wt + K86M, where only one measurement was performed). An immunoblot showing expression levels with the BXP-21 monoclonal anti-ABCG2 antibody is shown at the bottom of the figure (bands on the immunoblot are aligned with the corresponding columns of the Hoechst transport activity assay). The G553L mutant shows no transport of Hoechst 33342 (second column) and at the 50:400 ratio (fifth column), representing approximately equal protein expression levels for the mutant and the wild type, results in a 35% decrease in activity. While, in case of the K86M mutant the same ratio (last column) almost completely abrogates Hoechst transport.
Evaluating the effect of a charged residue at the 553 site, we replaced the glycine with glutamic acid (G553E) and transfected HEK 293 cells. In case of the Drosophila white protein, the corresponding, X-ray induced G589E mutation was described as presumably interfering with heterodimerization (22). Figure 9 shows, that, similarly to the leucine mutant, the G553E mutant did not traffic to the cell surface as verified by no staining with the 5D3 surface antibody on flow cytometry (Figure 9A). However, this mutant could also be cross-linked with the amine-reactive cross-linking agent DSG, which has a 7.7 Å arm length, demonstrated by the appearance of higher order multimers on immunoblot. (Figure 9B). The G553E mutant, like the G553L mutant, is represented by a double band on immunoblot, suggestive of impaired glycosylation, and in fact, following treatment with PNGase F only one lower molecular weight band was visible on immunoblot (data not shown).
Discussion
Composed of only one ATP-binding domain and one transmembrane domain, ABCG2 is considered a half-transporter and is presumed to function as a homodimer. In the present study, we have explored the potential role of a conserved glycine in the 5th TM helix near the extracellular surface in ABCG2 dimerization. We found that substitution of glycine 553 with either leucine (G553L) or glutamic acid (G553E) followed by transfection into HEK 293 cells resulted in markedly reduced protein expression with impaired glycosylation and predominant ER retention. In SF9 insect cells the G553L mutant trafficked to the cell surface but, nevertheless, was unable to hydrolyze ATP. Although the wild type protein was unable to rescue the mutant from its ER localization in the mammalian cells, chemical cross-linking studies indicated that the two G553L monomers are in close proximity. When Sf9 insect cells were coinfected with the G553L mutant and the wild type protein, the Hoechst transport activity was reduced by approximately 35%. Taken together, these studies suggest that this residue, understood to impair dimerization in the Drosophila, is critical for normal trafficking and positioning of the protein on the cell surface.
ABCG2 belongs to the G subfamily of human ABC transporters, members of which, like half-transporters from other species, are generally accepted to either homo- or heterodimerize. Overall, little is known about the exact mechanism and residues involved in the dimerization process. Accurate structural information could be obtained from a high-resolution crystal structure, but crystallization has so far proven to be very challenging for integral membrane proteins. To date, the crystal structure of only a small number of such proteins is available, including three ABC transporters from bacteria that have been crystallized with their transmembrane domains: the lipid A transporter MsbA from E. coli and from V. cholerae, and the vitamin B12 transporter BtuCD from E. coli (19; 21; 36). Unlike BtuCD with its twenty transmembrane segments, MsbA is similar to ABCG2 in being a half-transporter with six transmembrane helices, although its domain organization is reversed when compared to ABCG2.
In the case of the human ABCG subfamily, the ABCG5 and ABCG8 heterodimer is the most extensively characterized. There is a strong line of evidence suggesting that these proteins are obligate heterodimers (15). The genes encoding ABCG5 and ABCG8 are located head-to-head on chromosome 2, have similar expression profiles and mutations in either of them result in sitosterolemia. Furthermore, expression of both proteins is required for trafficking to the cell surface. Dimer formation is thought to take place in the ER and is required for surface expression (32; 37). Misfolded proteins and subunits of oligomeric complexes that fail to properly assemble are known to be retained in the ER and targeted for degradation. The fact that the ABCG2 G553L mutant protein does not leave the ER in mammalian cells suggests that it is recognized by the ER’s quality control system and is degraded, explaining the observed low expression levels (38). This finding is consistent with a failure to dimerize but could also be the result of improper folding, although the mutant’s ability to cross-link, its cell surface localization in insect cells, and the interference with wild type function argue against major defects in conformation.
In addition to the above-mentioned aberrant localization in the mammalian cells, the glycosylation pattern of the G553L and G553E mutants was also altered. Interestingly, N-linked glycosylation of only ABCG8 is essential for trafficking of the ABCG5/ABCG8 dimer from the ER to the plasma membrane, though ABCG5 was also found to be glycosylated (37). In case of ABCG2, previous studies have shown that the protein is glycosylated at asparagine 596 and that the glycosylation is not necessary for cell surface localization (39) or for function, suggesting that the G553L mutant is not retained in the ER merely due to failure to undergo glycosylation.
The fact that we found no evidence of colocalization on confocal microscopy when the G553L mutant was coexpressed with the wild type ABCG2 in HEK 293 cells suggests that there is no stable interaction between wild type and mutant proteins in the ER of mammalian cells, as would be expected if the wild type and mutant formed stable dimers. While the two proteins may not form a stable dimer, they may come in contact (note that the two do associate in Sf9 cells) and after failing to dimerize, the wild type ABCG2 finds another wild type molecule with which to form a stable dimer and traffic to the cell surface. The absence of colocalization or rescue of the mutant by the wild type thus argues against the presence of stable wild type/mutant dimers in the mammalian cells.
While several studies have presented evidence of chemical and disulfide cross-linking as a surrogate for dimerization (40) we would argue that these results are evidence of physical proximity and not of a functional interaction. We would define dimerization as an interaction that results in a properly localized, functional unit. We believe this distinction to be important because in the millieu of the ER or the plasma membrane, two ABCG2 molecules could be in sufficiently close proximity to be linked chemically, but yet not constitute a functional unit. Though the G553L mutant is confined to the ER, it must be in near proximity to another mutant protein because chemical cross-linking of two mutant proteins even at a distance of 7.7 Å was observed. We believe that our results suggest that in the case of ABCG2 chemical cross-linking is more appropriately a marker of proximity in the ER rather than functional dimerization.
In contrast to the results in mammalian cells, the evidence indicates that in insect cells the mutant is not held up in the ER, reaches the cell surface, and can interfere with wild type function. However no ATP hydrolysis could be detected for the mutant in the insect cells. Although this finding is compatible with either an isolated effect on dimerization or a broader abnormality in protein folding, the ability of the mutant to reach the cell surface and interfere with wild type transport activity argues against misfolding. In coexpression studies a dominant negative effect due to mutant/wild type dimer formation would be expected to provoke an approximately 75% decrease in transport activity. Although results with the catalytically inactive control K86M mutant were not precisely as expected based on the ratios transfected, the marked reduction in Hoechst transport activity is consistent with the reported dominant negative effect for this Walker A mutant (35). In contrast to the results observed with the K86M mutant/wild type dimer, a roughly 35% decrease was observed in the Sf9 cells expressing both the wild type and the G553L mutant protein. This limited, but reproducible, effect of the G553L mutant on the wild type protein argues that the mutant has a conformation that can associate with the wild type protein, although that association must be relatively weak.
Glycine 553 in ABCG2 is very well conserved in the human ABCG subfamily as well as in the mouse Abcg3 and in the Drosophila white protein. A growing body of mutational data suggest that the region including this glycine in TM5 of these proteins might play an important role in the dimerization process. In case of the Drosophila white protein, the residues corresponding to amino acids 552–554 of ABCG2 have been suggested to interfere with the white-brown and white-scarlet heterodimer formation (22). Mutation G589E in the white protein, analogous to our ABCG2 G553E mutant, apparently disrupts the white-brown heterodimer as suggested by significantly reduced red eye pigment levels. Furthermore, the naturally occurring ABCG8 G574E mutant, carrying a mutation corresponding to phenylalanine 551 of ABCG2, was also characterized as interfering with dimerization. When the ABCG8 G574E mutant was coexpressed with wild type ABCG5 in CHO-K1 cells, no protein in the position of a dimer was detected on native gels (37). On the other hand, the ABCG8 G574R mutant, although having some effect on ABCG8 maturation, did not prevent heterodimerization (33). In ABCG8 glycine 574 and the glycine corresponding to residue 553 in ABCG2 are adjacent (see alignment in Figure 1). However, in the case of ABCG2, the inactive L554P mutant was reported to be able to partially reverse the drug resistance of PA317 cells cotransfected with the mutant and wild type proteins, a result implying that residue 554 is critical for function, yet, mutating this residue does not prevent dimerization. Nevertheless, all these findings support an important role for this region of TM5 predicted to be near the extracellular surface.
Taken together, the present studies are consistent with a role for glycine 553 in the formation of a stable, functional ABCG2 homodimer. It is most interesting that the close proximity of the monomers, indicated by both chemical cross-linking and disulfide bridge formation in the mammalian cells, is not sufficient to allow correct trafficking and glycosylation, or proper functioning of ABCG2. Therefore, we would argue that the results presented here are consistent with an impairment of dimerization in the sense of formation of a fully functional dimeric protein that traffics normally to the cell surface. Note that in ABC transporters the dimer interface almost certainly involves multiple residues from more than one TM. We are suggesting that G553 in TM5 of ABCG2 might be one of these. It is hoped that as more data accrue, precise models such as those obtained by crystallography can be generated that will provide a better understanding of how the ABCG2 dimer is formed.
Acknowledgments
The authors would like to acknowledge Dr. Tito Fojo for helpful advice, and Dr. Yuan Ting for technical assistance with site-directed mutagenesis.
Footnotes
This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abreviations: ABC, ATP-binding cassette; BCRP, breast cancer resistance protein; MXR, mitoxantrone resistance protein; ABCP, ABC transporter expressed in placenta; ABC, ATP-binding cassette; CFTR, cystic fibrosis transmembrane conductance regulator; P-gp, P-glycoprotein; TMD, transmembrane domain; NBD, nucleotide-binding domain; TM, transmembrane segment; SDS, sodium dodecyl sulfate; HEK, human embryonic kidney; MBS, m-maleimidobenzoyl-N-hydroxysuccinimide ester; DSG, disuccinimidyl glutarate; SF9 insect cells, Spodoptera frugiperda ovarian cells; GFP, green fluorescence protein; wt, wild type; ER, endoplasmic reticulum; DSS, disuccinimidyl suberate; MX, mitoxantrone; DPBS, Dulbecco’s phosphate-buffered saline
References
- 1.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–17. [PubMed] [Google Scholar]
- 2.Stefkova J, Poledne R, Hubacek JA. ATP-binding cassette (ABC) transporters in human metabolism and diseases. Physiol Res. 2004;53:235–43. [PubMed] [Google Scholar]
- 3.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 4.van Veen HW, Konings WN. The ABC family of multidrug transporters in microorganisms. Biochim Biophys Acta. 1998;1365:31–6. doi: 10.1016/s0005-2728(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 5.Polgar O, Bates SE. ABC transporters in the balance: is there a role in multidrug resistance? Biochem Soc Trans. 2005;33:241–5. doi: 10.1042/BST0330241. [DOI] [PubMed] [Google Scholar]
- 6.Szakacs G, Chen GK, Gottesman MM. The molecular mysteries underlying P-glycoprotein-mediated multidrug resistance. Cancer Biol Ther. 2004;3:382–4. doi: 10.4161/cbt.3.4.743. [DOI] [PubMed] [Google Scholar]
- 7.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 8.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (MXR/BCRP/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–52. [PubMed] [Google Scholar]
- 9.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–54. [PubMed] [Google Scholar]
- 10.Kawabata S, Oka M, Shiozawa K, Tsukamoto K, Nakatomi K, Soda H, Fukuda M, Ikegami Y, Sugahara K, Yamada Y, Kamihira S, Doyle LA, Ross DD, Kohno S. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280:1216–1223. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 11.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64. [PubMed] [Google Scholar]
- 12.Berkower C, Michaelis S. Mutational analysis of the yeast a-factor transporter STE6, a member of the ATP binding cassette (ABC) protein superfamily. EMBO J. 1991;10:3777–85. doi: 10.1002/j.1460-2075.1991.tb04947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatia A, Schafer HJ, Hrycyna CA. Oligomerization of the human ABC transporter ABCG2: evaluation of the native protein and chimeric dimers. Biochemistry. 2005;44:10893–904. doi: 10.1021/bi0503807. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen U, Fog JU, Litman T, Gether U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J Biol Chem. 2005;280:36926–34. doi: 10.1074/jbc.M502937200. [DOI] [PubMed] [Google Scholar]
- 15.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–82. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 16.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–5. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 17.Cserepes J, Szentpetery Z, Seres L, Ozvegy-Laczka C, Langmann T, Schmitz G, Glavinas H, Klein I, Homolya L, Varadi A, Sarkadi B, Elkind NB. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: indications for heterodimerization. Biochem Biophys Res Commun. 2004;320:860–7. doi: 10.1016/j.bbrc.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz G, Langmann T, Heimerl S. Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res. 2001;42:1513–20. [PubMed] [Google Scholar]
- 19.Chang G, Roth CB. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science. 2001;293:1793–800. doi: 10.1126/science.293.5536.1793. [DOI] [PubMed] [Google Scholar]
- 20.van Veen HW, Venema K, Bolhuis H, Oussenko I, Kok J, Poolman B, Driessen AJ, Konings WN. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc Natl Acad Sci U S A. 1996;93:10668–72. doi: 10.1073/pnas.93.20.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang G. Structure of MsbA from Vibrio cholera: a multidrug resistance ABC transporter homolog in a closed conformation. J Mol Biol. 2003;330:419–30. doi: 10.1016/s0022-2836(03)00587-4. [DOI] [PubMed] [Google Scholar]
- 22.Mackenzie SM, Brooker MR, Gill TR, Cox GB, Howells AJ, Ewart GD. Mutations in the white gene of Drosophila melanogaster affecting ABC transporters that determine eye colouration. Biochim Biophys Acta. 1999;1419:173–85. doi: 10.1016/s0005-2736(99)00064-4. [DOI] [PubMed] [Google Scholar]
- 23.Polgar O, Robey RW, Morisaki K, Dean M, Michejda C, Sauna ZE, Ambudkar SV, Tarasova N, Bates SE. Mutational analysis of ABCG2: role of the GXXXG motif. Biochemistry. 2004;43:9448–56. doi: 10.1021/bi0497953. [DOI] [PubMed] [Google Scholar]
- 24.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at amino acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–8. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litman T, Jensen U, Hansen A, Covitz K, Zhan Z, Fetsch P, Abati A, Hansen P, Horn T, Skovsgaard T, Bates S. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim Biophys Acta. 2002;1565:6–16. doi: 10.1016/s0005-2736(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 26.Ozvegy C, Varadi A, Sarkadi B. Characterization of drug transport, ATP hydrolysis and nucleotide trapping by the human ABCG2 multidrug transporter: modulation of substrate specificity by a point mutation. J Biol Chem. 2002;277:47980–90. doi: 10.1074/jbc.M207857200. [DOI] [PubMed] [Google Scholar]
- 27.Ozvegy-Laczka C, Varady G, Koblos G, Ujhelly O, Cervenak J, Schuetz JD, Sorrentino BP, Koomen GJ, Varadi A, Nemet K, Sarkadi B. Function-dependent conformational changes of the ABCG2 multidrug transporter modify its interaction with a monoclonal antibody on the cell surface. J Biol Chem. 2005;280:4219–27. doi: 10.1074/jbc.M411338200. [DOI] [PubMed] [Google Scholar]
- 28.Muller M, Bakos E, Welker E, Varadi A, Germann UA, Gottesman MM, Morse BS, Roninson IB, Sarkadi B. Altered drug-stimulated ATPase activity in mutants of the human multidrug resistance protein. J Biol Chem. 1996;271:1877–83. doi: 10.1074/jbc.271.4.1877. [DOI] [PubMed] [Google Scholar]
- 29.Sarkadi B, Price E, Boucher R, Germann U, Scarborough G. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated ATPase. J Biol Chem. 1992;267:4854–4858. [PubMed] [Google Scholar]
- 30.Ozvegy C, Litman T, Szakacs G, Nagy Z, Bates S, Varadi A, Sarkadi B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem Biophys Res Commun. 2001;285:111–7. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 31.Abbott BL, Colapietro AM, Barnes Y, Marini F, Andreeff M, Sorrentino BP. Low levels of ABCG2 expression in adult AML blast samples. Blood. 2002;100:4594–601. doi: 10.1182/blood-2002-01-0271. [DOI] [PubMed] [Google Scholar]
- 32.Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110:659–69. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, Sugimoto Y. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97:626–30. doi: 10.1002/ijc.10100. [DOI] [PubMed] [Google Scholar]
- 34.Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–23. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- 35.Henriksen U, Gether U, Litman T. Effect of Walker A mutation (K86M) on oligomerization and surface targeting of the multidrug resistance transporter ABCG2. J Cell Sci. 2005;118:1417–26. doi: 10.1242/jcs.01729. [DOI] [PubMed] [Google Scholar]
- 36.Locher KP, Lee AT, Rees DC. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–8. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 37.Graf GA, Cohen JC, Hobbs HH. Missense mutations in ABCG5 and ABCG8 disrupt heterodimerization and trafficking. J Biol Chem. 2004;279:24881–8. doi: 10.1074/jbc.M402634200. [DOI] [PubMed] [Google Scholar]
- 38.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–4. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 39.Diop NK, Hrycyna CA. N-Linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry. 2005;44:5420–9. doi: 10.1021/bi0479858. [DOI] [PubMed] [Google Scholar]
- 40.Hogue DL, Liu L, Ling V. Identification and characterization of a mammalian mitochondrial ATP-binding cassette membrane protein. J Mol Biol. 1999;285:379–89. doi: 10.1006/jmbi.1998.2259. [DOI] [PubMed] [Google Scholar]