Abstract
The aim of this study was to create a gene expression profile to better define the phenotype of human adipose-derived stromal cells (HADSCs) during in vitro chondrogenesis, osteogenesis and adipogenesis. A novel aspect of this work was the analysis of the same subset of genes during HADSC differentiation into all three lineages. Chondrogenic induction resulted in increased mRNA expression of Sox transcription factors, COL2A1, COL10A1, Runx2 and Osterix. This is the first report demonstrating significant up-regulation in expression of osteogenesis-related transcription factors Runx2 and Osterix by TGF-β3 induction of HADSCs during in vitro chondrogenesis. These findings suggest that the commonly-used chondrogenic induction reagents promote differentiation suggestive of hypertrophic chondrocytes and osteoblasts. We conclude that alternative strategies are required to induce efficient articular chondrocyte differentiation in order for HADSCs to be of clinical use in cartilage tissue engineering.
Keywords: Human adipose-derived stromal cells (HADSC), mesenchymal stromal cell (MSC), mesenchymal stem cells, in vitro differentiation, chondrogenesis, TGF-β3, transcription factors, Runx2, Osterix
INTRODUCTION
Pluripotent mesenchymal stem cell-like populations that have the capacity to differentiate toward cells of connective tissue origins including bone, cartilage, adipose and muscle, have been isolated from a number of different sources including adult bone marrow aspirates, adipose, synovium and skeletal muscle [1–4]. By definition, true stem cells lack tissue-specific characteristics, maintain an undifferentiated phenotype until they are exposed to appropriate signals and have the capacity for extensive self-renewal. In terms of nomenclature, we use the term “stromal cells” rather than “stem cells” as this is a more appropriate term to describe the heterogeneous population of cells that are isolated from adult tissues and subsequently cultured in vitro to obtain cells with pluripotent qualities.
Standardized protocols have been developed for mesenchymal stromal cell (MSC) isolation, enrichment, expansion and differentiation. With respect to chondrogenesis, MSCs are normally cultured in high density, three-dimensional aggregates/pellets [5, 6] to attempt to mimic the mesenchymal cell condensations and cell to cell contacts that are required to initiate chondrocyte differentiation in vivo. Addition of transforming growth factor-β (TGF-β) is also commonly added to induction media to promote chondrocyte differentiation in vitro. Confirmation of proper chondrogenesis, osteogenesis and adipogenesis is usually determined by analysis of “end-stage” differentiation markers such as type II collagen (COL2A1) and aggrecan for chondrocytes and type I collagen (COL1A1) and alkaline phosphatase for osteoblasts. However, it is also necessary to understand the molecular mechanisms that regulate expression of these differentiation markers in order to generate a more complete picture of the intracellular pathways being activated in response to exogenous induction factors in vitro.
In the present study, we analyzed transcription factor expression as well as end-stage differentiation markers in human adipose-derived stromal cells (HADSCs) during in vitro chondrogenesis, osteogenesis and adipogenesis at early (day 3) and late time points (day 28) of differentiation. We examined expression levels of three Sox transcription factors (Sox5, Sox6 and Sox9) that are essential for cartilage formation [7–9], two osteogenesis-promoting transcription factors (Runx2 and Osterix) [10, 11] and two important adipogenic regulatory nuclear proteins (C/EBP α and PPAR-γ2) [12]. Our studies indicate that TGF-β3 chondrogenic induction of HADSCs results in a mixed population of cells including hypertrophic chondrocytes and osteoblasts, as evidenced by significant expression of COL10A1, Runx2 and Osterix. This suggests that commonly-accepted protocols being utilized to promote in vitro chondrogenesis of HADSCs do not induce an articular chondrocyte population and, as such, alternative methods are required to benefit the field of cartilage tissue regeneration.
MATERIALS AND METHODS
Isolation of mesenchymal stromal cells and primary articular chondrocytes
Human adipose-derived stromal cells (HADSCs) were obtained from subcutaneous adipose tissue of six patients (5 female, 1 male; median age 42.8 years) undergoing elective procedures (gastric bypass, panniculectomy and liposuction). All protocols were approved by Washington University School of Medicine Human Studies Committee. Adipose tissue was washed thoroughly with sterile phosphate-buffered saline (PBS), finely diced and digested with 0.05% collagenase solution (Worthington Biochemical Corporation) at 37°C for 60–90 minutes. After centrifugation (10 minutes, 1500rpm), the cell pellet was resuspended in proliferation media [DMEM-low glucose, 10% FBS, penicillin/streptomycin mixture and FGF-2 (10 ng/ml)] and plated at a density of 3ml processed lipoaspirate/cm2. Proliferation medium was replaced the following day and thereafter every 3 to 4 days. Cells were passaged 2–5 times at a density of 5000 cells/cm2 and FGF-2 supplementation was discontinued immediately prior to differentiation. Human primary articular chondrocytes were isolated using a previously published method [13].
Differentiation of HADSCs
HADSCs were induced toward adipogenic, chondrogenic, and osteogenic lineages using Poietics Mesenchymal Stem Cell Differentiation Systems according to the manufacturer’s instructions (Lonza; formerly Cambrex BioScience Walkersville Inc.). Refer to the User Manual for details of media components. Adipogenesis: HADSCs in monolayer (2.1×104 cells/cm2) were allowed to reach confluence (4–5 days) before incubation in adipogenic induction media for 3 days followed by adipogenic maintenance media for 1–3 days. This was repeated for a total of 3 cycles. Osteogenesis: HADSCs in monolayer (3.1×103 cells/cm2) were cultured in osteogenic induction media, which was changed every 3–4 days. Chondrogenesis: high density pellet cultures were generated (2.5 × 105 cells/pellet), plated in 96-well v-bottom, polypropylene plates and cultured in chondrogenic induction media containing TGF-β3 (10 ng/ml). Media was changed every 2–3 days.
Isolation of mRNA from Cells and Tissue
Total RNA was isolated using TRIzol reagent (Invitrogen). High density cell pellets were digested in 3 mg/ml collagenase (Worthington Biochemical Corporation), 0.25% trypsin (Sigma), 1 mg/ml hyaluronidase (Sigma) and 4mU/ml chondroitinase (Sigma) for 1–3 hours at 37°C followed by homogenization in TRIzol using a motorized pestle (Fisher Scientific). Following chloroform extraction, RNA was then purified using RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions.
Semi-quantitative RT-PCR
Reverse transcription of mRNA (1µg) was done using Superscript II Reverse Transcriptase (Invitrogen). Resulting cDNA was amplified by Taq polymerase PCR in the linear phase using a specific primer pair (see Supplementary Table 1 for the list of genes analyzed, primer sequences and PCR cycle numbers). mRNA expression was normalized to GAPDH and then averaged within each group. mRNA expression at days 3 and 28 of HADSC differentiation was calculated relative to mRNA expression of that same gene at day 0 from undifferentiated HADSCs. HADSCs from 2 to 4 donors were used to analyze gene expression at each time point.
Analysis of end-stage differentiation
Adipogenesis: At day 21, cells were fixed in 10% buffered formalin and stained with 1.8 mg/ml Oil red O (Sigma) in 60% isopropranol. Osteogenesis: At day 28,cultures were stained with von Kossa to detect calcified matrix. Cells were fixed in 10% buffered formalin, incubated in 5% AgNO3 for 1 hr in the absence of light, rinsed with 5% sodium thiosulfate and then incubated under UV light for 1 hr. Chondrogenesis: At day 21, HADSC pellets were fixed in 10% buffered formalin at room temperature for 4 hours. Alcian blue staining was performed on paraffin sections of cell pellets according to the manufacturer’s instructions (Rowley Biochemical Inc.) to detect the presence of proteoglycans.
Statistical Analysis
Comparison between groups was performed using a 2-tailed student’s t-Test, assuming unequal variances. P < 0.05 was considered significant. Error bars represent the standard error of the mean in all figures.
RESULTS
Proliferation and Differentiation of HADSCs
FGF-2 supplementation prior to differentiation was utilized in order to increase proliferation rate of HADSCs-and resulted in phenotypic stabilization over increasing passage numbers (Supplementary Fig. 1 and Supplementary Fig. 2). This self-renewal function of FGF-2 on HADSCs has previously been reported [14]. Analysis of end-stage differentiation revealed that the HADSCs were able to differentiate toward adipocytes (positive Oil Red O staining) and osteoblasts (positive von Kossa staining) (Supplementary Fig. 2). However, chondrogenic induced pellets weakly stained with alcian blue, indicating low levels of proteoglycan synthesis.
Expression Transcription Factors PPAR-γ2 and C/EBP-α (Adipocyte Markers)
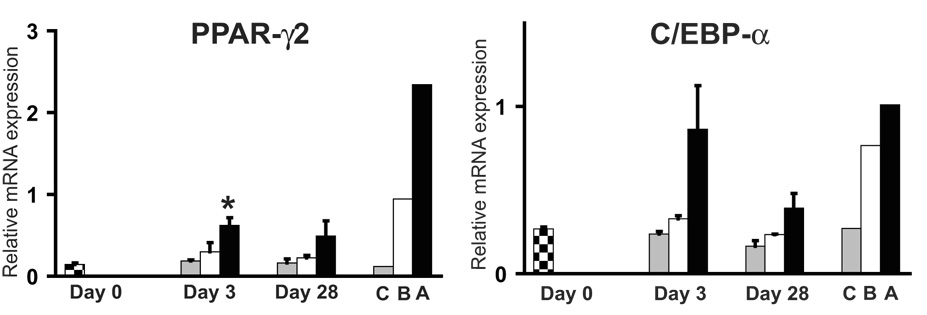
Higher levels of adipogenic transcription factors PPAR-γ2 and C/EBP-α were detected in HADSCs during adipogenesis at day 3 and day 28 of differentiation when compared to the other two lineages (Fig. 1). There was a statistically significant difference in PPAR-γ2 expression between unstimulated HADSCs (day 0) and day 3 of adipogenesis. Expression of these transcription factors was, as expected, higher in primary human adipose tissue when compared to expression in cortical bone or primary articular chondrocytes.
Fig. 1.
Expression of adipogenic transcription factors PPAR-γ2 and C/EBP-α during differentiation of HADSCs. Checkered bars represent levels of mRNA from undifferentiated HADSCs (Day 0). Gray, white and black bars represent mRNA levels from HADSCs after induction of chondrogenesis, osteogenesis and adipogenesis, respectively. C=human primary articular chondrocytes, B=human cortical bone, A=human subcutaneous adipose tissue.
* = P<0.05 relative to Day 0.
Expression of Sox Transcription Factors and COL2A1 (Chondrocyte Markers)
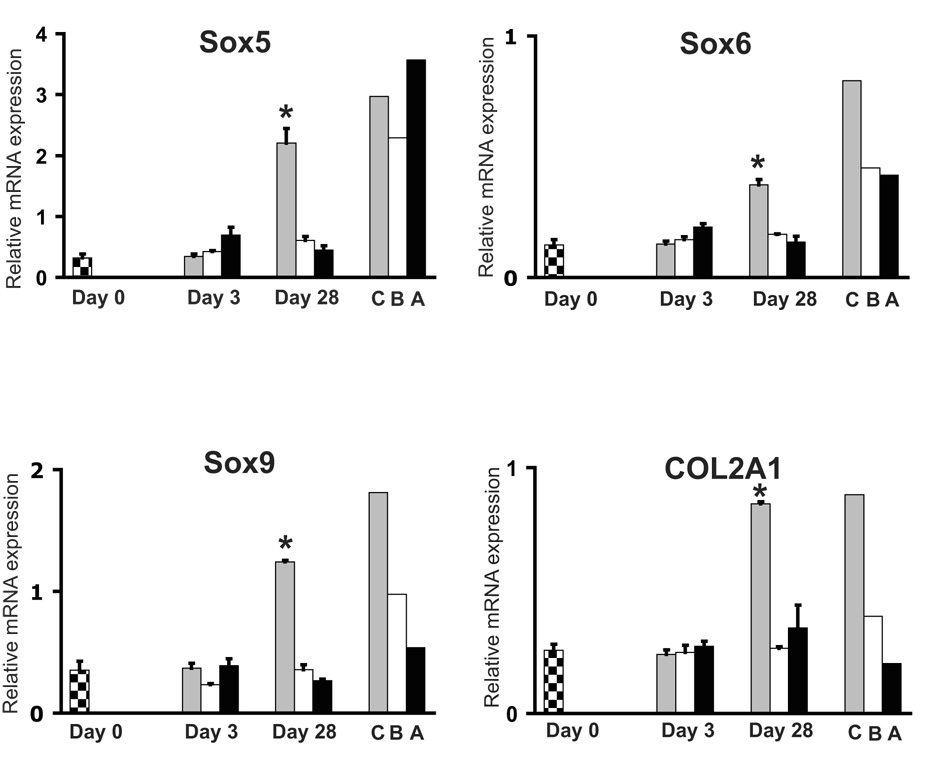
Expression levels of each Sox mRNA were significantly upregulated in HADSC pellets at day 28 of in vitro chondrogenesis when compared to unstimulated HADSCs (Fig. 2). Abundant expression of each was also detected in primary articular chondrocytes. Interestingly, high endogenous expression of Sox 5 was detected in human adipose tissue and bone tissue. COL2A1 gene expression was low in undifferentiated HADSCs and in all three lineages at day 3. However, there was a statistically significant increase in COL2A1 expression in pellet cultures of HADSCs after 28 days in chondrogenesis induction medium. COL2A1 expression was also shown to be higher in human primary articular chondrocytes when compared to levels in bone and adipose tissue.
Fig. 2.
Expression of chondrogenesis related genes during differentiation of HADSCs. Checkered bars represent levels of mRNA from undifferentiated HADSCs (Day 0). Gray, white and black bars represent mRNA levels from HADSCs after induction of chondrogenesis, osteogenesis and adipogenesis, respectively. C=human primary articular chondrocytes, B=human cortical bone, A=human subcutaneous adipose tissue.
* = P<0.05 relative to Day 0.
Expression of Indian Hedgehog and COL10A1 (Pre-Hypertrophic/Hypertrophic Chondrocyte Markers)
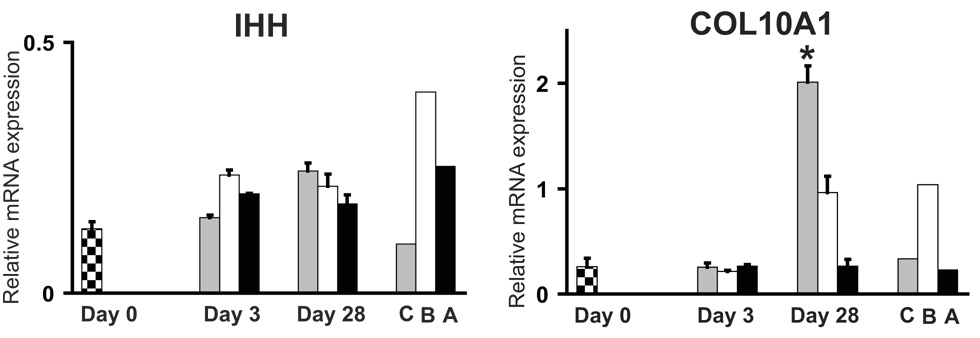
Expression patterns of the pre-hypertrophic marker, Indian Hedgehog, did not reveal any statistically significant changes during differentiation toward all three lineages (Fig. 3). However, there was a statistically significant increase in expression of the hypertrophic chondrocyte marker, type X collagen (COL10A1), at day 28 of chondrogenesis. COL10A1 expression was highest in bone rather than the primary chondrocyte cells (an expected result since the primary chondrocytes were isolated from articular cartilage rather than growth plate cartilage).
Fig. 3.
Expression of Indian Hedgehog (IHH) and type X collagen (COL10A1) during differentiation of HADSCs. Checkered bars represent levels of mRNA from undifferentiated HADSCs (Day 0). Gray, white and black bars represent mRNA levels from HADSCs after induction of chondrogenesis, osteogenesis and adipogenesis, respectively. C=human primary articular chondrocytes, B=human cortical bone, A=human subcutaneous adipose tissue.
* = P<0.05 relative to Day 0.
Expression of Runx2, Osterix, Alkaline Phosphatase and COL1A1 (Osteoblast Markers)
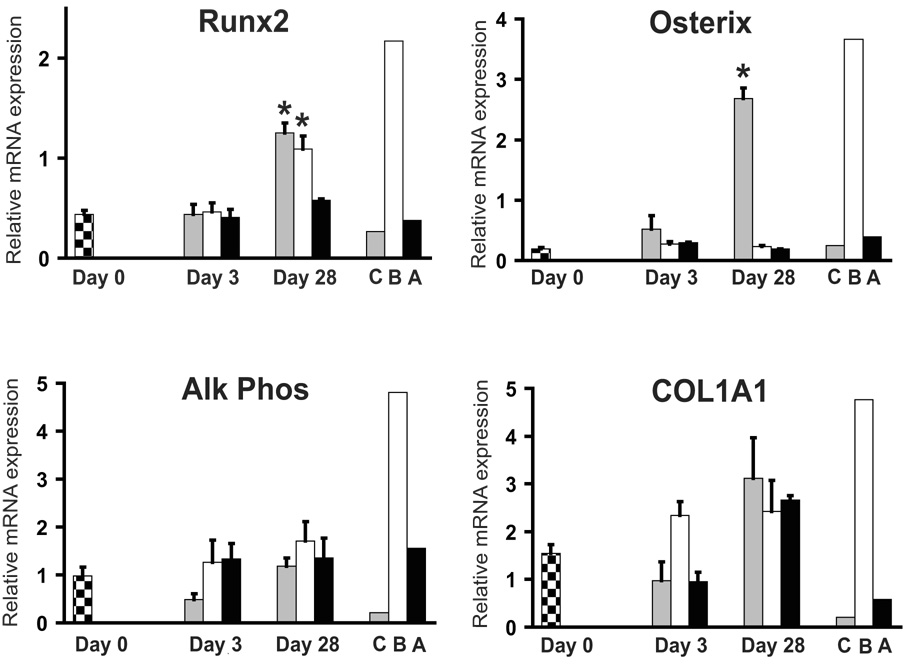
Levels of alkaline phosphatase and type I collagen (COL1A1) were high in human cortical bone tissue and negligible in primary articular chondrocytes and adipose tissue, as expected (Fig. 4). Although not statistically significant, we consistently found similar expression levels of COL1A1 in HADSCs after 28 days of chondrogenic and osteogenic induction. Levels of Runx2 and Osterix transcription factors were highest in human cortical bone when compared to articular chondrocytes and adipose tissue. At day 28 of differentiation, there was a statistically significant increase in Runx2 in osteogenesis-induced HADSCs as well as in chondrogensis-induced cell pellets. Interestingly, there was a significant increase in the expression of Osterix in chondrogenesis-induced cell pellets at day 28, while expression during osteogenesis was negligible at all time points analyzed.
Fig. 4.
Expression of osteogenesis related genes during HADSC differentiation. mRNA levels of transcription factors Runx2 and Osterix, and matrix markers alkaline phosphatase (Alk Phos) and type I collagen (COL1A1) were analyzed. Checkered bars represent levels of mRNA from undifferentiated HADSCs (Day 0). Gray, white and black bars represent mRNA levels from HADSCs after induction of chondrogenesis, osteogenesis and adipogenesis, respectively. C=human primary articular chondrocytes, B=human cortical bone, A=human subcutaneous adipose tissue. * = P<0.05 relative to Day 0.
DISCUSSION
Defining the molecular pathways of mesenchymal stromal cell (MSC) commitment into chondrocytes and osteoblasts has significant implications in orthopaedic trauma and skeletal repair [15]. In the present study, we carried out detailed gene expression analyses in human adipose-derived stromal cells (HADSCs) during in vitro chondrogenesis, osteogenesis and adipogenesis. In addition to monitoring “end-stage” differentiation markers, we also analyzed expression of transcription factors that are known to regulate chondrocyte, osteoblast and adipocyte differentiation. A novel aspect to this work was our analysis of each gene during differentiation toward all three lineages as well as in adult human tissue controls.
With respect to chondrogenesis, we observed a significant increase in COL2A1 and the three Sox transcription factors in chondrogenic induced HADSC pellet cultures, suggesting that some degree of chondrocyte differentiation had occurred. However, low levels of alcian blue staining (Supplemental Fig. 1), and type II collagen protein expression (results not shown) indicated inefficient in vitro chondrogenesis. Previous reports have demonstrated the chondrogenic potential of HADSCs [2, 16–18]. However, other studies have also shown that chondrogenic differentiation of MSCs from adipose tissue is not as effective as differentiation of MSCs from bone marrow aspirates when directly compared in the same study [19–22]. Therefore, it is apparent that the commonly-used, commercially available growth cocktail (containing TGF-β and dexamethasone) is not effectively conducive to chondrocyte differentiation in HADSCs.
Our results also showed a significant increase in type X collagen (COL10A1) mRNA during in vitro chondrogenesis. This has also been reported in many other studies [5, 6, 19, 23–25] and is indicative of advanced hypertrophic chondrocyte maturation that occurs prior to endochondral ossification in vivo, where matrix mineralization and blood vessel formation within hypertrophic cartilage occurs leading to invasion of osteoblasts and other cells to form bone and bone marrow [26]. Further indication of hypertrophy was also found in the present study by Runx2 upregulation. Knock-out mouse studies showed that, as well as hypertrophy, Runx2 also regulates osteoblast formation [10] therefore suggesting that some degree of osteogenesis had occurred in our cultures. Since the stroma fraction of adipose tissue contains a heterogeneous cell population, the expression of osteogenic genes may be due, in part, to the presence of osteoprogenitor cells. Direct induction of osteogenesis during chondrogenic induction of HADSCs was also suggested in a previous study [27] due to upregulation in COL1A1 expression; the present study also showed an increase in COL1A1 expression during in vitro chondrogenesis. Further evidence suggesting induction of osteogenesis in response to chondrogenic cues was shown by a significant increase in expression of Osterix. Osterix has been shown to function more specifically than Runx2 in regulating osteoblast differentiation, since Osterix knock-out mice lack bony skeletons while the cartilage anlagen are fully formed [11]. Interestingly, Osterix expression was not detected during our in vitro osteogenesis assays, even though calcified extracellular matrix was detected by von Kossa staining. This raises important questions regarding the quality of osteogenic differentiation that is occurring in vitro. It may be that expression of Osterix peaked at earlier time points, as has been reported in other studies of osteoblast differentiation [28, 29]. However, the fact that mature cortical bone control tissue was shown to express high levels of Osterix suggests that persistent Osterix expression is required for proper osteogenesis.
This is the first report to show significant up-regulation in expression of osteoblast-inducing transcription factors Runx2 and Osterix during TGF-β3 chondrogenic induction of HADSCs in vitro. Upregulation of Runx2 and Osterix provides a plausible regulatory mechanism for the hypertrophy and potential osteoblast differentiation occurring during in vitro chondrogenesis of HADSCs. Upregulation of Runx2 and Osterix may also partly explain the observed calcification and vascular invasion of chondrogenic induced bone marrow-derived MSC pellets when transplanted subcutaneously into SCID mice [25]. Therefore, it is apparent that these non-physiologic and artificial methods of chondrogenic induction in vitro do not result in articular chondrocytes consistent with hyaline cartilage, but rather result in hypertrophic chondrocytes and osteoblasts suggestive of endochondral ossification. Such “derailed” differentiation would have a deleterious effect on cartilage tissue regeneration as a treatment for conditions such as osteoarthritis where the ultimate goal is to generate permanent hyaline cartilage.
The concept of manipulating transcription factor levels to select for a specific differentiation pathway is an interesting one that is currently being tested. Recent reports have shown enhanced osteogenic activity of MSCs that were programmed to over-express either Runx2 or Osterix [30–32]. Similarly, over-expression of Sox9 in either embryonic stem cells or primary chondrocytes resulted in increased COL2A1 and proteoglycan expression [33, 34]. Clearly, we need to understand the specific factors that should be switched on/off to regulate articular chondrocyte differentiation in order to prevent production of “transient” or hypertrophic chondrocytes that are present in growth plate cartilage. We therefore propose that correct terminologies be used to clearly distinguish the type of chondrocyte differentiation being induced rather than using the general term “in vitro chondrogenesis”. Finally, we predict that adopting strategies to modulate expression of one or more tissue-specific regulatory factors will hold more promise for inducing articular chondrocyte differentiation than exposing MSCs to induction media containing exogenous recombinant growth factors, hormones and other reagents.
Supplementary Material
ACKNOWLEDGEMENTS
The present study was funded by a combination of the following grants: Arthritis Foundation Investigator Award (AM), Arthritis National Research Foundation grant (AM), NIH/NIDCD T32 DC000022 (JTR), NIH/NIAMS RO136994 (LS) and NHLBI RO1073256 (JN). We would also like to thank Carl Franz, Li Liang and Jan-Jan Liu for technical assistance and discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benayahu D, Akavia UD, Shur I. Differentiation of bone marrow stroma-derived mesenchymal cells. Curr Med Chem. 2007;14:173–179. doi: 10.2174/092986707779313363. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.Yokoyama A, Sekiya I, Miyazaki K, Ichinose S, Hata Y, Muneta T. In vitro cartilage formation of composites of synovium-derived mesenchymal stem cells with collagen gel. Cell Tissue Res. 2005;322:289–298. doi: 10.1007/s00441-005-0010-6. [DOI] [PubMed] [Google Scholar]
- 4.Usas A, Huard J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials. 2007;28:5401–5406. doi: 10.1016/j.biomaterials.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 7.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 8.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B, Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 10.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 13.Fukui N, Zhu Y, Maloney WJ, Clohisy J, Sandell LJ. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. The Journal of bone and joint surgery. 2003;85-A Suppl 3:59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- 14.Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells. 2006;24:2412–2419. doi: 10.1634/stemcells.2006-0006. [DOI] [PubMed] [Google Scholar]
- 15.Vilquin JT, Rosset P. Mesenchymal stem cells in bone and cartilage repair: current status. Regen Med. 2006;1:589–604. doi: 10.2217/17460751.1.4.589. [DOI] [PubMed] [Google Scholar]
- 16.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 17.Awad HA, Halvorsen YD, Gimble JM, Guilak F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003;9:1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 18.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 20.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 21.Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23:1383–1389. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 22.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson C, Brantsing C, Svensson T, Brisby H, Asp J, Tallheden T, Lindahl A. Differentiation of human mesenchymal stem cells and articular chondrocytes: analysis of chondrogenic potential and expression pattern of differentiation-related transcription factors. J Orthop Res. 2007;25:152–163. doi: 10.1002/jor.20287. [DOI] [PubMed] [Google Scholar]
- 25.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 26.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 27.Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222–1232. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 28.Kaback LA, Soung do Y, Naik A, Smith N, Schwarz EM, O'Keefe RJ, Drissi H. Osterix/Sp7 regulates mesenchymal stem cell mediated endochondral ossification. J Cell Physiol. 2008;214:173–182. doi: 10.1002/jcp.21176. [DOI] [PubMed] [Google Scholar]
- 29.Zou L, Zou X, Chen L, Li H, Mygind T, Kassem M, Bunger C. Multilineage differentiation of porcine bone marrow stromal cells associated with specific gene expression pattern. J Orthop Res. 2008;26:56–64. doi: 10.1002/jor.20467. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12:247–253. doi: 10.1016/j.ymthe.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Tu Q, Valverde P, Chen J. Osterix enhances proliferation and osteogenic potential of bone marrow stromal cells. Biochem Biophys Res Commun. 2006;341:1257–1265. doi: 10.1016/j.bbrc.2006.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L, Wu Y, Lin Y, Jing W, Nie X, Qiao J, Liu L, Tang W, Tian W. Osteogenic differentiation of adipose derived stem cells promoted by overexpression of osterix. Mol Cell Biochem. 2007;301:83–92. doi: 10.1007/s11010-006-9399-9. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Do HJ, Yang HM, Oh JH, Choi SJ, Kim DK, Cha KY, Chung HM. Overexpression of SOX9 in mouse embryonic stem cells directs the immediate chondrogenic commitment. Exp Mol Med. 2005;37:261–268. doi: 10.1038/emm.2005.35. [DOI] [PubMed] [Google Scholar]
- 34.Cucchiarini M, Thurn T, Weimer A, Kohn D, Terwilliger EF, Madry H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007;56:158–167. doi: 10.1002/art.22299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.