Abstract
In mammals, oocytes are packaged into compact structures—primordial follicles—which remain inert for prolonged intervals until individual follicles resume growth via a process known as primordial follicle activation. Here we show that the phosphoinositide 3-kinase (PI3K) signalling pathway controls primordial follicle activation through the forkhead transcription factor Foxo3. Within oocytes, Foxo3 is regulated by nucleocytoplasmic shuttling. Foxo3 is imported into the nucleus during primordial follicle assembly, and is exported upon activation. Oocyte-specific ablation of Pten resulted in PI3K-induced Akt activation, Foxo3 hyperphosphorylation, and Foxo3 nuclear export, thereby triggering primordial follicle activation, defining the steps by which the PI3K pathway and Foxo3 control this process. Inducible ablation of Pten and Foxo3 in adult oocytes using a new tool for genetic analysis of the germline, Vasa-CreERT2, showed that this pathway functions throughout life. Thus, a principal physiologic role of the PI3K pathway is to control primordial follicle activation via Foxo3.
Keywords: Akt, Forkhead transcription factors, germ cells, PI3K, Pten
Introduction
Primordial follicles, each comprised of a single oocyte and a surrounding layer of somatic granulosa cells, are formed early in life and represent a finite resource that must be safeguarded and efficiently utilized. The depletion of primordial follicles culminates in reproductive senescence or menopause, leading to the acceleration of many age-associated changes in women, such as reduced bone density (Block, 1952; Lobo, 2007). Primordial follicle activation is the irreversible, metered process by which primordial follicles are continually recruited to initiate follicle maturation (McGee and Hsueh, 2000). This process begins immediately after follicle assembly within a few days of birth and is gonadotropin-independent (Mason et al., 1986; Peters et al., 1973). Follicle growth is irreversible, and follicles that have initiated growth either complete the process (culminating in ovulation), or undergo atresia at some stage of their maturation. The irreversibility of this process and the limited supply of primordial follicles further suggest that primordial follicle activation is likely to be tightly regulated. Yet, the molecular pathway(s) regulating primordial follicle activation remain ill-defined, limiting our ability to understand this fundamental aspect of ovarian development and function that influences both fertility and the menopause.
The Foxo family of forkhead transcription factors (Foxo1, Foxo3, Foxo4, and Foxo6) participates in diverse processes including cell proliferation, apoptosis, stress resistance, differentiation, and metabolism (Accili and Arden, 2004). Foxo1, Foxo3, and Foxo4 are highly expressed in most tissues, and share partially overlapping functions, whereas Foxo6 expression appears to be restricted to the brain. Consistent with their participation in diverse processes, the Foxos are regulated by a variety of mechanisms including phosphorylation, acetylation, and ubiquitination (van der Horst and Burgering, 2007). Recently, we showed that the forkhead transcription factor Foxo3 is a master regulator and suppressor of primordial follicle activation, the first such factor to be defined. In Foxo3 knockout mice, primordial follicles are assembled normally (John et al., 2007) but then immediately undergo global activation, resulting in a distinctive syndrome of ovarian hyperplasia, follicle depletion, premature ovarian failure, and infertility (Castrillon et al., 2003; Hosaka et al., 2004). However, many basic questions regarding the regulation of Foxo3 and its role in the suppression of primordial follicle activation remain unanswered. For example, it is not known if Foxo3 is part of a mechanism that actively controls the decision to trigger oocyte growth, or if Foxo3 mutation promotes follicle activation via some other, less direct mode of action.
The phosphatidylinositol 3-kinase (PI3K)-Akt signalling pathway is an important regulator of cell proliferation and survival, widely studied because of its participation in cancer and other disease processes (Cully et al., 2006). More recently, PI3K pathway components including Pten have been implicated in stem cell maintenance and the regulation of organ size (Groszer et al., 2001; Yilmaz et al., 2006) suggesting that this pathway plays general, albeit incompletely understood roles in tissue maintenance. PI3K catalyzes the production of the phosphoinositide PI(3,4,5)P3 (PIP3) from PI(4,5)P2 in the plasma membrane, resulting in membrane recruitment, phosphorylation, and activation of Akt. Pten serves as a potent PI3K antagonist by removing the 3′ phosphate from PIP3 (Engelman et al., 2006), thereby inhibiting Akt. Activated, phosphorylated Akt in turn phosphorylates a wide range of direct intracellular targets containing a minimal Akt recognition motif, including Gsk3, Bad, Tsc2, and the Foxos, among many others; at least 20 bona fide Akt substrates have been identified (Brunet et al., 1999; Manning and Cantley, 2007). However, it has been difficult in most experimental systems to fully define the physiologically-relevant substrates and their relative contributions in mediating the biological effects of PI3K-Akt signalling.
In this paper, we show that the PI3K-Akt pathway has a key role in the initiation of oocyte growth (and hence in the maintenance of oocytes) and acts via Foxo3. Oocyte-specific Pten ablation resulted in Akt hyperactivation, Foxo3 hyperphosphorylation, and Foxo3 nuclear export, culminating in global primordial follicle activation and premature ovarian failure. Surprisingly, oocyte-specific ablation of Pten and Foxo3 resulted in virtually identical phenotypes of global primordial follicle activation, arguing that Foxo3 is the primary, if not sole effector of PI3K-Akt signalling in this physiologic context. Pharmacologic inhibition of PI3K suppressed the Pten but not the Foxo3 ovarian phenotype, further establishing that Foxo3 lies downstream of Pten. Our results demonstrate that Foxo3 is the prime effector of the PI3K-AKT pathway in the context of primordial follicle activation and that, surprisingly, the only essential role of Pten within the oocyte is to regulate Foxo3.
Materials and methods
Mouse Strains, Breeding, and Analysis
This study was approved by an Institutional Animal Care and Use Committee. All alleles were in an FVB/n background (backcrossed at least n = 6 generations). Genotyping of the Vasa-Cre, R26R, Pten, and Foxo3 alleles was performed on tail DNA using multiplexed PCR protocols as described (Castrillon et al., 2003; Gallardo et al., 2007; Li et al., 2002; Soriano, 1999); the protocol for Vasa-CreERT2 was the same as for Vasa-Cre. Vasa-Cre/+ mice were bred to Foxo3L/L homozygotes to obtain Vasa-Cre; Foxo3L/+ male progeny; males must be used for the second generation cross because of a potent maternal effect observed with Vasa-Cre (Gallardo et al., 2007). The Vasa-Cre transgene effects Cre-mediated recombination in germ cells and thus, Vasa-Cre carriers cannot transmit the L allele; e.g. Vasa-Cre; Foxo3L/+ mice can only transmit the null (−) or wt (+) Foxo3 alleles. Vasa-Cre; Foxo3L/+ males were crossed to Foxo3L/L females to generate experimental Vasa-Cre; Foxo3−/L females and sibling controls. An analogous strategy was employed to generate Vasa-Cre; Pten−/L animals and sibling controls. Pten floxed (Li et al., 2002) and R26R mice (Soriano, 1999) were purchased from Jackson Laboratories. Ovaries from at least n = 3 experimental and n = 3 control animals were evaluated for each timepoint in all analyses.
Tissue Processing, Immunohistochemistry and Immunofluorescence
Tissue sections from experimental and control samples were placed on the same slide to ensure identical processing; at least n = 3 slides were evaluated for each antibody. For immunohistochemistry, tissues were fixed in 10% formalin 1–12 hours, then processed and embedded in paraffin. 5 μ sections were deparaffinized in xylene, and hydrated in an ethanol series. Slides were subjected to antigen retrieval by boiling in 10mM NaCitrate and cooled at RTx20 min. Antibodies and titers used were: Foxo1 1:100 (Santa Cruz # sc-11350), Foxo4 1:200 (Santa Cruz # sc-5221), Pten 1:100 (Cell Signaling # 9559), p-Akt (Ser473) 1:50 (Cell Signalling # 9271), Foxo3 1:200 (Santa Cruz # sc-11351), p-Foxo3 (Thr32) 1:200 (Upstate # 07-695), p-mTOR (Ser2448) (Cell Signalling # 2976), p-S6 Ribosomal protein (Ser235/236) (Cell Signaling # 4857) and p-4E-BP1 (Thr70) (Cell Signaling # 9455). The detection system was Immpress (Vector, Burlingame, CA).
For immunofluorescence, tissues were fixed in 4% paraformaldehyde overnight at 4o and embedded in OCT. Frozen sections (5μ) were obtained and detection of PIP3 was performed as described (Kitamura et al., 2004) with Biotin-PIP3 (1:100) (Echelon Biosciences # Z-B345b) and streptavidin-alexa fluor 488 (1:200) (Invitrogen # S11223). Images were obtained on an Olympus BX51 microscope equipped for epifluorescence.
Wholemount X-gal staining was performed as described (Gallardo et al., 2007).
Organ Culture and Histomorphometry
Ovaries were photographed after explantation and at 4 and 8 days and the relative ovarian volume was approximated by the equation V = (long diameter x short diameter2). Average oocyte diameter was determined on H&E tissue sections per the long diameter of oocytes with nuclei in the plane of section (at least n>100 such oocytes were analyzed per ovary). Ovaries were cultured (37C, 5% CO2) on Transwell Permeable supports (Costar, catalog # 3413) in Waymouth’s medium (Invitrogen catalog # 11220-035) supplemented with 10% fetal bovine serum, 0.23 mM Pyruvic acid, 3 mg/ml BSA, 1x ITS supplement (Invitrogen, catalog # 51300-044) and 1x Antibiotic-Antimycotic (Invitrogen catalog # 15240-062). PI3K inhibition was performed by addition of 25 μM Ly294002 (Cell Signaling Technologies, catalog # 9901) to the media.
Generation of VasaCreERT2 Transgenic Mice and Tamoxifen Administration
To generate the pVasaCre-ERT2 construct used for transgenesis, the SV40 promoter fragment (SalI fragment) was removed from pCre-ERT2 (Feil et al., 1997) by SalI digestion/religation. A polylinker was then inserted by annealing oligos 5′-CTAGGTCGACGGCGCGCCGCGGCCGCTTAATTAA-3′ and 5′-GATCAATTAATTCGCCGGCGCCGCGCGGCAGCTG-3′ and cloning into the AvrII site. The Vasa promoter fragment from pVasaCre (Gallardo et al., 2007) was released with AscI and PacI and cloned into the AscI and PacI sites of the above polylinker. The resulting 11666 bp plasmid was linearized with SalI, purified on an Elutip-D column (Schleicher & Schuell) and microinjected into FVB/n oocytes by standard protocols (Nagy et al., 2003) at the UT Southwestern Transgenic Core Facility. Lines with transgene integration were identified by Southern blot, and further validated by Northern analysis. Total RNA (10 μg) was prepared from adult tissues using Tripure reagent (Roche), electrophoresed on a 1% formaldehyde gel, transferred to Hybond N+, probed with Cre and reprobed with GAPDH as a loading control. One line with high expression in testis and ovary (but not any other somatic tissues) was selected for further analysis and validation by crossing with R26R (Soriano, 1999). This line was backcrossed to FVB/n for 6 generations.
Tamoxifen (Sigma, catalog # T5648) was resuspended at 100 mg/ml in 100% ethanol and further diluted with corn oil (Sigma, catalog # C8267) to a final concentration of 20 mg/ml. Intraperitoneal injections (2mg tamoxifen/100 μl corn oil) were given qD for three consecutive days. Tissues were collected 24 hours following the last injection.
To exclude the possibility that tamoxifen might affect follicle growth, wild-type animals (n = 6) were subjected to tamoxifen treatment. There was no evidence of increased activation three weeks after treatment: there was no alteration in follicle counts including the ratio of primordial to growing follicles, and there was no increase in oocyte diameter or change in pregranulosa cell morphology (data not shown). To exclude the possibility that the Vasa-CreERT2 allele results in altered follicle growth dynamics, ovaries (n = 6 Vasa-CreERT2 females) were similarly analyzed and found to be morphologically unaltered (data not shown).
Results
Foxo3 Localizes to the Oocyte and Undergoes Nucleocytoplasmic Shuttling
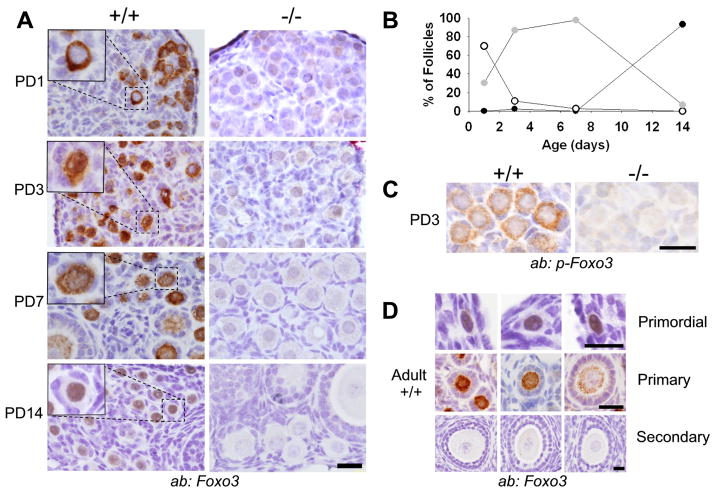
To begin to understand the role of Foxo3 in the suppression of primordial follicle activation, the Foxo3 protein was localized within mouse ovary tissue sections. Although prior mRNA in situ hybridization studies revealed widespread Foxo3 expression throughout the ovary (Castrillon et al., 2003; Richards et al., 2002), the protein itself is detectable only within oogonia and oocytes (Fig. 1A). The closely-related forkhead transcription factors Foxo1 and Foxo4 are not similarly localized to the oocyte (Fig. S1), rationalizing the unique, nonredundant role of Foxo3 in oogenesis (Paik et al., 2007; Tothova et al., 2007). At postnatal day (PD) 1, Foxo3 protein was cytoplasmic within most oogonia (Fig. 1A). By PD3, however, the protein had partially translocated to the nucleus and was detectable in both cytoplasm and nucleus in the great majority of follicles (Fig. 1B). By PD14, Foxo3 had completely translocated to the nucleus (Fig. 1A, B).
Fig. 1.
Foxo3 nucleocytoplasmic shuttling during ovarian development and early follicle growth. (A) Immunolocalization of Foxo3 in Foxo3+/+ and Foxo3−/− (negative control) ovaries, PD1–PD14. (B) Percent of follicles showing cytoplasmic (open circles), nuclear (black circles), or nuclear + cytoplasmic Foxo3 protein localization (gray circles) at PD1-14. (C) Immunodetection of p-Foxo3 (Thr32) at PD3. Foxo3−/−control confirms antibody specificity. (D) Nuclear to cytoplasmic translocation and degradation during early follicle growth, adult ovaries (6 weeks). Foxo3 localized to nucleus + cytoplasm in small primary follicles (2 left panels), but the protein was consistently cytoplasmic in larger primary follicles (right panel). Bars in all panels are 20 μ.
Foxo3 undergoes a number of post-translational modifications that regulate its activity, subcellular localization, and stability including acetylation, ubiquitylation, and phosphorylation by the kinases Ikkβ, Sgk, Cdk2, Dyrk1, Jnk, Ampk and Akt (Greer et al., 2007a; Greer et al., 2007b; van der Horst and Burgering, 2007). We speculated that the observed developmental translocation of Foxo3 protein was mediated by phosphorylation. Consistent with this hypothesis, a phospho-specific antibody that detects Foxo3 phosphorylated at its Akt consensus phosphorylation site RPRSCT (Thr32) detected p-Foxo3 (Thr32) in oocytes at PD3 (Fig. 1C). Furthermore, p-Foxo3(Thr32) localized exclusively to the cytoplasm, suggesting that phosphorylation of Foxo3 by Akt regulates its subcellular localization within oocytes (Fig. 1C).
The timing of Foxo3 cytoplasmic-to-nuclear import coincides with the formation of individualized primordial follicles, which is completed by PD3, implying that nuclear Foxo3 is essential for suppression of follicle activation. Consistent with this interpretation, and further suggesting that Foxo3 restricts primordial follicle activation throughout life (see below), Foxo3 retains its nuclear localization in adult primordial oocytes (Fig. 1D). In growing follicles in wild-type adults, Foxo3 protein underwent a reverse shift from the nucleus to the cytoplasm, and then appeared to be rapidly degraded by the secondary follicle stage (Fig. 1D). The biological basis of this Foxo3 protein degradation has not been explored, but may be the result of ubiquitylation (van der Horst and Burgering, 2007). A prior study found that enforced expression of a constitutively active form of Foxo3 in maturing oocytes disrupts follicle maturation (Liu et al., 2007), suggesting that Foxo3 degradation in growing follicles is physiologically significant.
Foxo3 Functions Specifically Within Oocytes to Suppress Primordial Follicle Activation
These results suggested that although the gene is broadly expressed, Foxo3 might function specifically within oocytes as a molecular switch to regulate primordial follicle activation. To confirm this genetically, we employed a conditional (floxed) Foxo3L allele and a Vasa-Cre (a.k.a. ddx4-Cre) deletor strain we previously generated (Castrillon et al., 2003; Gallardo et al., 2007; Paik et al., 2007). Vasa-Cre results in Cre-mediated recombination in >95% of oocytes in Rosa26β-galactosidase reporter (R26R) mice (Soriano, 1999) by PD3, with no recombination in other ovarian cell types, and minimal recombination in other tissues (Gallardo et al., 2007). Ovaries from 3 week-old Vasa-Cre; Foxo3−/L females were dramatically enlarged relative to sibling controls (Fig. 2A), and histologic analysis demonstrated that this ovarian enlargement was due to global primordial follicle activation (Fig. 2B). These results constitute formal genetic proof that Foxo3 functions specifically within oocytes to suppress primordial follicle activation, at least early in life.
Fig. 2.
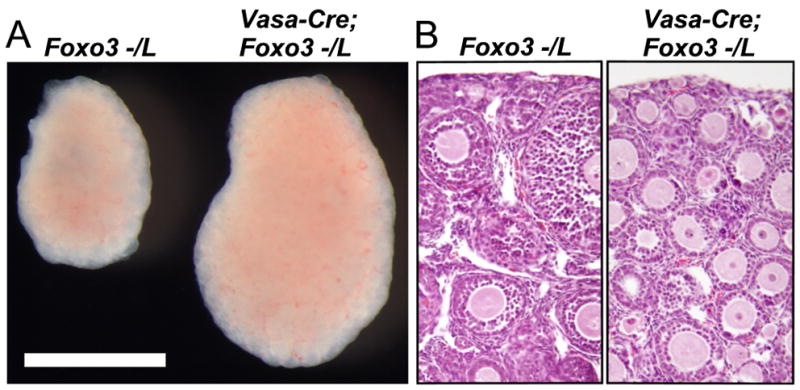
Foxo3 functions within oocytes to suppress primordial follicle activation. (A) Ovarian hypertrophy in Vasa-Cre; Foxo3−/L female relative to sibling control. Bar = 1 mm. (B) Global follicle activation in Vasa-Cre; Foxo3−/L ovary; H&E-stained tissue section.
Essential Role of Pten and PI3K-Akt Signalling in Primordial Follicle Activation
To determine if Foxo3 in the context of primordial follicle activation is regulated primarily by PI3K-Akt signalling, we conditionally deleted the Pten gene in oocytes with Vasa-Cre and the previously described floxed allele PtenL (Li et al., 2002). Consistent with prior results (Reddy et al., 2008), Vasa-Cre; Pten−/L ovaries, which have Pten-null oocytes, were dramatically enlarged by PD21; histologic analysis showed that this was due to widespread primordial follicle activation (Fig. 3). This confirms that Pten serves an essential physiologic role in the suppression of primordial follicle activation; in addition to Foxo3, it is the only other such factor identified (Castrillon et al., 2003). Surprisingly, in breeding tests with wild-type males, Vasa-Cre; Pten−/L females were initially fertile, although they exhibited a dramatic age-dependent decrease in fertility, having no more than two litters. Thus, within oocytes, Pten is specifically required for primordial follicle activation but not subsequent steps of oocyte growth, ovulation, or fertilization, a property it shares with Foxo3 (Castrillon et al., 2003; John et al., 2007). All progeny analyzed (n = 10) were heterozygous for the null Pten allele (data not shown), showing that the observed fertility was not due to “escaper” oocytes that failed to undergo Cre-mediated recombination. That the Vasa-Cre Foxo3 and Pten mutant phenotypes have identical global activation phenotypes argues that Foxo3 is the chief physiologic target of the PI3K-Akt signalling pathway in the context of primordial follicle activation.
Fig. 3.
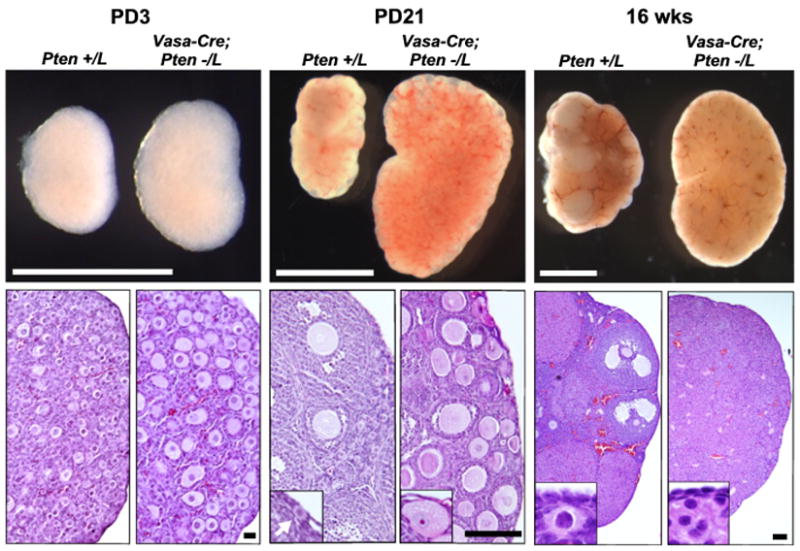
Pten maintains egg supply and prevents ovarian failure. Upper panels: Vasa-Cre; Pten−/L and control ovaries at PD3, PD21, and 16 weeks; size bars = 1mm. Lower panels, H&E-stained sections; size bars = (left to right) 20 μ, 100 μ, and 100 μ. At 16 weeks, controls have numerous primordial follicles whereas Vasa-Cre; Pten−/L ovaries are devoid of follicles, signifying complete ovarian failure. PD21 insets show primordial follicle in wild-type (arrow) and absence of primordial follicles in Vasa-Cre; Pten−/L ovary. 16 wk insets show normal primordial follicles in control ovary and stromal overgrowth/complete absence of follicles in Vasa-Cre; Pten−/L ovary. PD21 and 16 wk insets are not at same magnification.
Oocyte Foxo3 is Regulated by PI3K-Akt Signalling
To confirm this hypothesis, we analyzed the status and subcellular localization of pathway components within oocytes. In control ovaries, Pten was readily detectable in primordial oocytes, where it localized to both nucleus and cytoplasm, but was undetectable in Vasa-Cre; Pten−/L oocytes, as expected (Fig. 4). Pten loss resulted in a dramatic increase of membrane-associated PIP3 in oocytes at PD3, promoting Akt membrane recruitment and activation, as evidenced by increased membrane-associated p-Akt. This increased Akt activity led to Foxo3 hyperphosphorylation and nuclear export (Fig. 4). We observed no evidence of mTOR pathway activation (by analyzing p-mTOR, p-S6, p-S6K, and p-4EBP) in PD3 Vasa-Cre; Pten−/L oocytes (data not shown). Thus, although the mTOR pathway normally becomes activated at much later stages of follicle growth (John et al., 2007), it does not appear to be a part of the mechanism that triggers follicle growth, further underscoring the role of Foxo3 as the prime effector of PI3K-Akt signalling in primordial follicle activation.
Fig. 4.
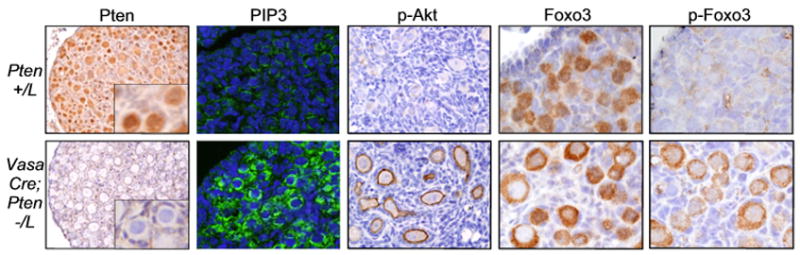
Pten regulates primordial follicle activation via PI3K and Foxo3. Pten ablation leads to increased PIP3, Akt activation, Foxo3 hyperphosphorylation, and nuclear export. Panels show Pten, PIP3, p-Akt (Ser473), Foxo3 and p-Foxo3 (Thr32) in PD3 ovaries counterstained with hematoxylin or DAPI (PIP3).
Pharmacologic Inhibition Studies Confirm Pathway Linearity and that Foxo3 is Downstream of Pten
To confirm that primordial follicle activation is regulated via the linear pathway (Pten/PI3K—Akt—Foxo3) suggested by these observations, ovaries were explanted at birth and cultured for 8 days in the presence of the PI3K inhibitor Ly294002. Untreated Vasa-Cre; Pten−/L and Vasa-Cre; Foxo3−/L ovaries grew more rapidly than controls. Ly294002 suppressed growth of Vasa-Cre; Pten−/L but not Vasa-Cre; Foxo3−/L ovaries, and measurements of average oocyte diameter, a more direct indicator of primordial follicle activation (Fig. 5) also showed that Ly294002 had a significant effect in Vasa-Cre; Pten−/L but not Vasa-Cre; Foxo3−/L ovaries (p = 2.6x10−7 vs. 0.26, Student’s T Test). Although it is possible that Ly294002 may have inhibited additional PI3K family members, these results demonstrate that the Pten (but not Foxo3) ovarian phenotype is PI3K-dependent, consistent with the above linear, ordered pathway where Pten regulates oocyte growth via PIP3, PI3K, Akt, and Foxo3. Furthermore, these findings and the equivalence of the Pten and Foxo3 ovarian phenotypes including their initial fertility strongly argue that the only essential physiologic role of Pten within oocytes is to forestall primordial follicle depletion by maintaining Foxo3 within the nucleus.
Fig. 5.
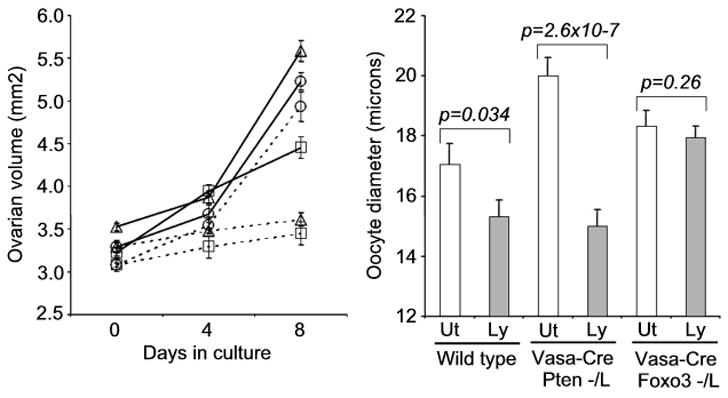
Pharmacologic inhibition shows PI3K activity is required for Pten but not Foxo3 ovarian phenotype. Ovaries were explanted at birth and cultured for 8 days. Solid lines, untreated; dashed bars, 25 μM LY294002. Squares = wild-type, triangles = Vasa-Cre; Pten−/L; circles = Vasa-Cre; Foxo3−/L. Graph on right shows oocyte diameters at the end of 8 day culture period. Ut = untreated, Ly = 25 μM LY294002. Note: The small apparent difference in untreated Pten−/L vs. Foxo3−/L oocytes is not significant (p = 0.40). Error bars = s. e. m.; p values calculated by Student’s T Test.
Generation and Validation of VasaCreERT2, a Novel Tool for Inducible Gene Ablation within the Mouse Germline
The above genetic analyses utilized a Vasa-Cre transgene that is active starting at around embryonic day 15, and results in near-total Cre-mediated recombination by PD3 (Gallardo et al., 2007). Our findings were thus consistent with two alternate models. In the first model, PI3K-Akt signalling acts during this narrow developmental window (E15-PD3) to endow primordial follicles with some factor(s) that suppress follicle activation later in life, or are required only prior to PD3. Alternatively, the PI3K-Akt pathway might function continually throughout life to balance primordial follicle activation and preservation. To formally distinguish between these two models, an inducible, germ cell-specific CreERT2 transgene was generated by placing a CreERT2 cDNA under the control of the murine Vasa (Ddx4) promoter (Vasa-CreERT2). The CreERT2 fusion protein is inactive until tamoxifen is administered (Feil et al., 1997). Tamoxifen treatment of Vasa-CreERT2; R26R female mice at 6 weeks of age resulted in efficient (90–95%) oocyte-specific recombination; there was no evidence of recombination prior to tamoxifen treatment (Fig. S2; see also Experimental Procedures for additional details and controls). This efficiency was similar to that observed in the male germline at 3–6 weeks of age (Fig. S2, B–D). Incidentally, since production of active β-galactosidase requires 1) tamoxifen-induced transport of Cre from the cytoplasm to the nucleus, 2) homologous recombination of loxP sites, 3) transcription, mRNA transport, protein translation and transport, this experiment reveals that primordial follicles are normally engaged in a surprisingly high degree of metabolic activity and turnover of cellular components.
The Pten-PI3K-Akt-Foxo3 Pathway that Regulates Primordial Follicle Activation Functions throughout Life
Vasa-CreERT2; Foxo3L/L and Vasa-CreERT2; PtenL/L females were generated by standard genetic crosses and treated at 6 weeks of age with tamoxifen. Ovaries were harvested following a three week interval to permit follicle growth and scoring of follicle growth phenotypes. Ablation of both Pten and Foxo3 in adult oocytes resulted in identical phenotypes of ovarian hypertrophy; histologic analyses confirmed global follicle activation (Fig. 6). Similar results were obtained at 3, 9, and 12 weeks of age (not shown). Thus, this pathway functions throughout life to regulate the utilization of the egg supply through Foxo3.
Fig. 6.

Ablation of Pten and Foxo3 in tamoxifen-treated adult Vasa-CreERT2 females results in global primordial follicle activation. (A) Pten (B) Foxo3. Bar = 1 mm. For both experiments, females were treated with tamoxifen and ovaries harvested 3 weeks later; controls were untreated siblings of the same genotype. Ablation of Pten and Foxo3 resulted in identical ovarian hypertrophy phenotypes; histological examination (H&E) confirmed that ovarian hypertrophy was due to global primordial follicle activation. Size bar = 50 μ. All corresponding panels in A and B are at the same magnification.
Discussion
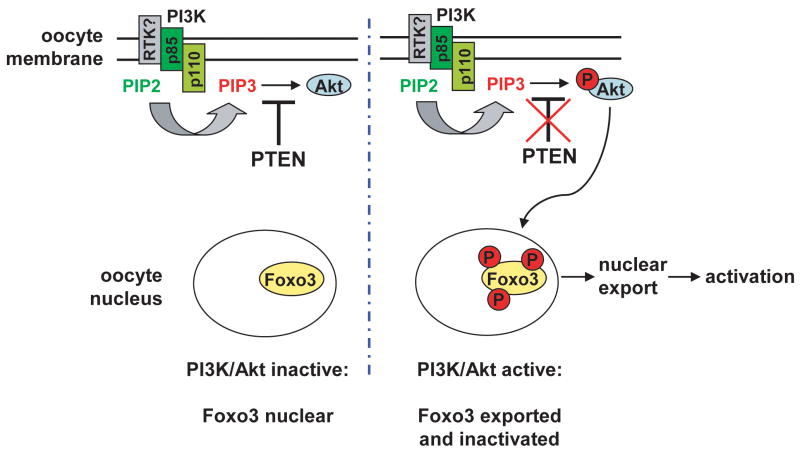
Primordial follicle activation appears to be a stochastic process, but the need to ration a finite supply of primordial follicles throughout reproductive life suggests that this process is tightly regulated. We speculate that activation is influenced by positive and negative feedback loops, perhaps mediated by secreted, diffusible factors. Although such factors have not been definitively identified (Skinner, 2005), our findings substantially advance our understanding of ovarian biology by demonstrating that such communication is integrated by Foxo3 and the PI3K signalling pathway acting within the oocyte itself (see Fig. 7 for model of Foxo3 function within oocytes).
Fig. 7.
Model of Foxo3 regulation by PI3K-Akt signaling pathway within oocytes in the control of primordial follicle activation. In resting state, absence of a presumptive ligand engaging receptor tyrosine kinase (RTK) complex or other cell surface receptor is associated with diminished Akt signalling in primordial oocytes. Foxo3 is unphosphorylated and thus localized to the nucleus, where Foxo3 acts to suppresses primordial follicle activation. Binding of presumptive ligand activates PI3K (consisting of p85 regulatory and p110 catalytic subunits), which phosphorylates the 3’OH group of PI[4,5]P2 (PIP2) to form PI[3,4,5]P3 (PIP3) at the oocyte membrane. Pten acts as a potent negative regulator of this pathway by dephosphorylating PIP3. Pten inactivation results in accumulation of PIP3 at the oocyte membrane and hyperactivation of Akt, resulting in Foxo3 phosphorylation and nuclear export, thereby triggering primordial follicle activation. In this model, Pten inactivation is functionally equivalent to the binding of presumptative ligand at a cell surface receptor.
Foxo1, Foxo3, and Foxo4 function similarly, are broadly expressed, and share partially redundant and overlapping functions, whereas Foxo6 is regulated differently and lacks a conserved Akt motif (van der Heide et al., 2005). In genetic analyses of Foxo1, Foxo3, and Foxo4 in mice, a prominent tumor suppressor phenotype was not apparent in single gene knockouts, whereas post-natal inactivation of all three loci resulted in lymphomas and endothelial cell neoplasms (Paik et al., 2007). Inactivation of two of three Foxo genes gave rise to intermediate phenotypes, clearly demonstrating that the Foxos can compensate for one another, at least in some cell types. Given this potential for genetic redundancy, it was initially surprising that Foxo3 has a unique, non-redundant role in the suppression of primordial follicle activation. This may now be explained by our finding that Foxo3 appears to be the predominant Foxo protein in oocytes. It is also notable that at the protein level, Foxo3 is readily detectable only within oocytes, whereas at the mRNA level, Foxo3 is much more broadly expressed within the ovary (Castrillon et al., 2003; Richards et al., 2002). This observation suggests that post-transcriptional mechanisms regulating Foxo protein stability are highly significant in vivo. Consistent with this idea, Foxo protein stability has been found to be regulated by ubiquitylation and proteosomal targeting in a wide range of experimental model systems and cell types (Huang and Tindall, 2007; Plas and Thompson, 2003; Schisler et al., 2007).
We discovered that within oocytes, Foxo3 is phosphorylated at an Akt site and that the phosphorylation status of this site tightly correlates with subcellular localization (cytoplasmic vs. nuclear) in vivo, arguing that Akt is the prime regulator of Foxo3 in the context of primordial follicle activation. Concordantly, oocyte ablation of Pten led to Akt hyperactivation, Foxo3 hyperphosphorylation, and export of Foxo3 from the nucleus to the cytoplasm, resulting in follicle activation. It is also possible that additional kinases or other types of post-translational modification contribute to the regulation of Foxo3 within oocytes, serving as additional layers of regulation, but our findings argue that such modifications are not the principal mechanism by which Foxo3 is regulated as a switch controlling primordial follicle activation.
The mTOR protein participates in two distinct complexes, mTORC1 and mTORC2, with different biological functions and specificities (Guertin and Sabatini, 2007). Our finding that oocyte Akt is phosphorylated at S473 argues that mTORC2 is active (or becomes activated) in primordial oocytes, since mTORC2 has recently been shown to be primary kinase regulating Akt via phosphorylation at this site. On the other hand, we found no evidence of increased phosphorylation of mTORC1 targets during follicle activation. Since at least some of these targets, including p-S6K (John et al., 2007) and p-4EBP (unpublished data) become hyperphophosphorylated in more advanced follicles, mTORC1 activation may be important in promoting increased protein translation during oocyte growth, but not during activation per se.
Along these lines, it is notable that Pten and Foxo3 are genetically equivalent with regard to primordial follicle activation. Conditional deletion of both genes within oocytes using the Vasa-Cre transgene led to nearly-identical phenotypes of global follicle activation. An even more striking demonstration of this genetic equivalence is that Vasa-Cre; PtenL/L and Vasa-Cre; Foxo3L/L females (as well as Foxo3−/− females) are initially fertile (Castrillon et al., 2003). Thus, neither Foxo3 nor Pten are essential for subsequent steps of follicle maturation, ovulation, fertilization, etc. These observations are further evidence that this pathway proceeds in a linear manner with Foxo3 downstream of Pten and PI3K/Akt, and that Foxo3 is the principal, if not sole output of this pathway. While the Foxos are known to be important effectors of PI3K/Akt signalling, we find it somewhat surprising that a single target, Foxo3, has such an overriding role as an effector of this pathway within oocytes, given the diversity of potential Akt substrates. It is also rather surprising that Pten inactivation and resultant Akt hyperactivation within oocytes does not disrupt subsequent steps of follicle maturation, ovulation, etc. Although it is likely that PI3K-Akt signalling participates in other aspects of oogenesis and follicle maturation, our findings demonstrate that this signalling pathway has evolved to serve a particularly critical role in primordial follicle activation.
Our investigations with Vasa-CreERT2 establish the feasibility of conditional gene targeting in primordial oocytes. This study represents the first example of conditional genetic inactivation in primordial oocytes in adult animals, and thus Vasa-CreERT2 should prove useful for a broad range of studies of oocyte activation, survival, and aging, as well as conditional genetic analysis of the male germ line (Fig. S2). It is also remarkable that the efficiency of recombination was so high (90–95% of primordial oocytes), at least up to 6 weeks of age. Primordial follicles are mitotically inactive (Hirshfield, 1991), and prior radiolabelling studies suggested that primordial oocytes are also metabolically quiescent (Bakken and McClanahan, 1978; Hirshfield, 1991; Moore and Lintern-Moore, 1974; Moore et al., 1974; Roversi and Silvestrini, 1963). However, these prior studies employed methods of limited sensitivity. Since production of active β-galactosidase requires 1) tamoxifen-induced transport of Cre from the cytoplasm to the nucleus, 2) homologous recombination of loxP sites, 3) ATP-intensive processes including transcription, mRNA transport, and protein translation, the high efficiency of Cre-mediated recombination argues that primordial follicles are normally engaged in a surprisingly high degree of metabolic activity and turnover of cellular components. In these respects, our observations argue that primordial oocytes resemble neurons, a finding with intriguing implications for the age-associated oocyte loss that leads to the menopause. We speculate that oocytes may be subject to some of the same aging mechanisms that contribute to age-associated neurodegeneration, including oxidative stress and the accumulation of damaged or malfolded proteins (Mattson and Magnus, 2006).
Lastly, our findings, including the demonstration that the pathway functions continually in adults and not just during a more limited stage of early development, further support the idea that defects in PI3K signalling may contribute to certain forms of female infertility due to primordial follicle depletion, such as premature ovarian failure and primary amenorrhea. These findings also raise the possibility that pharmacologic agents acting upon the PI3K pathway, several of which are in clinical trials (Granville et al., 2006), may be useful in controlling follicle activation, treating infertility, or forestalling the menopause.
Supplementary Material
Acknowledgments
We thank Meredith Shidler and Norman Sharpless for comments on the manuscript. This work was supported by grants from the National Institute of Child Health and Human Development (R01HD048690), the National Center for Research Resources (K26RR024196), and an award from the Lance Armstrong Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Bakken AH, McClanahan M. Patterns of RNA synthesis in early meiotic prophase oocytes from fetal mouse ovaries. Chromosoma. 1978;67:21–40. doi: 10.1007/BF00285645. [DOI] [PubMed] [Google Scholar]
- Block E. Quantitative Morphological Examinations of the Follicular System in Women. Acta Anatomica. 1952;14:108–123. doi: 10.1159/000140595. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, Castrillon DH. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis. 2007;45:413–417. doi: 10.1002/dvg.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granville CA, Memmott RM, Gills JJ, Dennis PA. Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2006;12:679–689. doi: 10.1158/1078-0432.CCR-05-1654. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007a;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007b;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 2007;133:855–863. doi: 10.1530/REP-06-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kitamura Y, Nakae J, Giordano A, Cinti S, Kahn CR, Efstratiadis A, Accili D. Mosaic analysis of insulin receptor function. J Clin Invest. 2004;113:209–219. doi: 10.1172/JCI17810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, Hennighausen L, Wu H. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129:4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134:199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Menopause. In: Katz VL, Lentz GM, Lobo RA, Gershenshon DM, editors. Comprehensive Gynecology. 5. Mosby Elsevier; Philadelphia: 2007. [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Moore GP, Lintern-Moore S. A correlation between growth and RNA synthesis in the mouse oocyte. J Reprod Fertil. 1974;39:163–166. doi: 10.1530/jrf.0.0390163. [DOI] [PubMed] [Google Scholar]
- Moore GP, Lintern-Moore S, Peters H, Faber M. RNA synthesis in the mouse oocyte. J Cell Biol. 1974;60:416–422. doi: 10.1083/jcb.60.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2003. [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H, Byskov AG, Lintern-Moore S, Faber M, Andersen M. The effect of gonadotrophin on follicle growth initiation in the neonatal mouse ovary. J Reprod Fertil. 1973;35:139–141. doi: 10.1530/jrf.0.0350139. [DOI] [PubMed] [Google Scholar]
- Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278:12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol. 2002;16:580–599. doi: 10.1210/mend.16.3.0806. [DOI] [PubMed] [Google Scholar]
- Roversi GD, Silvestrini R. Study on the protein metabolism of the evolutionary ovarian follicle. Experimental Cell Research. 1963;31:484–489. doi: 10.1016/0014-4827(63)90395-1. [DOI] [PubMed] [Google Scholar]
- Schisler JC, Willis MS, Patterson C. You Spin Me Round: MafBx/Atrogin-1 Feeds Forward on FOXO Transcription Factors (Like a Record) Cell Cycle. 2007;7 doi: 10.4161/cc.7.4.5451. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Jacobs FM, Burbach JP, Hoekman MF, Smidt MP. FoxO6 transcriptional activity is regulated by Thr26 and Ser184, independent of nucleo-cytoplasmic shuttling. Biochem J. 2005;391:623–629. doi: 10.1042/BJ20050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.