The Mannich reaction is one of the most widely utilized chemical transformations for the construction of β-amino carbonyl compounds and 1,2-amino alcohol derivatives, valuable synthetic intermediates for the synthesis of drugs and biologically active compounds.1 Only recently, several groups have reported a direct catalytic asymmetric Mannich reaction without resorting to preactivation of the pronucleophile using organocatalysts and metal catalysts,2,3 including our own dinuclear zinc complex 2.3 b Most of the examples reported to date are limited to reaction of unmodified ketone or hydroxyketone donors with imine acceptors. In addition, the cleavage of the N-protective group also requires harsh oxidizing conditions. Shibasaki, recently, reported pioneering work on the Et2Zn/(S,S)-linked-BINOL catalysis using an easily removable N-protective diphenylphosphinoyl (Dpp) imine and Boc-imine, which selectively provided either anti- and syn-β-amino alcohols, respectively.3c-d The successful donors are 2′- and 4′ - methoxy substituted hydroxyacetophenones and so far the successful imine acceptors have been limited to those derived from non-enolizable aldehydes, most notably aryl. In this paper, we report the application of our dinuclear zinc catalyst to the complementary direct catalytic asymmetric Mannich-type reaction of α-hydroxyketones using α-enolizable Dpp-imines4 and Boc-imines,5 which we have found to be stable at 0° at least for several days, to generate either anti- or syn-β-amino alcohols, respectively (Scheme 1).
Scheme 1.
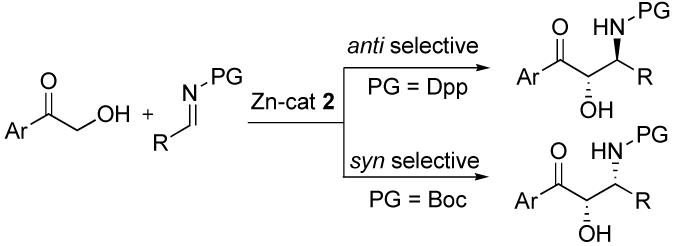
Anti- and Syn-β-Amino Alcohol Synthesis
We first examined the reaction of Dpp-imine 4a with hydroxy ketone 3a (Table 1) using dinuclear zinc catalyst 2a,6 which was prepared from chiral ligand 1a and 2 equiv of Et2Zn in THF (Scheme 2). Initially subjection of the catalyst 2a (3.5 mol%) to a mixture of 3a (1.4 equiv) and Dpp-imine 4a in the presence of 4ÅMS in THF afforded the desired amino alcohol 5a in reasonable yield but with poor dr (entries 1 and 2). Changing the sequence of addition by subjection of 3a and then 4a in THF to the suspension of the catalyst 2a and 4Å MS in THF and lowering the reaction temperature to -25 °C (entry 3) led to a significant increase in anti selectivity. Increasing the catalyst loading to 5 mol%, and the amount of ketone to 2.0 equiv, and stirring the reaction at -30 °C (entry 4) gave a high yield of 5a with high dr and ee. Increasing the size of the chiral ligand by switching from 1a (Ar = Ph) to 1b (Ar = 4-biphenyl) gave comparable yield and ee but with slightly increased dr (entry 5). On the other hand, using ligand 1 c decreased both yield and dr (entry 6). By lowering the catalyst loading to 3.5 and 2.5 mol% (entries 7 and 8), the desired product 5a was also obtained in high yield and selectivity. With 2.5 mol% 2a, however, a longer reaction time (36 h) was necessary (entry 8).
Table 1.
Optimization Studiesa
| entry | 3a (equiv) | 4a (equiv) | cat. 2 (mol%) | temp (°C) | time (h) | yieldb (%) | drc (anti:syn) | eed (%) (anti) |
|---|---|---|---|---|---|---|---|---|
| 1e | 1.4 | 1 | 2a (3.5) | 23 | 17 | 62 | 1:1 | ND |
| 2e | 1.4 | 1 | 2a (3.5) | -5 | 17 | 66 | 1:1 | (-)-67 |
| 3 | 1.4 | 1 | 2a (3.5) | -25 | 14 | 62 | 5:1 | (-)-96 |
| 4 | 2 | 1 | 2a (5.0) | -30 | 36 | 86 | 5:1 | (-)-94 |
| 5 | 2 | 1 | 2bf (5.0) | -30 | 36 | 80 | 6:1 | (+)-96 |
| 6 | 2 | 1 | 2c (5.0) | -30 | 36 | 75 | 4:1 | ND |
| 7 | 2 | 1 | 2a (3.5) | -30 | 24 | 86 | 5:1 | (-)-94 |
| 8 | 2 | 1 | 2a (2.5) | -30 | 36 | 90 | 5:1 | (-)-92 |
To mixture of catalyst 2, ketone 3a and 4Å MS in THF was added imine 4a in THF at the temperature shown in the Table.
Isolated yield.
Determined by the 1H NMR of the crude mixture.
Determined utilizing chiral HPLC.
To suspension of 3a, imine 4a, and 4Å MS in THF was added the catalyst 2 in THF.
(R,R)-catalyst 2b was used.
ND = not determined.
Scheme 2.
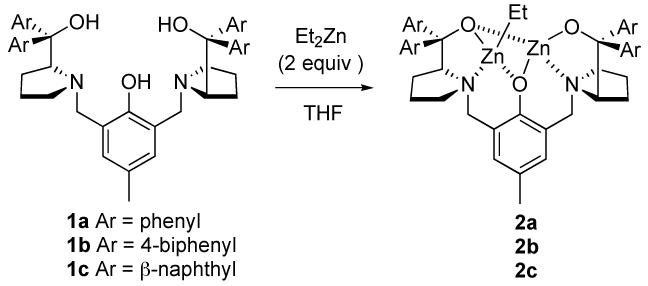
Generation of Dinuclear Zinc Catalyst
The optimized reaction conditions (Table 1, entry 7) was applicable to various aliphatic Dpp-imine 5, and the results are summarized in Table 2. Increasing the size of the α-substituents of the Dpp-imines increases the dr and ee. Reacting 3a with imine 4d-f (entries 4-6) derived from primary aldehydes (bearing both linear and β-branched aliphatic chains) also afforded the anti-amino alcohols 5d-f, respectively, in good yields and excellent ee with high diastereoselectivity (dr >4:1).
Table 2.
Aymmetric Mannich-type Reaction with Dpp-Iminea
| entry | Ar | R | product | yieldb (%) | drc (anti:syn) | eed (%) (anti) | |
|---|---|---|---|---|---|---|---|
| 1 | Ph | 3a cyclo-hexyl | 4a | 5a | 86 | 5:1 | 94 |
| 2 | Ph | 3a cyclo-propyl | 4b | 5b | 79 | 5:1 | 83 |
| 3 | Ph | 3a i-propyl | 4c | 5c | 83 | 6:1 | >99 |
| 4 | Ph | 3a i-butyl | 4d | 5d | 80 | 5:1 | 96 |
| 5 | Ph | 3a PhCH2CH2 | 4e | 5e | 76 | 4:1 | 96 |
| 6 | Ph | 3a n-hexyl | 4f | 5f | 71 | 4:1 | 96 |
| 7 | 2-furyl | 3b cyclo-hexyl | 4a | 5g | 73 | 3:1 | 83 |
| 8e | 3b | 4a | 5g | 85 | 4:1 | 90 | |
| 9 | 2-MeOC6H4 | 3c cyclo-hexyl | 4a | 5h | 65 | 2:1 | 56 |
| 10e | 3c | 4a | 5h | 70 | 1:1 | 57 | |
| 11 | 1-naphthyl | 3d i-propyl | 4c | 5i | 71 | 3:1 | 87 |
| 12e | 3d | 4c | 5i | 74 | 4:1 | 88 | |
| 13 | 2-naphthyl | 3e i-propyl | 4c | 5j | 69 | 3:1 | (-)-86 |
| 14e | 3e | 4c | 5j | 77 | 4:1 | (-)-95 | |
| 15f | 3e | 4c | 5j | 74 | 4:1 | (+)-95 | |
All reactions were performed using 3.5 mol% 2a and 2 equiv of 3 in THF at 0.3 M unless noted otherwise.
Isolated yield.
Determined by the 1H NMR of the crude mixture.
Determined utilizing chiral HPLC.
5 mol% catalyst 2a.
5 mol% (R,R)-catalyst 2a.
In an analogous manner, Mannich-type reaction with other hydroxyketone donors was then investigated to extend the scope of the reaction (Table 2, entries 7 to 13). The use of hetero-aromatic hydroxyketone was found to be applicable in our Mannich-type reaction. With 2-hydroxyacetylfuran 3b and imine 4a, an increase in both yield and stereoselectivity of the resultant amino alcohol 5g was observed with a higher catalyst load (entries 7 and 8). Surprisingly, hydroxyketone 3c (entries 9 and 10), the best ketone donor in Shibasaki’ results,3c-d saw a dramatic drop in both dr and ee. The hydroxyketones 3d and 3e (entries 11 to 15) were studied in order to gain insight on the origin of the observed selectivity. With ketone 3d and 4c using 3.5 mol% catalyst loading, dr was modest (entry 11). Increasing catalyst loading to 5 mol%, dr was significantly improved (entry 12). The use of hydroxyketone 3e and imine 4c in the presence of 5 mol% 2a also provided the Mannich adduct 5j in high ee (95%) with good anti selectivity (entry 14). Furthermore, the enantiomeric product was smoothly obtained in comparable yield and dr with completely reverse enantioselectivity when (R,R)-2a was used (entry 15). It is clear from our results that the methoxy substituent in the ortho-position plays a significant role in the loss of the yield and selectivity.
Another class of imine investigated was Boc-imine 6 (Table 3). Surprisingly the syn-β-amino alcohol 7a was selectively obtained in a ratio of 5 (syn, 94% ee) to 1 (anti) on treatment of imine 6a with 3a in the presence of 5 mol% catalyst 2a and 4Å MS in THF (entry 1). In this reaction, the undesired product 8a derived from alkoxide attack on the imine was isolated as a minor product (6%). The reaction of 3a with acyclic imine 6b also afforded the syn-7b in good yield and excellent ee. To the best of our knowledge, this is the first example of a direct catalytic asymmetric Mannich-type reaction using a Bocimine derived from an α-enolizable aldehyde.
Table 3.
Aymmetric Mannich-type Reaction with Boc-Iminea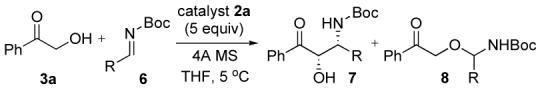
| entry | R | product | time(h) | yieldb (%) | drc (anti:syn) | eed (%) (anti,syn) | 8b(h) (%) | |
|---|---|---|---|---|---|---|---|---|
| 1 | cyclo-hexyl | 6a | 7a | 14 | 77 | 1:5 | ND,94 | 6 |
| 2 | i-propyl | 6b | 7b | 19 | 70 | 1:3 | 95,90 | 5 |
All reactions were performed using 5 mol% 2a and 2 equiv of 3a in THF at 0.3 M unless noted otherwise.
Isolated yield.
Determined by the 1H NMR of the crude mixture.
Determined utilizing chiral HPLC.
ND = not determined.
The relative and absolute stereochemistry were established by converting the amino alcohols into their corresponding 1,3-oxa-zolidin-2-one through NOE studies,7 and O-methyl mandelic amides, respectively.8 It is noteworthy that our dinuclear zinc catalyst 2 provides the Mannich adduct, anti-5 and syn-7, together with aldol adduct6 with the same absolute configuration at the α-position. On the other hand, the stereoselectivity at the β-position of the amino alcohol derivatives is differentiated. The observed stereoselectivities (see Scheme 1) can be understood by assuming the following mechanism. With the more bulky Dpp-imine, anti selectivity dominates to avoid the steric repulsion between the Dpp-group and the Zn-enolate.3d Conversely, to avoid the steric repulsion between a substituent (R group) of the less sterically demanding Boc-imine and zinc-enolate, the syn-amino alcohol 7 was observed in this case.
In summary, we have demonstrated the application of our dinuclear zinc catalyst for the synthesis of either syn- or anti-amino alcohols. Typically, with aliphatic Dpp-imines, the desired amino alcohols were obtained with anti-selectivity (yield up to 86, dr up to 6:1, ee up to >99%). On the other hand, syn selectivity was obtained in the reaction with Boc-imines. Detailed mechanistic studies of the present reaction, and further application of our catalyst with others hydroxyketone donors, and aliphatic Boc-imines are ongoing.
Supplementary Material
Acknowledgment
We thank the National Science Foundation and the National Institutes of Health, General Medical Sciences (GM-13598) for their generous support. The awards of the Royal Golden Jubilee Ph.D. Scholarship to JJ and a Senior Research Scholar to VR by the Thailand Research Fund are also gratefully acknowledged. We also thank the Higher Education Development project: PERCH for financial support. Mass spectra were provided by the Mass Spectrometry Regional Center of the University of California-San Francisco supported by the NIH Division of Research Resources.
References
- (1).Reviews:Denmark SE, Nicaise OJ-C. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Springer; Heidelberg: 1999. pp. 923–961.Kleinmann EF. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 2. Pergamon Press; New York: 1991. Chapter 4.1.Arend M, Westermann B, Risch N. Angew. Chem. Int. Ed. 1998;37:1044. doi: 10.1002/(SICI)1521-3773(19980504)37:8<1044::AID-ANIE1044>3.0.CO;2-E.Kobayashi S, Ishitani H. Chem. Rev. 1999;99:1069. doi: 10.1021/cr980414z.
- (2).A review of the direct Mannich reaction:Córdova A. Acc. Chem. Res. 2004;37:102. doi: 10.1021/ar030231l.
- (3).Selected examples for metal catalysts, see:Juhl K, Gathergood N, Jørgensen KA. Angew. Chem. Int. Ed. 2001;40:2995. doi: 10.1002/1521-3773(20010817)40:16<2995::AID-ANIE2995>3.0.CO;2-M.Trost BM, Terrell LR. J. Am. Chem. Soc. 2003;125:338. doi: 10.1021/ja028782e.Matsunaga S, Kumagai N, Harada S, Shibasaki M. J. Am. Chem. Soc. 2003;125:4712. doi: 10.1021/ja034787f.Matsunaga S, Yoshida T, Morimoto H, Kumagai N, Shibasaki M. J. Am. Chem. Soc. 2004;126:8777. doi: 10.1021/ja0482435.Harada S, Handa S, Matsunaga S, Shibasaki M. Angew. Chem. Int. Ed. 2005;44:4365. doi: 10.1002/anie.200501180.For organocatalysts, see:List B. J. Am. Chem. Soc. 2000;122:9336.List B, Pojarliev P, Biller WT, Martin HJ. J. Am. Chem. Soc. 2002;124:827. doi: 10.1021/ja0174231.Córdova A, Notz W, Zhong G, Betancort JM, Barbas CF., III J. Am. Chem. Soc. 2002;124:1842. doi: 10.1021/ja017270h.
- (4).Dpp-imine was prepared by treatment of N-(diphenylphosphinoyl)-α-(p-toluenesulfonyl) alkylamine 9 with sat. aq. NaHCO3 in CH2Cl2: see Supporting Information for details. For preparation of 9, see:Côté A, Boezio AA, Charette AB. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5405. doi: 10.1073/pnas.0307096101.
- (5).Boc-imine was prepared by treatment of N-(N-tert-butyloxy-carbonyl)-α-(phenylsulfonyl) alkylamine 10 with 1.5 M aq. K2CO3 in CH2Cl2: see Supporting Information for details. For preparation of 10, see:Pearson WH, Lindbeck AC, Kampf JW. J. Am. Chem. Soc. 1993;115:2622.
- (6).Leading reference:Trost BM, Ito H. J. Am. Chem. Soc. 2000;122:12003.
- (7).In support of our assignment, the relative stereochemistry of oxazolidinones can also be determined by examination of the J4-5 coupling constants:Murakami M, Ito H, Ito Y. J. Org. Chem. 1993;58:6766.
- (8).Trost BM, Bunt RC, Rulley SR. J. Org. Chem. 1994;59:4202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


