Abstract
The ubiquitously expressed Na–H exchanger NHE1 functions in regulating intracellular pH and cell volume. NHE1 activity is stimulated by hormones, growth factors, and activation of integrin receptors. We recently determined that NHE1 activity is also stimulated by activation of the low molecular weight GTPase RhoA and that increases in NHE1 activity are necessary for RhoA-induced formation of actin stress fibers. We now show that NHE1 acts downstream of RhoA to modulate initial steps in integrin signaling for the assembly of focal adhesions. Adhesion of CCL39 fibroblasts on fibronectin was markedly delayed in the presence of the NHE inhibitor ethylisopropylamiloride. In mutant PS120 cells, derived from CCL39 fibroblasts but lacking NHE1, adhesion was also delayed but was rescued in PS120 cells stably expressing NHE1. In the absence of NHE1 activity, cell spreading was inhibited, and the accumulation of integrins, paxillin, and vinculin at focal contacts was impaired. Additionally, tyrosine phosphorylation of p125FAK induced by integrin clustering was also impaired. Inactivation of RhoA with C3 transferase and inhibition of the Rho-kinase p160ROCK with the pyridine derivative Y-27632 completely abolished activation of NHE1 by integrins but not by platelet-derived growth factor. These findings indicate that NHE1 acts downstream of RhoA to contribute a previously unrecognized critical signal to proximal events in integrin-induced cytoskeletal reorganization.
INTRODUCTION
Cell contacts with the extracellular matrix (ECM)1 are important determinants of cell growth, differentiation, and migration (Clark and Brugge, 1995). These contacts, or focal adhesions, are mediated by the integrin family of cell surface receptors. Integrins are αβ heterodimeric transmembrane receptors that recognize and bind to many components of the ECM, as well as to some cell surface adhesion molecules. Cell attachment to ECM results in integrin clustering, which triggers the recruitment of focal adhesion proteins and results in the assembly of focal adhesions, in which integrins link to actin filaments through intracellular cytoskeletal complexes (Craig and Johnson, 1996). Besides playing an important role in stabilizing cell–matrix interactions, focal adhesions serve as signal transducers to multiple downstream cellular events, which include activation of protein tyrosine kinases (Kornberg et al., 1992; Burridge et al., 1992; Guan and Shalloway, 1992), elevations of intracellular pH (Schwartz et al., 1989; Ingber et al., 1990), activation of MAP kinase cascades (Chen et al., 1994; Miyamoto et al., 1995; Morino et al., 1995), and eventually gene expression and mitogenesis (Werb et al., 1989).
The GTPase RhoA also plays a prominent role in regulating the organization of the cytoskeleton by promoting the assembly of focal adhesions (Hotchin and Hall, 1995) and actin stress fibers (Ridley and Hall, 1992) and by activating the focal adhesion kinase FAK (Rankin et al., 1994). Aggregation of integrins induces the recruitment of RhoA to sites of integrin clustering (Miyamoto et al., 1995), and activation of RhoA in turn regulates signaling downstream of integrins (Chong et al., 1994; Schwartz et al., 1996; Barry et al., 1997). Recent evidence indicates that RhoA modulates initial steps in integrin signaling by regulating integrin clustering (Hotchin and Hall, 1995), most likely through changes in cell contractility (Burridge and Chrzanowska-Wodnicka,1996).
A common downstream signaling action of integrins and RhoA is activation of the ubiquitously expressed Na–H exchanger NHE1. NHE1 functions primarily in intracellular pH (pHi) homeostasis through an electroneutral exchange of extracellular Na+ for intracellular H+. Increases in NHE1 activity are correlated with increased cell proliferation (Kapus et al., 1994), differentiation (Rao et al., 1992), migration (Tilly et al., 1990), and neoplastic transformation (Gillies et al., 1990). Activation of integrin receptors (Schwartz et al., 1989, 1991; Ingber et al., 1990; Demaurex et al., 1996) and RhoA (Hooley et al., 1996) stimulates NHE1 activity. We recently determined that NHE1 activity is necessary for the formation of actin stress fibers induced by RhoA (Vexler et al., 1996). In fibroblasts, stress fiber assembly induced by expression of a constitutively activated RhoAV14 or by treatment with lysophosphatidic acid (LPA), which stimulates RhoA (Ridley and Hall, 1992), is markedly reduced in the presence of NHE1-selective inhibitors and in mutant fibroblasts that do not express Na–H exchangers. Stable expression of NHE1 in these mutant cells rescues LPA- and RhoAV14-stimulated stress fiber formation. A role for NHE1 in cytoskeletal events is also suggested by its predominant localization at focal contacts (Grinstein et al., 1993; Plopper et al., 1995) and by its F-actin–dependent activation by serum (Watson et al., 1992). NHE1 activity may have a specific role in RhoA-mediated cytoskeletal organization because, although Rac1, another member of the Rho family of GTPases, also acts upstream of NHE1 to stimulate its activity (Hooley et al., 1996), NHE1 does not regulate Rac1-induced lamellipodia formation (Vexler et al., 1996).
Although integrins have been shown to stimulate NHE1 activity, a role for the exchanger in cytoskeletal organization regulated by integrins has not been reported. The objective of the current study was to determine whether NHE1 activity regulates only actin stress fiber formation or whether it also acts on proximal events in integrin-mediated cytoskeletal remodeling. Although stress fibers are suggested to be required for the formation of mature focal adhesions, they may not be necessary for the initial recruitment and assembly of focal adhesion proteins at focal complexes (Nobes and Hall, 1995; Chihara et al., 1997). We now have found that RhoA is required for activation of NHE1 by integrins and that NHE1 activity is necessary for the assembly of focal adhesions and for tyrosine phosphorylation of FAK in response to integrin activation. Moreover, we determined that inhibition of NHE1 delays cell attachment and impairs cell spreading, suggesting that it acts upstream of stress fiber and focal adhesion formation by regulating initial steps in integrin activation.
MATERIALS AND METHODS
Cell Culture
CCL39 cells, a hamster lung fibroblast line that expresses only the NHE1 isoform (Lin et al., 1996); PS120 cells, an NHE-deficient clone derived from CCL39 cells (provided by J. Pouyssegur, University of Nice, France) (Pouyssegur et al., 1984); and PS120 cells stably expressing NHE1 (PS120N) (Vexler et al., 1996) were maintained at 5% CO2 in high glucose (4.5 g/liter) Dulbecco’s modified Eagle’s medium (DME), supplemented with 5% heat-inactivated FBS (Life Technologies, Gaithersburg, MD), streptomycin (100 μg/ml), and penicillin (100 U/ml). Before experiments, subconfluent cells were maintained in serum-free DME (SFM) for 20–24 h. Serum-starved cells were detached using a trypsin and EDTA solution, and trypsin was neutralized by adding soybean trypsin inhibitor (Sigma, St. Louis, MO) at 1 mg/ml in SFM. Cells were washed and resuspended in SFM and allowed to attach to dishes coated with poly-l-lysine (10 μg/ml) or fibronectin (FN) (10 or 20 μg/ml). For acute inhibition of NHE1, trypsinized CCL39 cells were treated with 25 μM ethylisopropylamiloride (EIPA) for 15 min before plating. Ltk− fibroblasts and the NHE-deficient LAP1 clone derived from Ltk− cells (Sardet et al., 1989) (provided by J. Pouyssegur) were maintained at 5% CO2 in MEM supplemented with 10% heat-inactivated fetal bovine serum, streptomycin (100 μg/ml), and penicillin (100 U/ml).
For C3 transferase treatment, CCL39 cells were plated at a density of 1.5 × 106 cells in 60-mm dishes and incubated with C3 transferase (provided by S. Narumiya, University of Kyoto, Japan) (Morii et al., 1995) at 7.5 μg/ml for 44 h in serum-containing DME and then for an additional 16 h in SFM. Cells were trypsinized, washed, and either plated on poly-l-lysine–coated glass coverslips to determine NHE1 activity or subjected to ADP-ribosylation reactions as previously described (Tominaga et al., 1993). The effects of the p160ROCK inhibitor Y-27632 (provided by S. Narumiya, Kyoto University) (Uehata et al., 1997) on NHE1 activity were determined by pretreating CCL39 cells for 30 min with 30 μM Y-27632.
Transient expression of mutationally active GTPases was performed as previously described (Hooley, et al., 1996). In brief, CCL39 and PS120 cells were plated on glass coverslips at 0.5 × 106 cells per 60-mm dish for 18 h and transfected using LipofectAMINE (Life Technologies) for 4 h with 1 μg of DNA. After transfections, cells were maintained for 18 h in SFM in the absence or presence of EIPA (25 μg) and then fixed for immunostaining as described below. pEXV-myc-RhoAV14, pEXVmyc-RacV12, and pCMVCdc42V12 constructs were produced as previously described (Hooley et al., 1996).
NHE1 Activity and Intracellular pH
NHE1 activity and pHi were determined as described previously (Voyno-Yasenetskaya et al., 1994; Vexler et al., 1996). Cells were maintained for 20–24 h in SFM, detached by trypsin, replated on poly-l-lysine–coated coverslips, and loaded with the acetoxymethyl ester of the H+-sensitive dye 2,7-biscarboxyethyl-5(6)-carboxyfluorescein (BCECF; 1 μM). BCECF fluorescence was measured using a Shimadzu RF5000 spectrofluorometer (Columbia, MD) by alternately exciting the dye at 500 and 440 nm at a constant emission of 530 nm. Fluorescence ratios were calibrated with 10 μM nigericin in 105 mM KCl (Thomas et al., 1979). NHE1 activity was determined in a nominally HCO3−-free HEPES buffer containing 145 mM NaCl, 5 mM KCl, 10 mM glucose, 1 mM MgSO4, 1 mM KHPO4, 2 mM CaCl2, and 20 mM HEPES (pH 7.4) by measuring the rates of pHi recovery (dpHi/dt) from a transient acid load induced by prepulsing cells for 10 min with 30 mM NH4Cl. Intracellular pH was determined in a 5% CO2-gassed buffer containing 130 mM NaCl, 5 mM KCl, 10 mM glucose, 1 mM MgSO4, 1 mM KHPO4, 2 mM CaCl2, and 25 mM NaHCO3 (pH 7.4). Intracellular pH measurements were obtained for untreated quiescent cells (resting pHi) and for quiescent cells treated with LPA (0.5 μM; Sigma), with platelet-derived growth factor (PDGF-BB; 25 ng/ml; Sigma), or with antibodies to activate α5β1 FN receptors. To activate these receptors, we preincubated cells with PB1 monoclonal antibody (mAb; provided by R. Juliano, University of North Carolina, Chapel Hill, NC) (Brown and Juliano, 1985) at 50 μg/ml for 20 min at 4°C with BCECF, washed the cells in HEPES buffer, and then treated the cells with rabbit anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) at 100 μg/ml for 10 min at 37°C to achieve antibody cross-linking. Data are expressed as the mean ± SEM of three to four separate cell preparations.
Adhesion Assays
Adhesion assays were performed in plastic 96-well plates as described previously (Zhang et al., 1996). Wells were coated overnight with 5 μg/ml FN at 4°C and washed with PBS, and then free-binding sites were blocked with 0.2% BSA-PBS. Trypsinized cells were resuspended at a density of 3 × 105 cells/ml in DME supplemented with 0.2% BSA, and 3 × 104 cells were added per well. The plates were centrifuged at 500 × g for 10 s to distribute the cells evenly and then were incubated in 5% CO2 for the indicated times. After vigorous agitation, weakly adherent and nonadherent cells were removed, and the attached cells were lysed with 0.5N NaOH. Cell adhesion was quantitated by determining protein concentrations with a microbicinchoninic acid assay (Pierce Chemical, Rockford, IL). Data are normalized to the number of cells attached to wells coated with BSA (nonspecific attachment) and are expressed as the percentage of total cells.
Immunocytochemistry
Immunofluorescence was performed as previously described (Vexler et al., 1996). To activate integrins, we plated serum-starved cells on FN-coated coverslips for the indicated times. The cells were washed with PBS, fixed in 4% paraformaldehyde, and permeabilized for 10 min in PBS containing 0.1% Triton X-100 before incubating with primary antibodies. Immunoreactive proteins were detected using FITC-labeled goat anti-mouse or anti-rabbit IgG (Jackson ImmunoResearch). Paxillin was stained with a mouse mAb (clone Z035; Zymed Laboratories, San Francisco, CA), vinculin was stained with a mouse mAb (VIN-11–5; Sigma), myc-tagged GTPases were stained with anti-myc polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), and actin was stained with rhodamine-conjugated phalloidin. Integrin localization was determined using a mouse mAb to hamster β1 integrin (7E2; provided by R. Juliano) (Brown and Juliano, 1988). The coverslips were examined on a Zeiss axiophot microscope using a Zeiss 63 × 1.4 oil-immersion objective. Fluorescence images were recorded on Kodak T-MAX 400ASA film. Confocal fluorescence images were obtained on an MRC 1000 laser-scanning (Bio-Rad, Richmond, CA) microscope (Nikon Optiphot; Garden City, NY).
Immunoblotting
Expression of vinculin, paxillin, and actin was determined by Western analysis of cell lysates. Cells were lysed and boiled in 10 mM Tris-HCl and 1% SDS (pH 7.4), and proteins (50 μg) were separated by SDS-PAGE and transferred to Immobilon membranes (Millipore, Bedford, MA). Membranes were blocked in PBS containing 0.2% Tween 20 (PBST) supplemented with 3% BSA and were incubated for 1 h at room temperature with 3% BSA in PBST containing anti-vinculin, anti-paxillin, or anti-actin (Amersham, Arlington Heights, IL) mAbs. After several washes, membranes were incubated for 45 min at room temperature with horseradish peroxidase-conjugated sheep anti-mouse antibodies (Jackson ImmunoResearch) in PBST. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham).
For analysis of Src and FAK expression, immunoprecipitates from whole-cell lysates were analyzed by immunoblotting. Cells were solubilized in lysis buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1 mM sodium orthovanadate [Na3VO4], 0.1 mM PMSF, 2 μg/ml leupeptin, and 2 μg/ml aprotinin) and clarified by centrifugation at 14,000 × g for 10 min. Equal amounts of protein (250 μg) were precleared with Protein A-agarose or with goat anti-mouse IgG-agarose (Sigma) for 1 h at 4°C and then were incubated with anti-Src polyclonal antibody (N-6; Santa Cruz Biotechnology) or anti-p125FAK antibody (2A7; Upstate Biotechnology, Lake Placid, NY), respectively at 2 μg/ml for 1 h at 4°C. Goat anti-mouse IgG-agarose was added (10 μl), and the lysates were incubated for an additional 1 h at 4°C. Immunoprecipitated proteins were washed four times with wash buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 1 mM Na3VO4, and 2 μg/ml leupeptin), separated by SDS-PAGE, and transferred to membranes for immunoblotting with anti-Src mAb (327; Calbiochem, San Diego, CA) or anti-FAK mAb (clone 77; Transduction Laboratories, Lexington, KY).
To determine integrin expression, we surface-labeled cells with EZ-link-Sulfo-LC-Biotin (Pierce Chemical) before lysis. Integrins were immunoprecipitated as described above, using the anti-hamster β1 integrin mAb 7E2 (Brown and Juliano, 1988) or the mouse mAb to human α5 integrin (BIIG2; provided by C. Damsky, University of California, San Francisco) (Werb et al., 1989). The immunoprecipitated proteins were separated by SDS-PAGE and transferred to membranes, and α5β1 abundance was detected with streptavidin–horseradish peroxidase (Amersham) followed by ECL.
Tyrosine Phosphorylation of FAK
Tyrosine phosphorylation of FAK was determined in response to integrin activation and LPA. To induce integrin clustering, we first plated cells on poly-l-lysine–coated dishes for 15 min at 37°C in 5% CO2, washed the cells with ice-cold DME supplemented with 20 mM HEPES (pH 7.4) (DME/HEPES), and incubated the cells on ice for 30 min with mAb to α5β1 integrins at 50 μg/ml. The cells were then washed in DME/HEPES and incubated for 10 min at 37°C with rabbit anti-mouse IgG at 100 μg/ml and with 100 μM Na3VO4. For LPA stimulation, cells attached to poly-l-lysine–coated dishes were treated with 1 μM LPA for 7.5 min at 37°C in 5% CO2. Cells treated with antibodies or LPA were washed with PBS supplemented with 1 mM Na3VO4, and FAK was immunoprecipitated, separated by SDS-PAGE, and transferred to membranes as described above. Tyrosine phosphorylation of FAK was determined by immunoblotting with anti-phosphotyrosine mAb (4G10; Upstate Biotechnology). The membranes were then stripped and reprobed with anti-FAK antibodies (Transduction Laboratories) to determine the abundance of immunoprecipitated FAK.
RESULTS
Integrin- and LPA-induced Increases in Intracellular pH Are Regulated Primarily by NHE1
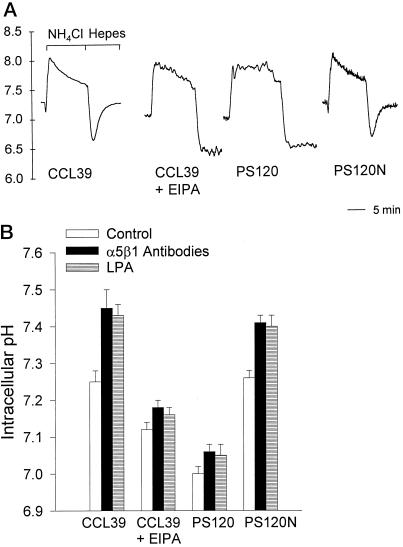
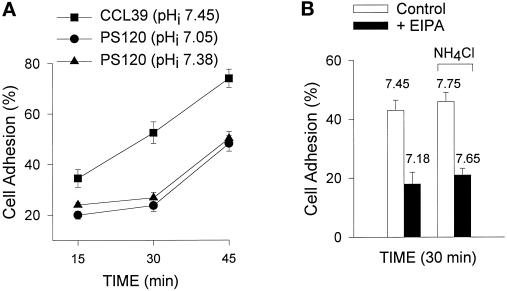
The role of NHE1 activity in cytoskeletal events induced by integrins and RhoA was determined using four cell models: CCL39 fibroblasts, which express only the NHE1 subtype (Lin et al., 1996); CCL39 fibroblasts pretreated with the amiloride analog EIPA, which inhibits Na–H exchange; NHE-deficient PS120 cells, which are derived from parental CCL39 cells (Pouyssegur et al., 1984); and PS120N cells, which are PS120 cells stably expressing NHE1 (Vexler et al., 1996). To confirm the absence of NHE1 activity in EIPA-treated CCL39 cells and in PS120 cells, we determined pHi recoveries from an NH4Cl-induced acid load. In a nominally HCO3−-free HEPES buffer, H+ extrusion from acid-loaded fibroblasts is primarily due to NHE1 activity (Vexler et al., 1996). In CCL39 and PS120N cells, there was a rapid pHi recovery after acid loading (Figure 1A). At pHi 6.70, the rate of pHi recovery (dpHi/dt) was 26.82 ± 1.96 × 10−4 pH/s for CCL39 cells and 23.51 ± 2.24 × 10 −4 pH/s for PS120N cells (mean ± SEM; n = 3 cell preparations). In EIPA-treated CCL39 cells and in PS120 cells, there was no measurable pHi recovery from an acid load (Figure 1A), indicating an absence of NHE1 activity.
Figure 1.
Integrin- and LPA-induced increases in pHi are regulated primarily by NHE1 activity. (A) Intracellular pH recoveries from an NH4Cl-induced acid load were determined in a nominally HCO3−-free HEPES buffer. The rates of pHi recoveries (dpHi/dt) were measured at intervals of 0.05 pH units. (B) Quiescent pHi and pHi induced by integrin activation and LPA were determined in cells maintained in SFM for 18–24 h and then replated on poly-l-lysine–coated coverslips in a HCO3-containing buffer. Integrin activation was induced by cross-linking antibodies to α5β1 integrins as described in MATERIALS AND METHODS. LPA effects on pHi were determined by treating quiescent cells with 0.5 μM LPA for 10 min. Data are expressed as the mean ± SEM of three to four separate cell preparations.
Although increased NHE1 activity is associated with an increase in pHi when cells are maintained in a HEPES buffer, in a HCO3−-containing buffer pHi can also be regulated by the Cl–HCO3− exchanger, an acid loader, and by the Na+-dependent Cl–HCO3− exchanger, an acid extruder. Because our studies on the role of NHE1 in cytoskeletal organization were performed in the presence of HCO3−, we determined the relative contribution of NHE1 activity to changes in pHi induced by integrin activation and LPA. In CCL39 cells, activation of integrin receptors by cross-linking antibodies to α5β1 integrins increased pHi from 7.25 ± 0.03 to 7.45 ± 0.05 (Figure 1B; n = 4 separate cell preparations). Similar integrin-induced increases in the pHi of fibroblasts maintained in HCO3− buffer were reported by Schwartz et al. (1991). In the presence of EIPA, however, the resting pHi of CCL39 cells was reduced to 7.12 ± 0.02, and antibody cross-linking increased pHi only slightly, to 7.18 ± 0.02 (Figure 1B). In PS120 cells, resting pHi and increases in pHi induced by integrin activation were significantly reduced (Figure 1B; p < 0.01; n = 4 separate cell preparations). Values in PS120N cells were similar to those in CCL39 cells. LPA treatment induced a significant increase in pHi in CCL39 and PS120N cells (Figure 1B; p < 0.01) but not in CCL39 cells pretreated with EIPA (p > 0.2) or in PS120 cells (p > 0.2). These findings indicate that in the presence of HCO3−, NHE1 activity in CCL39 cells has a predominant role in establishing resting pHi and increases in pHi induced by integrin activation and LPA.
NHE1 Activity Regulates Cell Adhesion and Spreading on FN
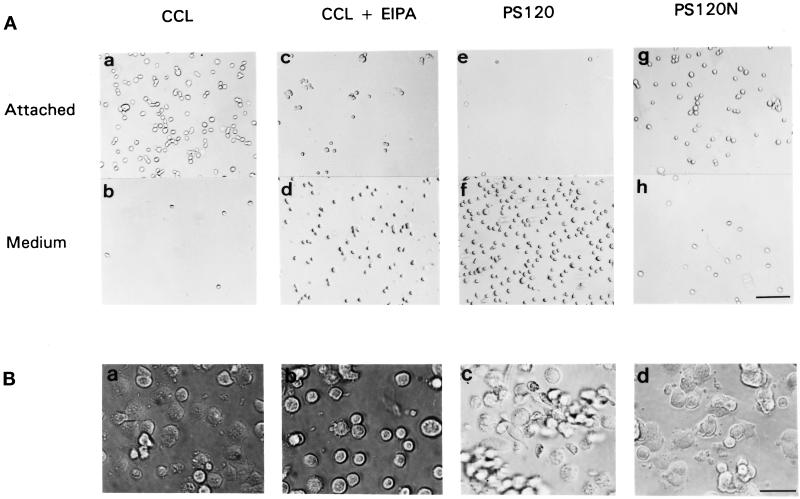
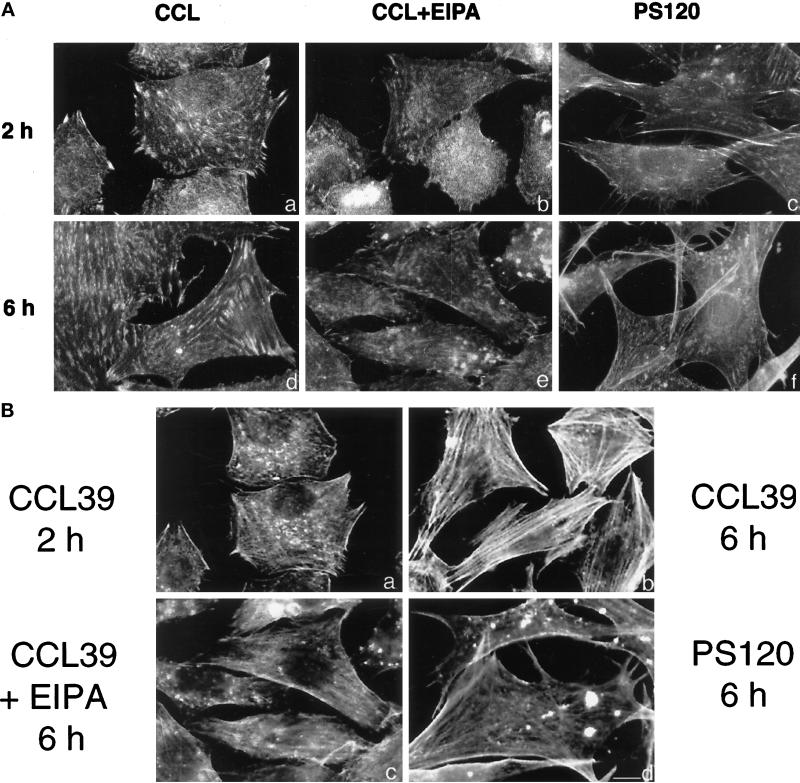
Downstream targets of integrins, including RhoA, can regulate events at the level of the integrin receptor through a process termed “inside-out signaling” (Kolanus and Seed, 1997). To determine whether NHE1 activity induced by integrins might also modulate events at the receptor level, we examined its effect on the attachment and spreading of cells plated on FN. The attachment of untreated CCL39 cells was compared with that of CCL39 cells pretreated with the amiloride analog EIPA and of NHE-deficient PS120 cells. Cells were serum-starved, detached by trypsinization, and plated on dishes coated with FN at 10 μg/ml. After 20 min on FN, the majority of CCL39 cells were attached, and few cells remained in the medium (Figure 2A, a and b). In contrast, the majority of EIPA-treated CCL39 cells (Figure 2A, c and d) and PS120 cells (Figure 2A, e and f) remained in the medium and were not attached to FN. Stable expression of NHE1 in PS120N cells resulted in a rate of attachment similar to that of CCL39 cells (Figure 2A, g and h). After 60 min, CCL39 and PS120N cells were attached and had begun to spread (Figure 2B, a and d). Although EIPA-treated CCL39 cells were attached by this time, most of these attached cells remained round (Figure 2B, b). After 60 min on FN, although some PS120 cells were still not attached, the cells that were attached failed to spread and maintained an elongated, fusiform morphology (Figure 2B, c). By 90 min, the majority of PS120 cells were attached to FN, but spreading was still inhibited (Tominaga and Barber, unpublished observations). We previously reported that after 24 h in culture, spreading of PS120 cells is impaired, and the cells retain a fusiform morphology (Vexler et al., 1996). We now have determined that when CCL39 cells are incubated with EIPA for 16 h, their morphology changes to more closely resemble the fusiform shape of PS120 cells (see Figure 5B, b and c).
Figure 2.
NHE1 activity regulates attachment and spreading of CCL39 fibroblasts on FN. (A) CCL39 cells (a and b), EIPA-treated CCL39 cells (c and d), PS120 cells (e and f), and PS120N cells (g and h) were plated on FN-coated dishes. After 20 min, cells attached to FN (a, c, e, and g) and unattached cells in the medium (b, d, f, and h) were photographed. Bar, 30 μm. (B) Cells were plated on FN-coated dishes and photographed at 60 min. Adhesion and spreading of EIPA-treated CCL39 cells (b) were delayed compared with that in NHE1-expressing CCL39 cells (a) and in PS120N cells (d). (c) After 60 min, many PS120 cells had still not attached. Bar, 10 μm.
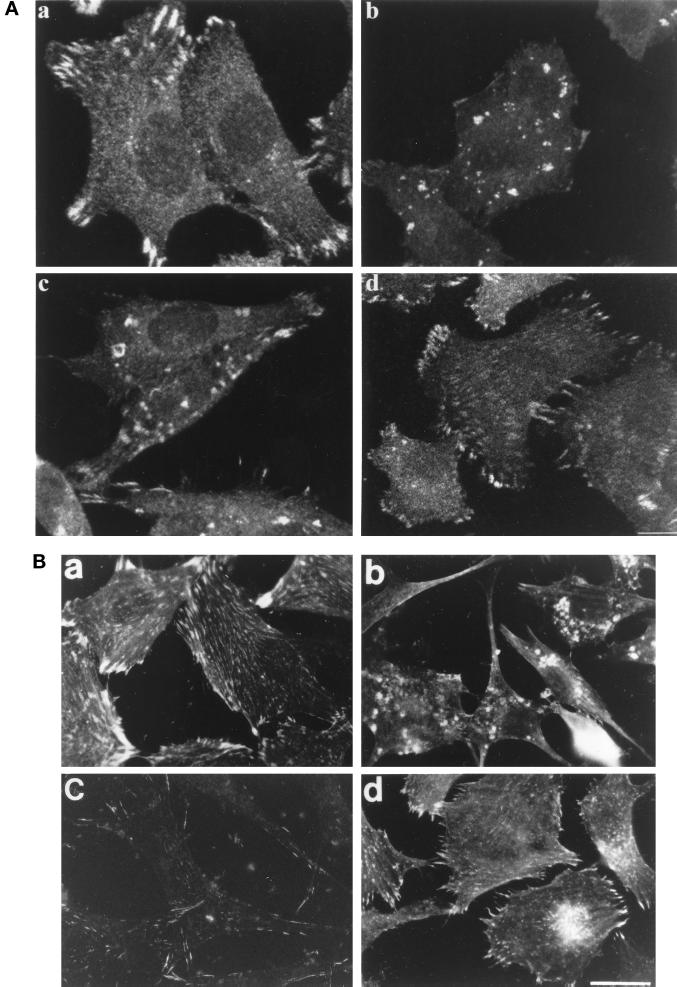
Figure 5.
NHE1 activity regulates the assembly of focal adhesions. Cells plated on FN-coated coverslips in SFM were fixed at 2 h and stained with anti-paxillin or fixed at 16 h and stained with anti-vinculin antibodies to visualize focal adhesions. EIPA-treated cells were pretreated with EIPA for 15 min before plating and allowed to attach on coverslips in the presence of EIPA. (A) Confocal images of paxillin after 2 h on FN in CCL39 cells (a), EIPA-pretreated CCL39 cells (b), PS120 cells (c), and PS120N cells (d). Bar, 1.5 μm. (B) Immunostaining for vinculin after 16 h on FN in CCL39 cells (a), EIPA-pretreated CCL39 cells (b), PS120 cells (c), and PS120N cells (d). Bar, 4 μm.
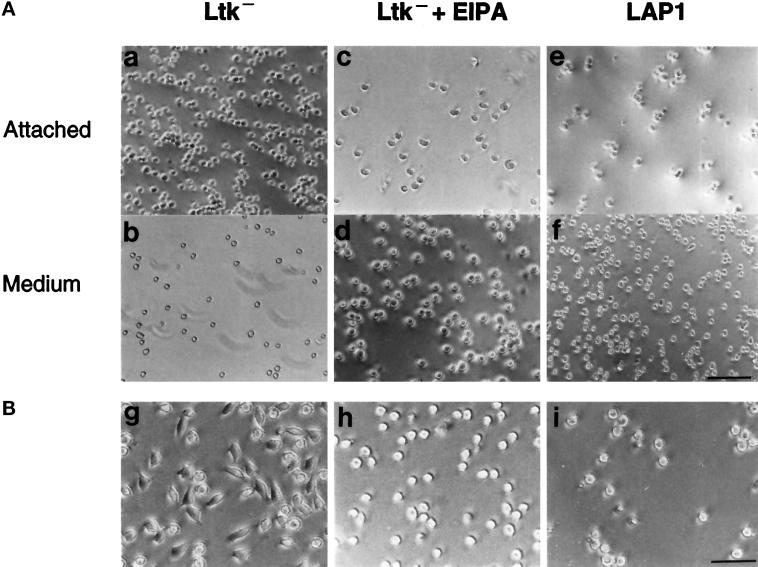
To confirm further that NHE1 activity regulates cell adhesion, we determined the attachment and spreading of Ltk− fibroblasts on FN. Like the CCL39 fibroblasts, after 20 min on FN, the majority of Ltk− cells were attached to FN (Figure 3A, a and b), and after 60 min, the attached cells were spread (Figure 3B, g). Pretreating Ltk− cells with EIPA (25 μM) markedly reduced the number of adherent cells at 20 min (Figure 3A, c and d), and at 60 min, the attached cells remained round and had not spread (Figure 3B, h). LAP1 cells, an NHE-deficient clone derived from parental Ltk− cells (Sardet et al., 1989), also were not attached at 20 min (Figure 3A, e and f), and although most cells were attached at 60 min, the attached cells were not spread (Figure 3B, i). These findings indicate that in the absence of NHE1 activity, cell attachment on FN is delayed and cell spreading is inhibited. They also suggest that the effect of NHE1 on cell attachment is not specific to CCL39 fibroblasts.
Figure 3.
NHE1 activity regulates attachment and spreading of Ltk− fibroblasts on FN. (A) Ltk− cells (a and b), EIPA-treated Ltk− cells (c and d), and LAP1 cells (e and f) were plated on FN-coated dishes. After 20 min, cells attached (a, c, and e) and unattached (b, d, and f) to FN were photographed. Bar, 30 μm. (B) Cells were plated on FN-coated dishes, and attached cells were photographed at 60 min. Bar, 20 μm.
Adhesion assays were used to quantify the differences we observed in adherence. Cells were plated on FN and briefly centrifuged, and after nonadherent cells were aspirated, the adherent cells were quantitated. At 15, 30, and 45 min, adhesion of PS120 cells was significantly lower than that of CCL39 cells (Figure 4A; p < 0.01; n = 3). Pretreating CCL39 cells with EIPA resulted in a significant decrease in cell adhesion (Figure 4B; p < 0.01; n = 5). To determine whether the effect of NHE1 on cell adhesion is mediated by pHi, we used two different approaches to modify cytosolic pH. First, PS120 cells were maintained at 12% CO2 in DMEM containing 50 mM HCO3− for 7–10 d. Although this culture condition increased the pHi of PS120 cells on FN from 7.05 to 7.38, it had no effect on their rate of attachment (Figure 4A). Second, CCL39 cells, in the absence and presence of EIPA, were plated on FN-coated dishes in DMEM supplemented with 30 mM NH4Cl. As demonstrated in Figure 1A, incubating cells with 30 mM NH4Cl induces a rapid increase in pHi as the influx of NH3 complexes with intracellular H+. Addition of NH4Cl had no effect on the rate of attachment of CCL39 cells, and although it increased the pHi of EIPA-treated cells to 7.65, it did not rescue the delay in attachment to FN (Figure 4B). The effect of NHE1 on cell attachment, therefore, may be independent of pHi.
Figure 4.
Cell attachment is not regulated by changes in intracellular pH. Cells were allowed to attach to plates coated with 5 μg/ml FN for the indicated times at 37°C, and the extent of cell adhesion was quantitated as described in MATERIALS AND METHODS. Assays were performed using (A) CCL39 cells (▪), PS120 cells maintained in 25 mM HCO3− at 5% CO2 (•), and PS120 cells maintained for 7–10 d in 50 mM HCO3− at 12% CO2 (▴) and (B) CCL39 cells in the absence (open bars) or presence (filled bars) of EIPA plated in DME and in DME supplemented with 30 mM NH4Cl to increase pHi. Results are expressed as the mean ± SEM of three separate cell preparations.
NHE1 Activity Regulates the Assembly of Focal Adhesions
The process of cell spreading is regulated by the clustering of integrins and by the recruitment of focal adhesion proteins into mature focal complexes. To determine whether the NHE1-dependent effect on cell spreading is associated with the regulation of these processes, we used immunostaining to assess the distribution of integrin receptors and the focal adhesion proteins paxillin and vinculin after plating cells on FN. At 2 h, CCL39 cells, in the absence and presence of EIPA, and PS120 cells had attached to FN. In CCL39 cells, paxillin staining was predominantly localized at peripheral focal adhesions at 2 h (Figure 5A, a). When CCL39 cells were maintained in the continuous presence of EIPA, however, this staining pattern changed dramatically. There was little staining for paxillin at the periphery; however, discrete aggregates of staining accumulated in the cells (Figure 5A, b). A similar staining pattern was seen in PS120 cells plated on FN for 2 h: staining was predominantly in irregular dense aggregates, and there were few focal contacts (Figure 5A, c). In NHE1-competent PS120N cells, paxillin staining was similar to that of CCL39 cells (Figure 5A, d). Immunostaining for vinculin revealed that after 16 h on FN, abundant focal adhesions extended throughout the periphery and at the ventral surface of CCL39 cells (Figure 5B, a). This staining pattern was markedly different in the presence of EIPA. In EIPA-treated CCL39 cells, there was an absence of focal adhesions, and vinculin staining accumulated in large aggregates within the cell (Figure 5B, b). In PS120 cells, there was weak staining for vinculin that was restricted to the periphery (Figure 5B, c). Vinculin staining in PS120N cells resembled that of CCL39 cells (Figure 5B, d).
To determine whether integrin clustering, an event that initiates the recruitment of focal adhesion proteins, is also regulated by NHE1, we determined the localization of the β1 integrins by using the β1 subunit-specific antibody 7E2 (Brown and Juliano, 1988). In CCL39 cells plated on FN, integrin staining revealed extensive focal contacts at the periphery and over the ventral surface at 2 h. At 6 h, the intensity of staining was enhanced at abundant focal adhesions along the ventral surface (Figure 6A, a and d). In the presence of EIPA, integrin staining in CCL39 cells at 2 h was diffuse and revealed that there were few focal contacts compared with those in untreated CCL39 cells (Figure 6A, b and e). At 6 h, there were still few focal contacts, and staining accumulated in irregular aggregates. In PS120 cells, there was weak integrin staining at 2 and 6 h that was restricted to small and narrow focal adhesions located at the edge of spike-like extensions (Figure 6A, c and f). Together, these findings suggest that inhibition of NHE1 activity impairs the localized distribution of integrin receptors, which inhibits the recruitment of focal adhesion proteins and cell spreading.
Figure 6.
NHE1 activity regulates integrin localization and actin stress fiber formation. Cells plated on FN-coated coverslips in SFM were fixed at 2 and 6 h and stained using anti-β1 integrin antibodies (A; a–f) and phalloidin to visualize actin stress fibers (B; a–d). Bar, 3 μm.
We previously determined that in adherent cells, NHE1 activity is critical for the formation of actin stress fibers induced by lysophosphatidic acid and by RhoA (Vexler et al., 1996). Although the processes of stress fiber formation and focal adhesion assembly occur simultaneously, recent findings suggest that the initial assembly of focal adhesions can still occur in the absence of stress fibers (Nobes and Hall, 1995; Chihara et al., 1997). We therefore examined the time course of stress fiber formation in cells plated on FN in the absence of growth factors. Cells immunostained for β1 integrins were costained for actin with phalloidin. In CCL39 cells, stress fibers were not readily apparent at 2 h (Figure 6B, a), although at this time paxillin was clearly localized in focal adhesions (see Figure 5A, a). Abundant stress fibers, however, were observed in CCL39 cells after 6 h on FN (Figure 6B, b). In the presence of EIPA, stress fibers had not developed in CCL39 cells at 6 h (Figure 6B, c), and in PS120 cells, actin accumulated in cytoplasmic aggregates and was not organized in stress fibers (Figure 6B, d). Additionally, in PS120 cells double-stained for paxillin and actin, we observed that staining was colocalized in these aggregates (Tominaga and Barber, unpublished observations).
Expression of Vinculin and Paxillin Is Regulated in PS120 Cells
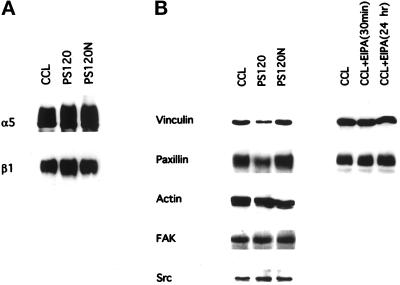
Inhibition of NHE1 activity in cells plated on FN was associated with impaired cell attachment and with the inhibition of cell spreading and focal adhesion assembly. To confirm that these effects in PS120 cells were not due to a downregulation of α5 or β1 receptor subunits, we examined the abundance of these subunits in CCL39, PS120, and PS120N cells. Cells were biotin-labeled, and integrin subunits were immunoprecipitated with anti-β1 mAb 7E2 (Brown and Juliano, 1988), and with BIIG2, an anti-α5 mAb (Werb et al., 1989). Immunoblotting of immunoprecipitated proteins indicated that the expression of both integrin subunits in all three cell lines was similar (Figure 7A).
Figure 7.
Expression of vinculin and paxillin is regulated in PS120 cells. (A) Expression of α5 and β1 integrins is shown. Biotin-labeled lysates obtained from CCL39, PS120, and PS120N cells were incubated with antibodies to α5 and β1 integrins. Immunoprecipitated integrins were detected by streptavidin-horseradish peroxidase. (B) Expression of vinculin, paxillin, and actin was determined by immunoblotting whole-cell lysates (50 μg). Expression of FAK and c-Src was determined by immunoblotting immunoprecipitates of whole-cell lysates (250 μg).
We also determined whether prolonged absence of NHE1 activity in PS120 cells regulated the expression of focal adhesion-associated proteins. Immunoblot analysis revealed that the abundance of vinculin and paxillin in CCL39 cells was reduced by 75 and 50%, respectively, in PS120 cells but was rescued in PS120N cells (Figure 7B). EIPA treatment of CCL39 cells for up to 24 h, however, had no effect on vinculin or paxillin expression (Figure 7B). Hence, acute inhibition of NHE1 activity with EIPA may inhibit the recruitment of vinculin and paxillin to focal contacts, and long-term absence of NHE1 activity in PS120 cells also downregulates their expression. The effect of NHE1 activity appeared to be specific for vinculin and paxillin expression, because the expression of actin, FAK, and Src was similar in CCL39, PS120, and PS120N cells (Figure 7B).
NHE1 Activity Regulates the Tyrosine Phosphorylation of FAK
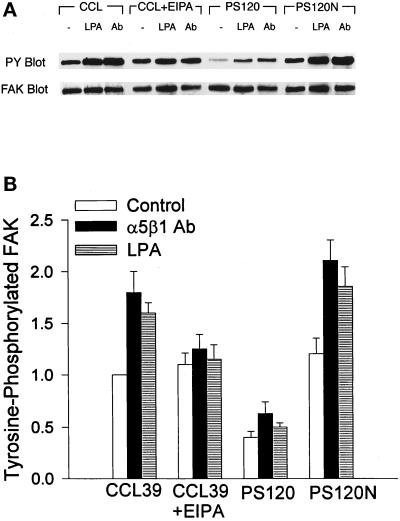
The process of cell adhesion is regulated by integrin receptor occupancy and by receptor clustering. Integrin-induced phosphorylation of FAK requires receptor aggregation but can occur independently of receptor occupancy (Miyamoto et al., 1995). If NHE1 activity regulates integrin clustering, as our results with β1 immunostaining suggest, then it should also regulate downstream effects of clustering. Hence, we investigated whether NHE1 activity regulates tyrosine phosphorylation of FAK. Cells were plated on poly-l-lysine, and tyrosine phosphorylation of FAK was induced by cross-linking of α5β1 antibodies, which results in integrin receptor clustering (Kornberg et al., 1992), and by treating cells with LPA, which activates RhoA (Kumagai et al., 1993; Ridley and Hall, 1992). Antibody-induced activation of integrin receptors and LPA treatment increased the tyrosine phosphorylation of FAK in CCL39 cells (Figure 8). In CCL39 cells pretreated with EIPA, integrin- and LPA-induced increases in FAK phosphorylation were significantly inhibited (Figure 8; p < 0.01; n = 3). In PS120 cells, the basal level of tyrosine-phosphorylated FAK was significantly lower than that in parental CCL39 cells (Figure 8; p < 0.01; n = 5), and although increases in FAK phosphorylation stimulated by integrin and LPA were observed, the absolute level of phosphorylation was reduced compared with that in CCL39 cells (Figure 8). In PS120N cells, basal and stimulated levels of FAK phosphorylation were rescued to the levels of CCL39 values (Figure 8). In CCL39 and PS120N cells, the basal levels of tyrosine-phosphorylated FAK were relatively high, which may be due to constitutive activation of integrins and Rho in quiescent cells. As previously suggested by Hotchin and Hall (1995), cells may have residual RhoA activity that cannot be inhibited after serum starvation. The reduced basal level of tyrosine-phosphorylated FAK in PS120 cells may be due to prolonged inhibition of integrins and RhoA.
Figure 8.
NHE1 activity regulates the tyrosine phosphorylation of FAK. Cells plated on poly-l-lysine were treated by cross-linking α5β1 integrin antibodies or by adding LPA. (A) Tyrosine phosphorylation of FAK was determined by immunoblotting with anti-phosphotyrosine (PY) antibodies. The membranes were then stripped and reprobed with anti-FAK antibodies to determine the abundance of immunoprecipitated FAK. (B) Quantitation of tyrosine-phosphorylated FAK is shown. Data are expressed relative to the abundance of phosphorylated FAK in untreated CCL39 cells and represent the mean ± SEM of three to five separate cell preparations.
RhoA Is Required for Activation of NHE1 by Integrins
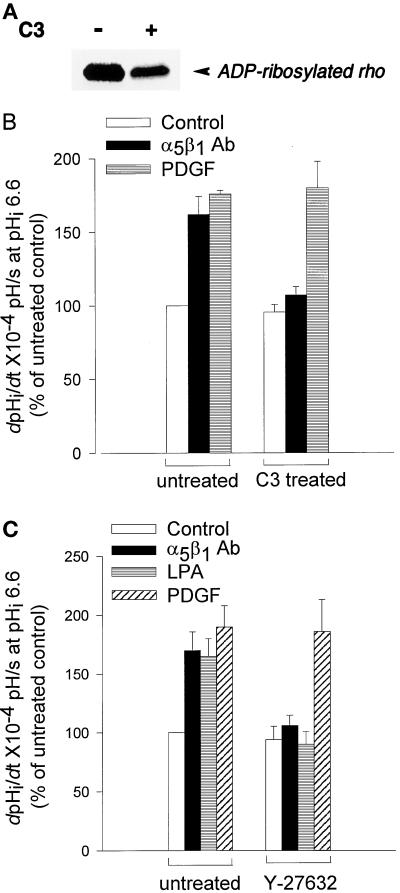
The clustering of integrins and the downstream events of focal adhesion assembly and FAK phosphorylation induced by this clustering are regulated by RhoA (for review, see Burridge and Chrzanowska-Wodnicka, 1996). Because NHE1 activity is stimulated by a RhoA-dependent pathway (Hooley et al., 1996; Vexler et al., 1996), we sought to determine whether activation of RhoA was necessary for integrin-stimulated exchanger activity. The first approach we used to answer this question was to treat CCL39 cells with C3 transferase. C3 transferase specifically ADP-ribosylates RhoA, thereby inactivating it (Morii and Narumiya, 1995). As shown in Figure 9A, the amount of ADP-ribosylation substrate decreased ∼70% in C3-treated cells. The effect of C3 treatment on NHE1 activity in quiescent and stimulated cells was determined by measuring the rate of pHi recovery from an acid load. C3 treatment had no effect on the basal NHE1 activity of quiescent cells; however, it almost completely inhibited increases in NHE1 activity in response to integrin activation induced by cross-linking antibodies to the α5β1 receptor (Figure 9B). In contrast, C3 treatment had no effect on NHE1 activity stimulated by PDGF (Figure 9B). The second approach we used to determine whether a Rho-dependent pathway regulates integrin activation of NHE1 was to inhibit activity of the Rho-kinase p160ROCK. p160ROCK acts directly downstream of RhoA to mediate RhoA-dependent cell contractility and stress fiber assembly (Ishizaki et al., 1997), and we recently determined that p160ROCK mediates activation of NHE1 by RhoA (Tominaga et al., 1998). CCL39 cells were pretreated for 30 min before pHi determinations with the pyridine derivative Y-27632 (30 μM), which selectively inhibits p160ROCK activity (Uehata et al., 1997) and the ability of RhoA, but not Rac or Cdc42, to activate NHE1 (Tominaga et al., 1998). Y-27632 had no effect on basal or PDGF-stimulated NHE1 activity; however, it blocked increases in NHE1 activity in response to activation of α5β1 integrins and to LPA. Together, our findings indicate that integrin activation of NHE1 requires a Rho-dependent pathway, and they suggest that NHE1 acts downstream of RhoA to regulate events at the level of the integrin receptor.
Figure 9.
RhoA and p160ROCK mediate activation of NHE1 by integrins. (A) The decrease in ADP-ribosylation substrate in C3 transferase–treated CCL39 cells. Cells were maintained in the absence or presence of C3 transferase (7.5 μg/ml) for 60 h. The remaining ADP-ribosylation substrate (unmodified RhoA) in homogenized cells was ADP-ribosylated in vitro with C3 transferase in the presence of [32P]NAD+ and was analyzed by SDS-PAGE and autoradiography. (B and C) The rate of pHi recovery (dpHi/dt) determined in a HEPES buffer in untreated cells and in cells treated with C3 transferase (B) or the p160ROCK inhibitor Y-27632 (C). Integrin activation was induced as described in the legend of Figure 1B, and PDGF (25 ng/ml) and LPA (1 μM) were added 10 min before acid loading. Data are expressed relative to control (quiescent) recovery rates in untreated cells and represent the mean ± SEM of three to four separate cell preparations.
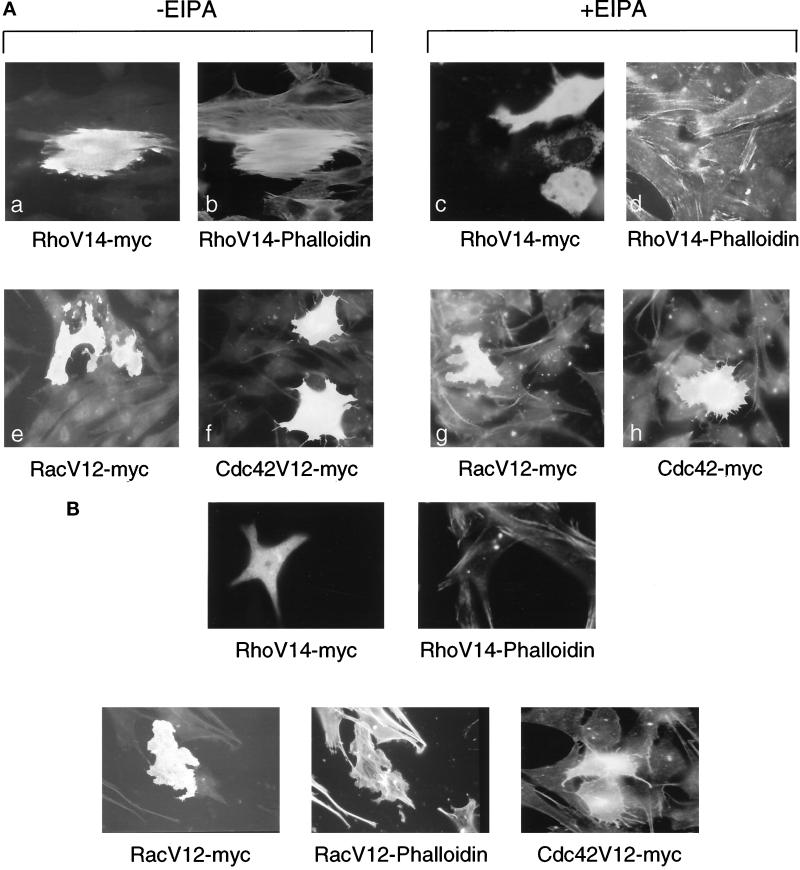
To demonstrate that NHE1 activity selectively acts downstream of RhoA-mediated cytoskeletal remodeling, we transiently expressed myc-tagged, mutationally activated RhoAV14, RacV12, and Cdc42V12 in CCL39 cells maintained for 16 h in the absence or presence of EIPA. We previously determined that although activated Rac and Cdc42 stimulate NHE1 activity, they act through a mechanism that is dependent on the mitogen-activated protein kinase kinase 1 (MEKK1) but independent of RhoA (Hooley et al., 1996). In the absence of EIPA, RhoAV14-induced stress fibers, RacV12-induced lamellipodia, and Cdc42V12-induced filopodia were similar to previously reported phenotypes (Ridley and Hall, 1992; Nobes and Hall, 1995) (Figure 10A). In the presence of EIPA, stress fiber formation in cells expressing RhoAV14 was specifically blocked; however, lamellipodia formation in RacV12-expressing cells and filopodia formation in Cdc42V12-expressing cells were not impaired (Figure 10A). Additionally, in NHE-deficient PS120 cells, although the induction of stress fiber formation was absent in cells expressing RhoAV14, lamellipodia formation in response to RacV12 and filopodia formation in response to Cdc42 were still observed (Figure 10B). These findings argue against EIPA having nonspecific effects on cytoskeletal organization, and they indicate that NHE1 activity selectively regulates cytoskeletal remodeling in response to activation of RhoA.
Figure 10.
Inhibition of NHE1 selectively inhibits RhoA-induced stress fiber formation. Cells transiently expressing myc-tagged RhoAV14, RacV12, or Cdc42V12 were immunostained with c-myc polyclonal IgG and fluorescein and were costained with rhodamine-conjugated phalloidin 16 h after transfections. (A) CCL39 cells maintained in the absence (a, b, e, and f) or presence (c, d, g, and h) of EIPA (25 μM) after transfection. (B) PS120 cells.
DISCUSSION
Integrin-induced cell adhesion and cytoskeletal remodeling occur through an ordered series of events (for review, see Parsons, 1996). Integrin engagement with the ECM results in receptor clustering. Integrin clustering triggers the recruitment and phosphorylation of FAK and the recruitment of actin-binding proteins and actin stress fibers, which promote cell spreading and the assembly of focal adhesions. Focal adhesions enhance cell adhesiveness, and they also function as a scaffold for the initiation of other signaling cascades. These integrin-induced cytoskeletal effects are modulated by the GTPase RhoA (for review, see Burridge and Chrzanowska-Wodnicka, 1996). We previously determined that RhoA acts upstream of NHE1 to stimulate activity of the ion exchanger (Hooley et al., 1996) and that in adherent cells, NHE1 activity is necessary for RhoA-induced actin stress fiber formation (Vexler et al., 1996). We now have determined that NHE1 selectively acts downstream of RhoA to regulate cell attachment and spreading on FN. NHE1-dependent effects on cell spreading were likely due to its ability to promote the clustering of integrins receptors and the recruitment and assembly of focal adhesion proteins.
Cell adhesion has previously been determined to stimulate NHE1 activity (Schwartz et al., 1989; Demaurex et al., 1996). The ability of NHE1 to regulate attachment and spreading on FN suggests that a reciprocal regulation occurs between integrins and NHE1. A similar reciprocal function between cell attachment and protein kinase C (PKC) activity has been demonstrated (Vuori and Ruoslahti, 1993). Cell attachment on FN stimulates PKC activity, and inhibition of PKC activity inhibits the rate of cell adhesion and spreading. A reciprocal function between cell attachment and RhoA has also been suggested. RhoA regulates integrin clustering in fibroblasts (Hotchin and Hall, 1995), and the aggregation of integrins induces the recruitment of RhoA to sites of integrin clustering (Miyamoto et al., 1995). Furthermore, through inside-out signaling, RhoA regulates cell attachment in platelets (Morii et al., 1992), lymphocytes (Tominaga, et al., 1993), and leukocytes (Laudanna et al., 1996). Integrin-mediated cell adhesion can be regulated at several levels, including the affinity of receptors for ECM ligands, the clustering of receptors, and the interaction of receptors with cytoskeletal proteins (Gumbiner, 1996). Although it remains to be determined whether NHE1 activity regulates integrin affinity, our findings suggest that it does act downstream of RhoA to regulate integrin activation.
The impaired spreading and fusiform morphology of cells lacking NHE1 activity resemble what is observed in cells expressing mutations in the cytoplasmic domain of integrin β subunits (Ylänne et al., 1993; Peter and O’Toole, 1995). These mutations prevent the interaction of integrins with cytoskeletal proteins, resulting in impaired focal adhesion assembly and cell spreading. The decreased expression of vinculin and paxillin in PS120 cells could contribute to impaired spreading, because adhesion and spreading on FN are impaired in vinculin-null cells (Coll et al., 1995). Treating CCL39 cells with EIPA, however, impaired adhesion and inhibited spreading, but it had no effect on the abundance of vinculin and paxillin. Reduced expression of focal adhesion–associated proteins, therefore, probably results from long-term decreases in cell adhesion, as previously reported (Bendori et al., 1987; Lee and Otto, 1996). The relationship between NHE1 activity and cell spreading, however, may be cell-type specific. In neutrophils, cell spreading precedes the activation of NHE1, and inhibition of NHE1 activity has no effect on spreading (Demaurex et al., 1996). In contrast, in fibroblasts, insoluble FN stimulates NHE1 activity independently of cell spreading (Schwartz et al., 1991), and we now have shown that NHE1 activity regulates cell spreading. Hence, one interpretation for differences in these findings is that NHE1 activity may be more critical for the spreading of fibroblasts than for neutrophils. In the neutrophil study, however, NHE1 was inhibited at the time of plating, whereas in our study, cells were pretreated with EIPA for 15 min before plating. A more likely interpretation, therefore, is that preinhibition of NHE1 inhibits cell attachment and then subsequently cell spreading.
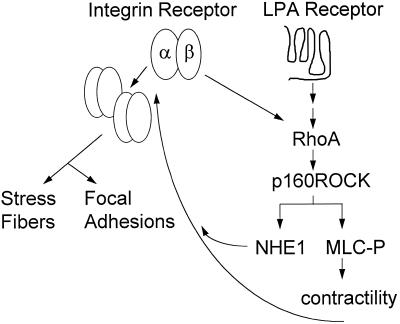
How does NHE1 act downstream of RhoA to regulate integrin function? The current model of integrin signaling suggests that RhoA regulates integrin actions via changes in cell contractility induced by phosphorylation of myosin light chain (MLC) (for review, see Burridge and Chrzanowska-Wodnicka, 1996). RhoA-induced phosphorylation of MLC is mediated by the RhoA target Rho-kinase (Leung et al., 1995; Ishizaki et al., 1997; Matsui et al., 1996), which increases MLC phosphorylation by inhibiting myosin phosphatase (Kimura et al., 1996). It is currently unclear whether NHE1 acts in a RhoA pathway regulating contractility; however, a functional link between MLC and NHE1 activity has been reported (Shrode et al., 1995), and we recently determined that a Rho-kinase homolog, p160ROCK (Ishizaki et al., 1997), mediates RhoA activation of the exchanger (Tominaga et al., 1998). Our current findings also indicate that a RhoA-p160ROCK pathway mediates activation of NHE1 by integrins. Hence, it is likely that NHE1 activity acts cooperatively with this myosin-based mechanism to mediate RhoA actions and cytoskeletal remodeling by integrins (Figure 11). Inhibition of myosin contractility, however, does not dramatically alter cell morphology (Chrzanowska-Wodnicka and Burridge, 1996), indicating that cell adhesion is maintained by another mechanism. This latter mechanism, involving integrin activation, might also be regulated by NHE1 activity. A fundamental defect we consistently observe in the absence of NHE1 activity is that F-actin accumulates in aggregates in the cytoplasm, which suggests that perhaps the bundling of actin filaments is a target of NHE1 activity. Impaired bundling of actin filaments in the absence of NHE1 activity could inhibit stress fiber formation, the clustering of integrin receptors, and the recruitment of FAK and other focal adhesion proteins to focal contacts.
Figure 11.
A proposed pathway of integrin–RhoA–NHE1 signaling for induction of focal adhesions and stress fibers. MCL-P, phosphorylated myosin light chain.
The primary function of NHE1 is thought to be pHi homeostasis. Integrin-induced increases in pHi were predominantly mediated by increases in NHE1 activity, suggesting that increases in pHi may be a critical signal mediating integrin actions on the cytoskeleton. In the absence of NHE1 activity, however, the delay in cell adhesion was not rescued by increasing cytosolic pH. Additionally, Ingber et al. (1990) previously determined that integrin-induced increases in DNA synthesis are mediated in part through increases in NHE1 activity but independently of increases in cytosolic pH. NHE1 is localized at sites of focal contact (Grinstein et al., 1993; Plopper et al., 1995), suggesting that if H+ is an important signal, then perhaps localized pHi gradients are important in cytoskeletal remodeling, and these cannot be mimicked by increasing cytosolic pHi. Our studies were performed in the presence of HCO3−, suggesting that if changes in intracellular H+ regulate cell adhesion and the organization of the cytoskeleton, then this signal is generated primarily by NHE1 and not by anion exchangers. In the presence of HCO3−, anion exchangers were unable to compensate for changes induced by EIPA or for the absence of NHE1 in PS120 cells, indicating a specific requirement for NHE1 activity.
Another possible signal might be generated by a physical association of NHE1 with the cytoskeleton. Osmotic activation of NHE1 occurs through changes in cell shape (Krump et al., 1997), and F-actin assembly is required for activation of NHE1 by serum (Watson et al., 1992). Hence, although speculative, it is possible that NHE1 is structurally linked to the cytoskeleton by binding actin-associated proteins. AE1, the erythrocyte Cl–HCO3− exchanger, functions not only in pHi regulation but also in maintaining cytoskeletal integrity. The N-terminal cytoplasmic domain of AE1 binds ankyrin (Ding et al., 1996) and protein 4.1 (An et al., 1996), which link AE1 to the actin cytoskeleton, and this association is thought to play a role in regulating cell shape (Wong, 1994). If NHE1 is a cytoskeleton-associated protein, the putative docking site would most likely be localized to the C-terminal cytoplasmic domain of NHE1, which is known to regulate ion translocation by the transmembrane domain (Wakabayashi et al., 1992, 1994). We recently identified a novel Ca2+-binding protein, CHP, that binds to the cytoplasmic domain of NHE1 (Lin and Barber, 1996), and overexpression of CHP in CCL39 cells inhibits RhoA-induced stress fiber formation (Vexler and Barber, unpublished observations). CHP could potentially provide a Ca2+-dependent link between NHE1 and the cytoskeleton.
In summary, our findings indicate that NHE1 activity acts downstream of RhoA to regulate a number of cytoskeletal events initiated by activation of integrins in fibroblasts. Of particular importance, we found that NHE1 activity regulated the attachment and spreading of cells on FN, suggesting that it regulates initial steps in integrin activation. The ability of NHE1 activity to regulate the downstream actions of integrins suggests that it is likely to play a significant role in cytoskeletal-dependent processes associated with morphogenesis, wound healing, and anchorage-dependent cell growth.
ACKNOWLEDGMENTS
We are grateful to C. Damsky, D. Ilic, R. Juliano, and S. Narumiya for valuable suggestions and reagents. We also thank P. Steen and P. Sargent for help with confocal microscopy and Z. Vexler and S. Denker for help with immunostaining. This work was supported by National Institutes of Health grants DK-40259 and GM-47413. D.L.B. is an Established Investigator for the American Heart Association.
Abbreviations used:
- BCECF
2,7-biscarboxyethyl-5(6)-carboxyfluorescein
- CHP
calcineurin homologous protein
- DME
Dulbecco’s modified Eagle’s medium
- ECM
extracellular matrix
- EIPA
ethylisopropylamiloride
- FAK
focal adhesion kinase
- FN
fibronectin
- LPA
lysophosphatidic acid
- MLC
myosin light chain
- Na3VO4
sodium orthovanadate
- NHE
Na-H exchanger
- PBST
PBS containing 0.2% Tween 20
- PDGF
platelet-derived growth factor
- pHi
intracellular pH
- PKC
protein kinase C
- PS120N
PS120 cells stably expressing NHE1
- SFM
serum-free DME
REFERENCES
- An XL, Takauwa Y, Nunomura W, Manno S, Mohandas N. Modulation of band 3-ankyrin interaction by protein 4.1. Functional implications in regulation of erythrocyte membrane mechanical properties. J Biol Chem. 1996;271:33187–33191. doi: 10.1074/jbc.271.52.33187. [DOI] [PubMed] [Google Scholar]
- Barry ST, Flinn HM, Humphries MJ, Critchley DR, Ridley AJ. Requirement for Rho in integrin signalling. Cell Adhes Commun. 1997;4:387–398. doi: 10.3109/15419069709004456. [DOI] [PubMed] [Google Scholar]
- Bendori R, Salomon D, Geiger B. Contact-dependent regulation of vinculin expression in cultured fibroblasts: a study with vinculin-specific cDNA probes. EMBO J. 1987;6:2897–2905. doi: 10.1002/j.1460-2075.1987.tb02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Juliano RL. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985;228:1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Juliano RL. Monoclonal antibodies to distinctive epitopes on the α and β subunits of the fibronectin receptor. Exp Cell Res. 1988;177:303–318. doi: 10.1016/0014-4827(88)90464-8. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and Pp 125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Kaibuchi K. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by rho-kinase. J Biol Chem. 1997;272:25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Coll JL, Ben-Ze’ev A, Ezzell RM, Rodriguez Fernandez JL, Baribault H, Oshima RG, Adamson ED. Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology, adhesion, and locomotion. Proc Natl Acad Sci USA. 1995;92:9161–9165. doi: 10.1073/pnas.92.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig SW, Johnson RP. Assembly of focal adhesions: progress, paradigms, and portents. Curr Opin Cell Biol. 1996;8:74–85. doi: 10.1016/s0955-0674(96)80051-2. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Downey GP, Waddell TK, Grinstein S. Intracellular pH regulation during spreading of human neutrophils. J Cell Biol. 1996;133:1391–1402. doi: 10.1083/jcb.133.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Kobayashi S, Kopito R. Mapping of ankyrin binding determinations on the erythroid anion exchanger, AE1. J Biol Chem. 1996;271:22494–22498. doi: 10.1074/jbc.271.37.22494. [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Martinez-Zaguilan R, Martinez GM, Serrano R, Perona R. Tumorigenic 3T3 cells maintain an alkaline intracellular pH under physiological conditions. Proc Natl Acad Sci USA. 1990;87:7414–7418. doi: 10.1073/pnas.87.19.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S, Woodside M, Waddell TK, Downey GP, Orlowski J, Pouyssegur J, Wong DC, Foskett JK. Focal localization of the NHE-1 isoform of the Na+/H+ antiport: assessment of effects on intracellular pH. EMBO J. 1993;12:5209–5218. doi: 10.1002/j.1460-2075.1993.tb06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hooley R, Yu C-Y, Symons M, Barber DL. Ga13 stimulates Na-H exchange through distinct Cdc42-dependent and Rho-dependent pathways. J Biol Chem. 1996;271:6152–6158. doi: 10.1074/jbc.271.11.6152. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Prusty D, Frangioni JV, Cragoe EJ, Lechene C, Schwartz MA. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J Cell Biol. 1990;110:1803–1811. doi: 10.1083/jcb.110.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Kapus A, Grinstein S, Wasan S, Kandasamy R, Orlowski O. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. ATP dependence, osmotic sensitivity, and role in cell proliferation. J Biol Chem. 1994;269:23544–23552. [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi KK. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kolanus W, Seed B. Integrins and inside-out signal transduction: converging signals from PKC and PIP3. Curr Opin Cell Biol. 1997;9:725–731. doi: 10.1016/s0955-0674(97)80127-5. [DOI] [PubMed] [Google Scholar]
- Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- Krump E, Nikitas K, Grinstein S. Induction of tyrosine phosphorylation and Na+/H+ exchanger activation during shrinkage of human neutrophils. J Biol Chem. 1997;272:17303–17311. doi: 10.1074/jbc.272.28.17303. [DOI] [PubMed] [Google Scholar]
- Kumagai N, Morii N, Fujisawa K, Nemoto Y, Narumiya S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J Biol Chem. 1993;268:24535–24538. [PubMed] [Google Scholar]
- Laudanna C, Campbell JJ, Butcher EC. Role of rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- Lee SW, Otto JJ. Differences in turnover rates of vinculin and talin caused by viral transformation and cell density. Exp Cell Res. 1996;227:352–359. doi: 10.1006/excr.1996.0284. [DOI] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;27:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Lin X, Barber DL. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc Natl Acad Sci USA. 1996;93:12631–12636. doi: 10.1073/pnas.93.22.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Voyno-Yasenetskaya T, Hooley R, Lin C, Orlowski J, Barber DL. Ga12 differentially regulates Na-H exchanger isoforms. J Biol Chem. 1996;271:22604–22610. doi: 10.1074/jbc.271.37.22604. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;231:791–807. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii N, Narumiya S. Preparation of native and recombinant Clostridium botulinum C3 ADP-ribosyltransferase and identification of Rho proteins by ADP-ribosylation. Methods Enzymol. 1995;256:196–206. doi: 10.1016/0076-6879(95)56024-6. [DOI] [PubMed] [Google Scholar]
- Morii N, Teru-uchi T, Tominaga T, Kumagai N, Kozaki S, Ushikubi F, Narumiya S. A rho gene product in human blood platelets. J Biol Chem. 1992;267:20921–20926. [PubMed] [Google Scholar]
- Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Integrin-mediated signalling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- Peter K, O’Toole TE. Modulation of cell adhesion by changes in alpha L beta 2 (LFA-1, CD11a/CD18) cytoplasmic domain/cytoskeleton interaction. J Exp Med. 1995;18:315–326. doi: 10.1084/jem.181.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J, Sardet C, Franchi A, Allemain G, Paris A. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci USA. 1984;81:4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S, Morii N, Narumiya S, Rozengurt E. Botulinum C3 exoenzyme blocks the tyrosine phosphorylation of p125FAK and paxillin induced by bombesin and endothelin. FEBS Lett. 1994;354:315–319. doi: 10.1016/0014-5793(94)01148-6. [DOI] [PubMed] [Google Scholar]
- Rao GN, Sardet C, Pouyssegur J, Berk BC. Na+/H+ antiporter gene expression increases during retinoic acid-induced granulocytic differentiation of HL60 cells. J Cell Physiol. 1992;151:361–366. doi: 10.1002/jcp.1041510217. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56:271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Both G, Lechene C. Effect of cell spreading on cytoplasmic pH in normal and transformed fibroblasts. Proc Natl Acad Sci USA. 1989;86:4525–4529. doi: 10.1073/pnas.86.12.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Lechene C, Ingber DE. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin α5β1, independent of cell shape. Proc Natl Acad Sci USA. 1991;88:7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Toksoz D, Khosravi-Far R. Transformation by Rho exchange factor oncogenes is mediated by activation of an integrin-dependent pathway. EMBO J. 1996;15:6524–6530. [PMC free article] [PubMed] [Google Scholar]
- Shrode LD, Klein JD, O’Neill WC, Putnam RW. Shrinkage-induced activation of Na+/H+ exchange in primary rat astrocytes: role of myosin light-chain kinase. Am J Physiol. 1995;269:C257–C266. doi: 10.1152/ajpcell.1995.269.1.C257. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurement in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Tertoolen LG, Remorie R, Ladoux A, Verlaan I, de Laat SW, Moolenaar WH. Histamine as a growth factor and chemoattractant for human carcinoma and melanoma cells: action through Ca2+-mobilizing H1 receptors. J Cell Biol. 1990;110:1211–1215. doi: 10.1083/jcb.110.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga, T., Ishizaki, T., Narymiya, S., and Barber, D. (1998). p160 ROCK mediates RhoA activation of Na-H exchange. EMBO J. (in press). [DOI] [PMC free article] [PubMed]
- Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Symons M, Barber DL. Activation of Na+-H+ exchange is necessary for rhoA-induced stress fiber formation. J Biol Chem. 1996;271:22281–22284. doi: 10.1074/jbc.271.37.22281. [DOI] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya T, Conklin BR, Gilbert RL, Hooley R, Bourne HR, Barber DL. Ga13 stimulates Na-H exchange. J Biol Chem. 1994;269:4721–4724. [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Activation of protein kinase C precedes alpha 5 beta 1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- Wakabayashi S, Bertrand B, Shigekawa M, Fafournoux P, Pouyssegu J. Growth factor activation and “H+-sensing” of the Na+/H+ exchanger isoform 1 (NHE1) J Biol Chem. 1994;269:5583–5588. [PubMed] [Google Scholar]
- Wakabayashi S, Fafournoux P, Sardet C, Pouyssegur J. The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls “H+-sensing.”. Proc Natl Acad Sci USA. 1992;89:2424–2428. doi: 10.1073/pnas.89.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AL, Levine S, Donowitz M, Montrose MH. Serum regulates Na+/H+ exchange in Coco-2 cells by a mechanism which is dependent on F-actin. J Biol Chem. 1992;267:956–962. [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtson O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. Mechanism of control of erythrocyte cell shape: a possible relationship to band 3. J Theor Biol. 1994;171:197–205. doi: 10.1006/jtbi.1994.1223. [DOI] [PubMed] [Google Scholar]
- Ylänne J, Chen Y, O’Toole TE, Loftus JC, Takada Y, Ginsberg MH. Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Wang H-G, Reed JC, Ruoslahti R. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]