Abstract
Impaired language is a prominent behavioral marker of autism spectrum disorders (ASD), but its neurobiological underpinnings are incompletely understood. We studied letter and category fluency in 14 high functioning ASD individuals and 14 age-matched controls. Each fluency condition was compared to self-paced repetition of the word “nothing.” Responses were recorded to monitor performance. In letter fluency, the ASD group had significantly greater activation than controls in the right frontal and right superior temporal lobe. Between-group differences were not observed in left prefrontal cortex. By examining functional asymmetry in frontal cortex, we found that the ASD group had significantly reduced lateralization of activation patterns in letter fluency compared to the controls. In category fluency, no between-group differences in lateralization were found, in light of greater bilateral activation in controls. These findings indicate reduced hemispheric differentiation for certain verbal fluency tasks in ASD, consistent with some previous evidence of atypical functional and structural asymmetries in autism. Abnormal functional organization may contribute to the language impairment seen in ASD.
Keywords: FMRI, asymmetry, letter fluency, category fluency, frontal lobes
Introduction
Atypical language development is a prominent behavioral marker of autism spectrum disorders (ASD). In young autistic children, language deficits are among the most salient overt symptoms. Lack of spoken language by two years of age is often the first indicator to impel parents to seek professional advice (De Giacomo and Fombonne, 1998) or to be recognized as a significant risk factor by pediatricians. The timing of language acquisition is a key predictor of functional outcome; acquisition of useful speech by 5-6 years of age has been associated with better educational and functional attainment in adulthood (Howlin et al., 2000).
The severity of language deficits in individuals with ASD varies markedly. Approximately half of all individuals with autistic disorder remain nonverbal throughout life, while other individuals may develop fluent language and extensive vocabularies (Volkmar et al., 2000). However, even in high functioning individuals with ASD, difficulty with acquisition of complex syntax and morphology (Tager-Flusberg and Joseph, 2003) and aspects of pragmatic knowledge such as prosody and discourse. Given the role of language abilities in functional outcome and in mediating and facilitating social communication, an improved understanding of the brain organization underlying disordered language may shed light more generally on the neural basis of ASD and factors related to clinical severity.
Structural and functional neuroimaging studies of language areas have provided evidence that differences in lateralization may underlie language and communication difficulties in individuals with ASD (Bigler et al., 2007; Boddaert et al., 2003; Chandana et al., 2005; Chiron et al., 1995; Flagg et al., 2005; Herbert et al., 2002; Herbert et al., 2005). For example, a series of volumetric studies by Herbert and colleagues (Herbert et al., 2002; Herbert et al., 2005), demonstrated that brain asymmetry patterns differed in children with high functioning autism compared to controls. Notably, children with autism showed rightward asymmetry (relatively greater volume in the right-versus-left hemisphere) in frontal language areas (i.e., pars opercularis) whereas controls showed leftward asymmetry (Herbert, 2005). Neuroanatomical studies of the temporal lobes in autism have reported reduced volume in left planum temporale (Rojas et al., 2002), increased leftward asymmetry of planum temporale (Herbert et al., 2005), and increased rightward asymmetry in middle and inferior temporo-occipital gyrus (Herbert et al., 2005). Previously conducted functional neuroimaging studies of language processing in ASD have not consistently found a similar pattern of reversed hemispheric dominance. Several studies have reported reduced activation in the left inferior frontal gyrus during language tasks (Gaffrey et al., 2007; Just et al., 2004; Kana et al., 2006; Muller et al., 1999a; Muller et al., 1998a); one study reported atypical activation in the right frontal lobe (Muller et al., 1999a). However, it is important to note that the language tasks utilized in these studies do not yield strongly lateralized frontal and temporal lobe activation in typically developing individuals.
The goal of the current study was to determine whether abnormal functional asymmetries in the frontal lobes are present in autism during single word production. Two widely used behavioral measures of language functioning, letter fluency and category fluency, were selected for the fMRI experiment. The letter fluency task relies on basic word knowledge and initiation of efficient lexical retrieval strategies to name appropriate items whereas the category fluency task depends to a greater extent on overlearned semantic knowledge. Both tasks are mediated by left prefrontal cortex in most typically developing individuals (Abrahams et al., 2003; Fu et al., 2002; Gaillard et al., 2000; Gourovitch et al., 2000; Paulesu et al., 1997; Phelps et al., 1997; Szaflarski et al., 2002). Individuals with ASD show impairments on behavioral measure of the letter fluency task relative to typically developing controls (Rumsey and Hamburger, 1988; Rumsey and Hamburger, 1990; Turner, 1999, but see Minshew et al., 1997) adults with severe dyslexia (Rumsey and Hamburger, 1990) and clinical norms (Kleinhans et al., 2005). In contrast, category fluency appears to be less affected in ASD. Children with autistic disorder and Asperger's disorder are not impaired on category fluency relative to typically developing children (Boucher, 1988; Dunn et al., 1996; Manjiviona and Prior, 1999) or clinical norms (Kleinhans et al., 2005), although they may produce a higher number of uncommon category members (e.g., “yak” for animal) than expected (Dunn et al., 1996).
Verbal fluency tasks have been found to correlate with frontal and temporal lobe functioning. Impaired letter fluency and intact category fluency is typically ascribed to frontal-subcortical dysfunction and intact temporal lobe functioning (Henry and Crawford, 2004). As such, impaired performance on fluency tasks in ASD may be related to known impairments in frontal-subcortical systems (for review see Courchesne et al., 2004; Courchesne et al., 1999). However, the understanding of the link between cognitive deficits and developmental neuropathology remains limited. It is possible that the neural underpinnings of cognitive deficits present in a neurodevelopmental disorder such as ASD may not mirror those of acquired disorders (Thomas and Karmiloff-Smith, 2002). Thus, in addition to comparing group differences in clusters of activation using standard FMRI methodology, we conducted a lateralization study to investigate individual patterns of activation in the frontal lobes in order to better characterize the functional organization of language in ASD. Unlike the standard methodology, activation need not overlap spatially across individuals as long as it is localized within the frontal lobe region of interest. Based on previous findings in the neuropsychological and neuroimaging literature, we predicted that the ASD group would show reduced activation in the left prefrontal cortex and increased activation in the right prefrontal cortex.
Results
Behavioral
D-KEFS Verbal Fluency Test
In order to facilitate comparisons to behavioral performance during FMRI, the statistical analyses were limited to the mean number of words generated in the first 45 s of each task (i.e., specific letter or specific category). Consistent with previous studies, the ASD group generated significantly fewer words per letter than the control group in the Letter Fluency Test [ASD M (SD) = 7.4 (2.3); Control M (SD) = 13.0 (2.7); p < .00001] and fewer words per category in the Category Fluency Test [ASD M (SD) = 9.8 (2.8); Control M (SD) = 12.6 (2.4); p < .01]. However, the ASD group performed in the average range according to the clinical norms on the D-KEFS category fluency task. Group averages of standardized scores on these measures are presented in Table 2.
Table 2.
Neuropsychological and demographic information
| M | ASDa SD |
Range | M | Control a SD |
Range | |
|---|---|---|---|---|---|---|
| Age (years) | 23.79 | 9.58 | 14-44 | 22.41 | 8.67 | 14-43 |
| Weschler Abbreviated Scale of Intelligence | ||||||
| Verbal IQ | 92.43 | 14.93 | 68-115 | 110.29 | 12.98 | 87-125 |
| Performance IQ | 105.79 | 14.68 | 79-127 | 114.07 | 12.83 | 89-129 |
| Full Scale IQ | 98.14 | 11.84 | 80-117 | 113.43 | 12.91 | 88-130 |
| Delis-Kaplan Executive Function System | ||||||
| Letter Fluency | 7.3 | 2.6 | 2-12 | 13.6 | 3 | 9-19 |
| Category Fluency | 8.8 | 4.3 | 1-16 | 13.4 | 3.2 | 8-19 |
| ADI-Rb | ||||||
| Social (cutoff = 10; max = 30) | 23.21 | 4.58 | 15-30 | N | N | N |
| Verbal (cutoff = 8; max = 26) | 17.50 | 3.55 | 9-22 | N | N | N |
| Restricted Interests & Repetitive Behavior (cutoff = 3, max = 12) | 7.36 | 2.65 | 3-12 | N | N | N |
n =14
Autism Diagnostic Interview-Revised
Note. N = not applicable. Scaled scores (mean = 10, SD = 3) are reported for the DKEFS fluency tests.
fMRI verbal fluency performance
Between-group comparisons of mean number of correct words generated per Fluency block were conducted. The ASD group produced significantly fewer correct words per Fluency block than the control group during the letter fluency [ASD M (SD) = 10.4 (3.5); control M (SD) = 13.0 (2.4); p < .001] and category fluency paradigms [ASD M (SD) = 10.8 (2.3); control M (SD) = 16.2 (4.0); p < .001]. However, no significant between-group differences were found in the number of errors during the letter fluency [ASD M (SD) = 0.37 (0.36); control M (SD) = 0.61 (.63); p > .05] or category fluency [ASD M (SD) = 0.77 (0.83); control M (SD) = .71 (1.17); p > .05] blocks. Responses were coded as errors if they were a repetition of a previously stated word, a non-target item, or a neologism. Words that could not be understood were not included in the error score.
FMRI
Corrected head motion
Between-group differences in the amount of detected head motion were investigated by statistically comparing the motion parameters provided by the 3dvolreg output files. We statistically compared the absolute value of degrees in the roll, pitch, and yaw direction, and mean mm displacement in the x, y, and z direction. A conservative approach was utilized, in that all time points were included, even those that were censored in the FMRI analyses due to visually detected motion. No significant between-group differences in overall head movement were found for Letter Fluency [ASD M(SD) = 0.25 (0.213), control M(SD) = 0.18 (0.009); t (17.79) = 1.081, p = .294] or Category Fluency [ASD M(SD) = 0.40 (0.112), control M(SD) = 0.14 (0.005); t (18.70) = 1.081, p = .086] scans.
Within group analyses
Letter fluency
Clusters of significant task-related activity for both groups are shown in Figure 1. The specific brain regions underlying the significant clusters of activation are detailed in Table 1. Both the ASD and the Control group showed significant (p<.05, two-tailed, corrected) activation in the left middle frontal gyrus.
Figure 1.
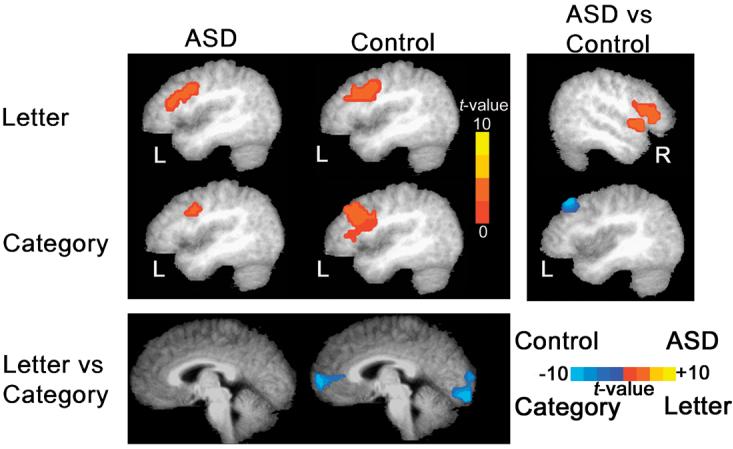
Areas of significant activation during letter and category fluency, superimposed on a standardized group-averaged brain. Results are organized by task (rows) and group (columns). Both groups exhibited significant (voxel height p < .005, voxel extent p < .05) left prefrontal activation during each verbal fluency task. In the letter fluency task, the ASD group evidenced significantly (voxel height p < .05, voxel extent p < .05) greater activation than the control group in right prefrontal cortex and right temporal cortex. There were no brain regions in which the controls had significantly greater activation than the ASD group. In the category fluency task, the controls had significantly (voxel height p < .05, voxel extent p < .05) greater activation in the left middle frontal gyrus. The ASD did not have greater activation than the control group in any brain region in the category fluency task. Within-group comparisons of letter fluency vs. category fluency revealed significantly (voxel height p < .05, voxel extent p < .05) increased medial frontal and occipital lobe activation during category fluency in the control group. No task related differences were observed in the ASD group. No brain area was significantly more active in the letter fluency task than the category fluency task in either group. Statistical significance for all comparisons was determined at the whole brain level using a Monte Carlo based procedure (AlphaSim) in AFNI. Clusters of activation that reflected differences in amount of reverse activation were not reported.
Table 1.
Within and between-group brain activation for letter and category fluency.
| Location (approximate Brodmann area) |
Mean Intensityd |
Cluster Volume |
Talairach coordinates | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Letter Fluency | ||||||
| ASDa | ||||||
| L middle frontal G. (9) | 0.26 | 6208 | −41 | 15 | 25 | |
| Controla | ||||||
| L middle frontal G. (9) | 0.30 | 6016 | −42 | 9 | 31 | |
| ASD > Control b | ||||||
| R frontal (44) | 0.39 | 7808 | 49 | 14 | 7 | |
| R inferior frontal G. (45) | 48 | 25 | 7 | |||
| R superior temporal G. (22) | 54 | 7 | 4 | |||
| ASD > Control c | ||||||
| R frontal (44) | 0.41 | 2176 | 50 | 11 | 4 | |
| R inferior frontal G. (45) | 0.38 | 1024 | 46 | 24 | 11 | |
| Category Fluency | ||||||
| ASDa | ||||||
| L insula | 0.20 | 4992 | −30 | 19 | 11 | |
| L inferior frontal G (9) | 0.36 | 1920 | −50 | 10 | 29 | |
| L medial frontal G. | 0.36 | 1664 | −4 | 1 | 52 | |
| Controla | ||||||
| L middle frontal G. (46,9) | 0.29 | 14272 | −44 | 17 | 26 | |
| L inferior frontal G (45) | −37 | 26 | 5 | |||
| L medial frontal G. (32) | 0.23 | 1792 | −6 | 3 | 54 | |
| Control > ASD b | ||||||
| L middle frontal G. (8) | −0.5816 | 13760 | −42 | 23 | 44 | |
| ASD > Control c | ||||||
| R inferior frontal G. (47) | 0.50 | 4672 | 52 | 19 | −3 | |
| L inferior frontal G. (44) | 0.33 | 2176 | −55 | 3 | 17 | |
| L medial frontal G. | 0.22 | 1600 | −10 | −15 | 51 | |
| Control > ASD c | ||||||
| L middle frontal F. (8) | −0.525 | 5248 | −42 | 23 | 44 | |
| Category > Letter Fluency | ||||||
| Controlb | ||||||
| Bilateral lingual gyrus (18) | −0.4552 | 51648 | −10 | −87 | −14 | |
| Bilateral cuneus (18) | −2 | −94 | 6 | |||
| Bilateral medial frontal G. | −0.4958 | 20928 | −9 | 53 | 8 | |
| Left inferior frontal G. | 36 | −39 | 1 | |||
| Left middle frontal G. | 44 | −47 | 4 | |||
Note. Talairach coordinates for other brain areas within the cluster are listed separately and italicized.
Voxel height p < .005, α = .05 (cluster volume corrected)
Voxel height p < .05, α = .05 (cluster volume corrected)
Results for ASD and controls groups matched on handedness. Voxel height p < .05, α = .05 ( uncorrected).
Intesity values correspond to the mean percent signal change between the task and control blocks.
Category fluency
The ASD had significant (p< .05, two-tailed, corrected) activation in the left insula, left inferior frontal gyrus and left medial frontal gyrus. The control group had significant clusters of activation in the left middle, inferior, and medial frontal gyri.
Letter versus category fluency
In the control group, a direct comparison of letter fluency and category fluency yielded significantly greater activity in left inferior, middle, and medial frontal cortex, and bilateral occipital cortex (BA 18) (see Figure 1). No brain region was significantly more active during the letter fluency condition. Significant clusters reflecting task differences in amount of reverse activation were not reported. No significant differences in activation were found between the letter fluency tasks and category fluency tasks in the ASD group.
Between-group analyses
Letter fluency
The ASD group had significantly (p < .05, two-tailed, corrected) greater activation than the control group centered in the right inferior frontal lobe. The control group did not activate any brain region significantly more than the ASD group.
Category fluency
The control group had significantly (p < .05, two-tailed, corrected) greater activation than the ASD group centered in the left middle frontal lobe (BA 8). The ASD group did not activate any brain region significantly more than the control group.
Between-group analyses matched on age and handedness
Post-hoc analyses were conducted on a subset of the ASD and control groups matched on handedness (n=12). One left-handed and one ambidextrous individual were excluded from the ASD group and two right-handed, age-matched controls were excluded. Statistical significance was set at p < .05, uncorrected. Only brain regions which yielded statistically significant results in analyses with the entire group were investigated (left frontal, right frontal, medial frontal and occipital).
Letter fluency
The specific brain regions underlying the significant clusters of activation are detailed in Table 1. The ASD group had significantly (p < .05, two-tailed) greater activation than the control group centered in the right inferior frontal lobe. The control group did not activate any brain region significantly more than the ASD group.
Category fluency
The specific brain regions underlying the significant clusters of activation are detailed in Table 1. The control group had significantly (p < .05, two-tailed) greater activation than the ASD group centered in the left middle frontal lobe (BA 8). The ASD group had significantly greater activation than the control group in the right inferior frontal (BA 47), left inferior frontal (BA44), and medial frontal gyri.
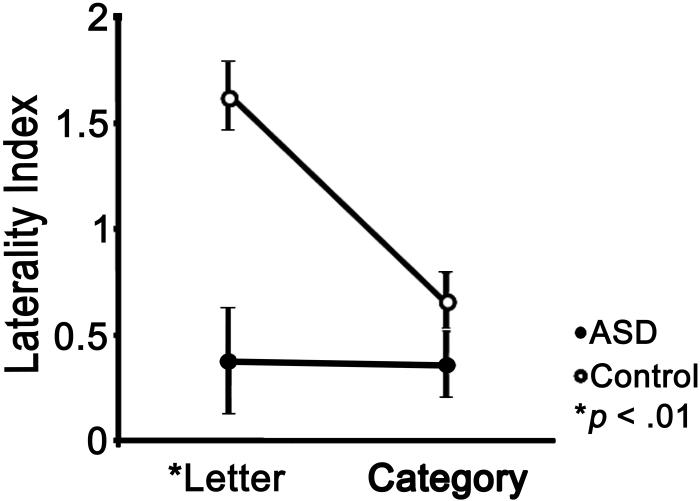
Laterality study
The laterality index used here resulted in a scale from -2 to 2 on which positive values indicate leftward lateralization (the higher the stronger) and negative values indicate rightward lateralization (the lower the stronger).
Letter fluency
T-tests were performed to examine between-group differences in mean volumes of significant activation for left and right frontal lobe ROIs (analyzed separately), and in degree of functional asymmetry as measured by the laterality index (LI). Individuals without significant activation in either ROI were excluded from the LI computation, but not the mean cluster volume comparison. The ASD group showed significantly weaker left-lateralized activation than the control group (ASD M = .38, Control M = 1.68, p < .001). No significant between-group differences were found in mean total cluster volume for either the left (ASD = 18106 μL, 16303 μL, p>.05) or right (ASD = 22078 μL, control = 21480 μL, p>.05) frontal ROIs.
Category fluency
T-tests were performed to determine if significant between-group differences were present in mean left hemisphere volume of significant activation, mean right hemisphere volume of significant activation, and in degree of functional asymmetry as measured by the LI. All study participants had significant activation in at least one of the ROIs. No significant group differences in lateralization of activation was found (ASD M = .36, control M = .66, p > .05). The control group showed weaker left-lateralization in the category fluency condition compared to the letter fluency condition. The ASD group did not show task-related differences in lateralization. No significant between-group differences were found in mean total cluster volume for either the left (ASD = 103986 μL, 92360 μL, p>.05) or right (ASD = 66979 μL, control = 53114 μL, p>.05) frontal ROIs.
Letter vs. category fluency
Task-related and group related differences in the laterality index were further investigated. We conducted statistical analyses using a one-factorial split-plot within subjects design. The within-subjects factor was task (letter, category) and the between-subjects factor was group membership (ASD, control). Main effects and interactions between factors were tested. When LIs were combined across fluency tasks, the ASD group showed significantly weaker left lateralization than the control group (M ASD = .353, M control = 1.141; F1,23= 13.4, p= .001).
Significantly greater leftward lateralization was observed in the letter fluency task compared to the category fluency task (F1,23=8.11, p = .007). However, a significant task by group interaction was found, such that the relationship between lateralization and verbal fluency task was dependent on group (F1,23=6.872, p = .015). A follow up analysis looking at the simple effect of task in the ASD group was not significant (M letter = .383, M category = .323; F1,10=.036, p > .05), indicating that lateralization did not differ in ASD when performing the letter and category fluency task. In contrast, the control group showed significantly greater leftward lateralization in the letter fluency task than in the category fluency task (M letter = 1.623, M category = .659; F1,13=78.998, p < .0001). These results indicate that typically developing controls, but not individuals with autism spectrum disorders, display significantly stronger leftward asymmetry during letter fluency compared to category fluency (see Figure 2).
Figure 2.
Task-related differences in mean frontal lobe activation laterality. Error bars indicate standard error of the mean. A larger laterality index (LI) indicates stronger left-lateralization of activation. Significantly weaker left-lateralization was observed in category fluency compared to letter fluency in the control group. In the ASD group, significant task-related differences in lateralization were not observed.
Between-group lateralization analyses matched on handedness
Post-hoc analyses were conducted on a subset of the ASD and control groups matched on handedness (n=12). One left-handed and one ambidextrous individual were excluded from the ASD group and two right-handed, age-matched controls were excluded.
Letter fluency
Individuals without significant activation in either ROI were excluded from the LI computation but the not mean cluster volume comparison. As in the previous analysis, ASD group (n=9) showed significantly weaker left-lateralized activation than the control group (n=12) (ASD M = 0.54., Control M = 1.56, p = .011). No significant between-group differences were found in mean total cluster volume for either the left (ASD = 23,830 μL, control=22,220 μL, p>.05) or right (ASD = 18,497 μL, control = 9,140 μL, p>.05) frontal ROIs.
Category fluency
No significant group differences in the LI (ASD M = 0.42., Control M = 0.60 p < .05) or mean total cluster volume (left: ASD = 110,057 μL, control=92,047 μL, p>.05; right: ASD = 68,083 μL, control = 56,433 μL, p>.05) of the frontal ROIs was found.
Discussion
This study used two frontally mediated language tasks to measure the functional organization of language in ASD with fMRI. The present study showed reduced hemispheric differentiation for verbal fluency tasks in individuals with autism spectrum disorders. At the group level, robust activation in left prefrontal cortex was observed in the ASD group during both verbal fluency conditions. However, a direct group comparison of the letter fluency task showed that the ASD group had greater right hemisphere activation than controls in frontal (BA 44, 45), insular, and temporal (BA 20, 21, 22, 37) regions. Between-group differences were not observed in left prefrontal cortex. A follow-up analysis conducted to further characterize the abnormal functional lateralization in prefrontal cortex for letter fluency found significantly greater leftward asymmetry in controls than in the ASD group, a difference which persisted when groups were matched on handedness. In fact, all controls showed leftward asymmetry with 10 of 14 exclusively activating the left frontal lobe, whereas 12 of the 14 ASD individuals evidenced right, bilateral, absent, or weak left lateralized activation patterns. These findings indicate that impairments in verbally mediated executive functioning tasks may be due to abnormal functional organization of language in prefrontal cortex.
Unlike the current study, decreased activation in the left inferior frontal gyrus and increased left temporal lobe activation during sentence comprehension (Just et al., 2004; Kana et al., 2006), semantic processing (Gaffrey et al., 2007; Harris et al., 2006) has been previously reported in FMRI studies of language in ASD. Increased temporal lobe activation during language processing was interpreted to reflect an unusual strength in single word processing (Just et al., 2004). It is possible that reduced left frontal activation was not found in the current study because we used a task that required generating single words, a skill that in this paradigm is mediated by the frontal (instead of temporal) lobes. The differences between the current results and previous studies may also be due to differences in language ability. The participants with ASD in the current study were considerably more language impaired than those in Just et al, Kana et al. or Harris et al. Thus, it is possible that atypical language dominance is related to language impairment in autism, rather than specific to individuals with autism across all language abilities (Herbert et al., 2005). Furthermore, in the Just et al study, individuals who did not have strongly left lateralized activation were excluded, which may also account for the lack of increased right hemisphere activation reported in their study. Interestingly, increased right frontal activation was reported in two studies that investigated language comprehension tasks with a social attribution component in children (mean age 11) with ASD (Takeuchi et al., 2004; Wang et al., 2006). The children with ASD in these studies were high functioning. Although speculative, one possible interpretation of the current findings in adults and children with ASD is that high functioning children may eventually acquire typical language dominance while individuals with greater language impairments do not. Therefore, the atypical lateralization findings by Wang et al and Takeuchi et al may reflect a transient state in ASD, reflecting perhaps delayed maturation of the frontal lobes (Zilbovicius et al., 1995) and language lateralization processes (see below).
Task-Related Differences in Activation: Letter Fluency vs. Category Fluency
The between-group differences in lateralization of activation present in the letter fluency condition were not observed in the category fluency condition. In contrast to the strong left-lateralized activation present in controls during letter fluency (M LI = 1.62), the control group recruited right prefrontal cortex to a significantly greater extent during category fluency, resulting in weaker lateralization (M LI = .66). The direct comparison of letter fluency to category fluency in the control group did not yield a significant cluster of activation in right prefrontal cortex related to the category fluency condition, indicating the loci of right hemisphere activation(s) were variable in the control participants.
The presence of stronger left lateralization in letter fluency compared to category fluency in typically developing controls was reported by one group (Billingsley et al., 2004) but not others (Gourovitch et al., 2000; Mummery et al., 1996) (Paulesu et al., 1997). It is possible that previous studies did not find this effect because group results, rather than individual-based analyses of lateralization, were provided. Right hemisphere activation was present in all controls during the category fluency task, compared to only 4 controls in the letter fluency task. This is consistent with previous studies that have reported right hemisphere involvement in tasks which require semantic processing (Bookheimer, 2002; Gold and Kertesz, 2000; Kang et al., 1999; Seger et al., 2000; Seghier et al., 2004).
Greater activation in BA 8 was also found in category fluency compared to letter fluency in the control group in the direct comparison of the fluency tasks. Activation in left superior frontal gyrus has been reported in other studies involving lexical-semantic processes (Binder et al., 1997; Braver et al., 1997; Demonet et al., 1992; Muller et al., 2003) and has been suggested to reflect working memory demands (Muller et al., 2003). Greater working memory demands were likely present in the category fluency task compared to the letter fluency task given the significantly greater number of words produced by participants during category fluency. When more words are generated, the process of monitoring responses in order to avoid repeating a previously stated word increases working memory demands. Thus, the significantly greater activation in BA 8 in category fluency in this study likely reflects task-related differences in working memory.
Lateralization and Behavioral Impairment in ASD
In individuals with ASD, absence of strongly, left-lateralizing activation during the letter fluency task may underlie performance difficulties. Task-related differences in lateralization of activation were not present in the ASD group (M letter = .383, M category = .323), indicating reduced hemispheric specialization for linguistically driven tasks compared to typically developing individuals. It is also notable that between-group differences in activation in the letter fluency task were more striking than in the category fluency task, which corresponds to behavioral performance on these tasks (i.e., letter fluency behavioral performance was clinically impaired while category fluency was not). Thus, it is possible that individuals with ASD may have more difficulty performing tasks in which unilateral hemispheric specialization confers an advantage. Lateralization of functions is potentially advantageous, because greater efficiency may result from transferring information within, as opposed to across, cerebral hemispheres (Toga and Thompson, 2003). Another possible hypothesis is that lack of hemispheric specialization may contribute to reduced abilities in right hemisphere-specialized tasks as well, which include processing the prosodic, emotional, and melodic aspects of language, and interpreting figurative meanings in language, humor, and metaphor (Toga and Thompson, 2003). Behavioral studies report deficits in these skills in autism (e.g., McCann and Peppe, 2003), which suggests that further study may be warranted in this area.
Early Brain Growth Abnormalities and Lateralization of Language
Atypical organization of language functions may result from early neural insults as demonstrated by experiments with perinatal focal lesions and early onset epilepsy (Adcock et al., 2003; Muller et al., 1999b; Muller et al., 1998b) and has been reported in a preliminary study of five individuals with autism (Muller et al., 1999a). As such, the atypical functional organization of language found in the current study may be due to the aberrant neurodevelopmental processes which have been recently identified in ASD. Recent studies demonstrated that rapid, excessive brain growth occurs in the first years of life in autism (Courchesne et al., 2003; Dementieva et al., 2005; Webb et al., 2007). In the Courchesne et al. study, head circumference measures showed that autistic newborns were in the 25th percentile for head size at birth, which was significantly smaller than the normative sample. However, head circumference measurements increased dramatically beginning at 2 to 3 months of age, and by 6 to 14 months of age, had reached the 86th percentile relative to normative databases. From age 2 to 4 years, brain growth appears to slow down in autism, but absolute brain size is still enlarged compared to typically developing controls (Courchesne et al., 2001). In this 2 to 4-year-old age period, increased grey matter volume in the cerebrum and increased white matter volume in the cerebrum and cerebellum is present (Courchesne et al., 2001; Hazlett et al., 2005). Early brain overgrowth is followed by premature arrested development, with maximum brain size being attained at approximately 4-5 years of age (Courchesne et al., 2001). Thus, brain growth in autism appears to stop approximately 9 years earlier than brain growth in typically developing children, who do not reach maximum brain size until 12-16 years of age (Courchesne et al., 2000; Giedd et al., 1999).
The course of early brain growth and development in autism contrasts sharply with typically developing children. In typically developing children, brain growth is a slow, experience modulated process (Huttenlocher, 2002) with regional variation in the timing of the development of cerebral cortex (Huttenlocher and Dabholkar, 1997). The right hemisphere develops faster than in the left hemisphere in the first year of life (Toga and Thompson, 2003) and remains dominant until approximately 3 years of age (Chiron et al., 1997). Putative language areas (BA 44/45) exhibit adult-like cytoarchitectonic asymmetry at an even later point in development than the emergence of generalized left hemisphere dominance (Amunts et al., 2003). Left greater than right structural asymmetry is not reached in BA 45 until 5 years of age and in BA 44 until 11 years of age in typically developing children (Amunts et al., 2003). Amunts and colleagues suggested that delayed maturation is the microstructural reflection of the development of language abilities and that language experience may in turn influence brain anatomy.
Prematurely arrested brain growth in autism may have specific consequences for language development. Notably, brain growth in autism ceases before the mature pattern of left hemisphere dominance is reached in typically developing children (Chiron et al., 1997), and in the majority of children with autism spectrum disorders, prior to the emergence of complex language (Charman et al., 2003). Thus, unlike typical development where extended periods of brain growth coincide with language acquisition and mastery, in autism, language acquisition typically occurs subsequent to brain growth, without the benefits of neuroplasticity to promote the development of adaptive neural connections. Courchesne and colleagues (2003) suggested that such aberrantly rapid and disordered growth may lead to an excessive amount of connections that may be maladaptive. Consistent with that theory, the widespread bilateral language activation in ASD found in the current study suggests that excessive, maladaptive connections and failure to develop typical lateralization may underlie disordered language in ASD. However, because volumetric analysis was not performed, we cannot determine whether reduced lateralization is also present at the morphological level in this group.
Limitations
The inclusion of a diagnostically mixed group covering a wide age range was both a strength and weakness. The generalizability of the current findings to individuals across the broader, higher functioning autism spectrum is strengthened. Although the individuals in the current study differed in early developmental history and level of autistic symptomatology, as a group, consistent functional abnormalities were observed. However, given the evidence that language ability may be linked to neural abnormalities, it is possible that differences in degree or type of neural abnormality may lead to these diagnostic outcomes. Future studies that look specifically at differences in early language history and current language functioning may help determine the role these factors play in neurofunctional language organization. In addition, developmental changes in language organization throughout adolescence and adulthood in ASD were not addressed in the current study. Given the wide age-range of our sample, it is important to note that the lateralization differences reported here may not be observed to the same extent at all ages; such a possibility should also be addressed in future studies. Finally, because our ASD group was not matched on verbal ability to the control group, the neural abnormalities identified in this study may reflect language impairment generally rather than an abnormality that is specific to individuals on the autism spectrum. Further, it should be noted that reduced cognitive ability may underlie language impairment in ASD. Thus, both language impairment and general intelligence may be associated with reduced lateralization of cognitive functions.
Conclusion
In summary, the ASD group had significantly greater activity than controls in right frontal and temporal lobes in the letter fluency task. Between-group differences were not observed in left prefrontal cortex. A lateralization analysis of prefrontal activation found significantly greater leftward asymmetry in controls than in the ASD group in the letter fluency task. In fact, all controls showed leftward asymmetry whereas 12 of the 14 ASD individuals evidenced right, bilateral, absent, or weak left lateralized activation patterns. Between-group differences in lateralization were not found in the category fluency task, due to the significantly greater right hemisphere activation present in controls on this task. The lack of between-group differences in lateralization corresponds to the lack of clinical impairment found on the category fluency test in ASD. Overall, these data indicate reduced hemispheric differentiation for verbal fluency tasks in ASD. While lack of hemispheric specialization for higher-order tasks may have broad implications across many language abilities in ASD, greater behavioral impact may be observed in tasks which are strongly lateralized in typically developing controls (e.g., letter fluency). Abnormal functional organization may be related to early, rapid overgrowth of frontal lobes and subsequent arrested brain development recently reported in autism. Such growth dysregulation may disrupt the protracted developmental progression by which the left hemisphere becomes dominant for language, and in turn contribute to the language impairment seen in autism.
Experimental Procedure
Participants
Sixteen adults adult and adolescent males diagnosed with an autism spectrum disorder (ASD) participated in the experiment. Two individuals were excluded from the study due to excessive head motion; their data are not reported. Thus, fourteen adult and adolescent males diagnosed with an autism spectrum disorder (ASD) were included in the final sample. Eight participants in the ASD group met criteria for autistic disorder, three met criteria for Asperger's disorder, and three met criteria for pervasive developmental disorder, not otherwise specified (PDD-NOS) according to DSM –IV criteria (American Psychiatric Association, 1994). In addition, participants all met autism spectrum disorder criteria on the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994) and Autism Diagnostic Observation Schedule - General (ADOS-G) (Lord et al., 2000). The mean age of the ASD group was 24.1 years (range = 14-44 years). Thirteen individuals were Caucasian and one was Hispanic. All had full scale IQs above 80 (see Table 2 for diagnostic and demographic information on the ASD group). Two individuals were left-handed, one individual was ambidextrous, and 11 were right-handed. Handedness was determined by self-report.
Typical developing participants were screened for a history of developmental, psychiatric, or neurologic disorders. Participants were recruited from existing subject databases and through personal contacts. Two individuals were excluded from the study due to excessive head motion during fMRI scanning and one individual was excluded because of a technical failure related to recording his responses. Control participants were matched to the ASD participants according to age. Three individuals with technically acceptable data were excluded following the matching procedure because other participants yielded a better age match. Fourteen typically developing individuals were included in the study. The mean age difference between the matched pairs was 1.29 years (SD = 1.56 years). Eleven individuals were Caucasian, two were Asian, and one was African-American. One individual was left handed; all others were right-handed. Handedness was determined by self-report. In addition, none of the study participants had obvious structural pathology on the anatomical brain scan.
This study was approved by the University of California, San Diego, San Diego State University, and Children's Hospital of San Diego Institutional Review Boards. Informed written consent was obtained from the participants and if applicable, their parents. All participants were paid $20 per hour of participation.
Design and Procedure
Prior to scanning, all participants were administered the Verbal Fluency Test from the Delis-Kaplan Executive Function System (Delis et al., 2001) following standard administration procedures. Participants performed two verbal fluency tasks (letter and category) during echo planar imaging acquisition. During the letter fluency task, participants generated as many words as possible that began with the letter on the screen (B,H,R,F). For category fluency, participants generated as many items as possible from the category on the screen (animals, clothes, buildings, vehicles). The comparison condition for both FMRI experiments was self-paced repetition of the word “nothing,” which was guided by the visual presentation “Nothing.” Short rest periods were interspersed between the Nothing and Fluency conditions. Each run consisted of four 45-second Fluency blocks (F), four 26-second Nothing blocks (N), and four 10-second Rest blocks (R). The experimental design for each run was: FNRFNRFNRFNR which followed a 5.2 second delay. Responses were recorded during the FMRI experiment to verify task performance. Responses were transcribed and scored for accuracy.
FMRI Data Acquisition
MR Imaging was performed with a 1.5 T Siemens Symphony MR scanner (Erlangen, Germany) equipped with the standard clinical head coil. Functional whole-brain T2*-weighted images were acquired using a single-shot gradient-recalled echo-planer imaging sequence (TR = 2600 ms; TE = 36 ms; flip angle = 90°; FOV = 256 mm) with a matrix size of 64 × 64 (in-plane resolution = 4 × 4 mm). Twenty-eight contiguous 5 mm slices in the axial plane were acquired during each image using interleaved slice acquisition. One hundred and thirty-four images were collected per verbal fluency run. The letter fluency paradigm always preceded the category fluency paradigm. This order was maintained in order to match the clinical administration procedure. The first two volumes were discarded to control for signal inhomogeneities which occur at the beginning of the scan. A high-resolution 3D MP-RAGE (magnetization prepared-rapid gradient echo; TR = 11.08 ms; TE = 4.3 ms; flip angle = 45°; FOV = 256 mm; matrix 256 × 256; 180 slices; resolution = 1 mm3) structural scan was acquired during the scanning session for anatomical localization.
FMRI Data Analysis
Individual processing and analyses
Motion was systematically evaluated in all participants. First, a printout of outlier values in the raw data was obtained from AFNI (http:afni.nimh.nih.gov/afni, Cox, 1996). Timepoints with large spikes in the number of outliers may indicate the presence of head movement, but visual inspection is necessary for confirmation. Next, a research technician scrolled through the time series, and noted timepoint by timepoint, which ones had visibly detectable motion. Image registration and functional analyses were then conducted using AFNI (http:afni.nimh.nih.gov/afni, Cox, 1996). Motion correction and registration were done using an automated alignment program (3dvolreg), which co-registers each volume in the timeseries to a fiducial volume using an iterative process (Cox and Jesmanowicz, 1999). The fiducial volume was individually selected for each participant and corresponded to the volume closest to the midpoint without visibly detectable motion. Then, those time points with noticeable motion in the raw data were inspected again, to determine whether the registration program had been able to correct the motion such that it was no longer visibly detectable. If motion was still visibly detectable following the registration procedure, then the scan was excluded. If the motion was no longer detectable, then the scan was included, but the timepoints with detectable motion prior to registration were censored from the statistical analysis. The functional image time series was smoothed with a Gaussian filter (full-width, half-maximum = 8 mm) resampled into Talairach coordinates (Talairach and Tournoux, 1988) according to the AFNI hand-landmarking procedure (resampled volumes = 4 mm3).
Statistical analysis of the individual functional imaging data was conducted through calculation of the cross-correlation of the reference waveforms and the measured timeseries data on a voxel-by-voxel basis. Boxcar timeseries models were created that modeled the verbal fluency condition and the “nothing” control condition. The modeled timeseries were shifted to account for the delay in hemodynamic response in fitting the ideal model. Timepoints with apparent motion, determined through visual inspection, were censored on an individual basis and excluded from all statistic analyses. Percent MR signal change of the verbal fluency condition relative to the control condition was calculated and correlated with the modeled timeseries using the program 3dfim+. The linear trend and global mean were removed from the FMRI timeseries. Motion parameters, corresponding to mm of adjustment in the x, y, and z axes and degrees of adjustment in the roll, pitch, and yaw direction per timepoint, obtained from the motion correction procedure detailed above, were included as orthogonal regression coefficients.
Group level analyses
The voxelwise group mean signal change and associated t value in the combined fluency versus “nothing” conditions for the ASD group and the control group separately were calculated using a one sample t-test. An unpaired t-test comparing the ASD group to the control group was also conducted. Group differences that stemmed from differences in the control task (deactivations) were excluded. Statistical significance was determined for the whole brain using a Monte Carlo based voxel-cluster threshold technique.
Laterality studies
A region of interest (ROI) approach was used to conduct the laterality studies for the Letter and Category fluency paradigms. Right and left hemisphere ROIs which included the superior, middle, and inferior frontal gyri and the insula were created with the Talairach Daemon program. A liberal search area was utilized because the ASD participants' frontal lobe activations were anticipated to be more heterogeneous than typically developing individuals (Müller, Kleinhans, Kemmotsu et al., 2003). Significant cluster(s) of activation in the ROI for each individual were identified using Monte Carlo simulation at p< .05, one-tailed. A one-tailed distribution was utilized because the investigation was limited to identifying positive percent signal change in the fluency task relative to the control task. Significant clusters were summed, per hemisphere, on an individual basis. The laterality index (LI) was computed using the total cluster volume(s) per ROI: (L-R)/0.5(L+R).
Acknowledgements
The authors were supported by funds from the National Institute of Mental Health (RO1-MH36840) awarded to Eric Courchesne and (RO1-DC6155) awarded to R-Axel Müller. Portions of this work were presented at the International Neuropsychological Society, St. Louis, MO, February 2005 and the International Meeting for Autism Research, Boston, MA, May 2005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams S, et al. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp. 2003;20:29–40. doi: 10.1002/hbm.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock JE, et al. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–38. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Amunts K, et al. Broca's region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Bigler ED, et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–38. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, et al. Spatio-temporal cortical dynamics of phonemic and semantic fluency. Journal of clinical and experimental neuropsychology. 2004;26:1031–43. doi: 10.1080/13803390490515333. [DOI] [PubMed] [Google Scholar]
- Binder JR, et al. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–62. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert N, et al. Perception of Complex Sounds: Abnormal Pattern of Cortical Activation in Autism 10.1176/appi.ajp.160.11.2057. American Journal of Psychiatry. 2003;160:2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Boucher J. Word fluency in high-functioning autistic children. J Autism Dev Disord. 1988;18:637–45. doi: 10.1007/BF02211881. [DOI] [PubMed] [Google Scholar]
- Braver TS, et al. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Chandana SR, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. International Journal of Developmental Neuroscience Autism: Modeling Human Brain Abnormalities in Developing Animal Systems. 2005;23:171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Charman T, et al. Measuring early language development in preschool children with autism spectrum disorder using the MacArthur Communicative Development Inventory (Infant Form) J Child Lang. 2003;30:213–36. doi: 10.1017/s0305000902005482. [DOI] [PubMed] [Google Scholar]
- Chiron C, et al. The right brain hemisphere is dominant in human infants. Brain. 1997;120(Pt 6):1057–65. doi: 10.1093/brain/120.6.1057. [DOI] [PubMed] [Google Scholar]
- Chiron C, et al. SPECT of the brain in childhood autism: evidence for a lack of normal hemispheric asymmetry. Dev Med Child Neurol. 1995;37:849–60. doi: 10.1111/j.1469-8749.1995.tb11938.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, et al. Evidence of brain overgrowth in the first year of life in autism. Jama. 2003;290:337–44. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–82. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Courchesne E, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–54. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, et al. The autistic brain: birth through adulthood. Curr Opin Neurol. 2004;17:489–96. doi: 10.1097/01.wco.0000137542.14610.b4. [DOI] [PubMed] [Google Scholar]
- Courchesne E, et al. Biological and behavioral heterogeneity in autism: Role of pleiotropy and epigensis. In: Broman SH, Fletcher JM, editors. The Changing Nervous System: Neurobehavioral Consequences of Early Brain Disorders. Oxford University Press; New York: 1999. pp. 292–338. [Google Scholar]
- De Giacomo A, Fombonne E. Parental recognition of developmental abnormalities in autism. Eur Child Adolesc Psychiatry. 1998;7:131–6. doi: 10.1007/s007870050058. [DOI] [PubMed] [Google Scholar]
- Dementieva YA, et al. Accelerated head growth in early development of individuals with autism. Pediatr Neurol. 2005;32:102–8. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Demonet JF, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115(Pt 6):1753–68. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Dunn M, et al. Prototypicality of responses of autistic, language disordered, and normal children in a word fluency task. Child Neuropsychology. 1996;2:99–108. [Google Scholar]
- Flagg EJ, et al. Language lateralization development in children with autism: Insights from the late field magnetoencephalogram. Neuroscience Letters. 2005;386:82–87. doi: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Fu CH, et al. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demand. Neuroimage. 2002;17:871–9. [PubMed] [Google Scholar]
- Gaffrey MS, et al. Atypical [corrected] participation of visual cortex during word processing in autism: an fMRI study of semantic decision. Neuropsychologia. 2007;45:1672–84. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, et al. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–5. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gold BT, Kertesz A. Right hemisphere semantic processing of visual words in an aphasic patient: an fMRI study. Brain Lang. 2000;73:456–65. doi: 10.1006/brln.2000.2317. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, et al. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14:353–60. doi: 10.1037//0894-4105.14.3.353. [DOI] [PubMed] [Google Scholar]
- Harris GJ, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006 doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–76. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284–95. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Large brains in autism: the challenge of pervasive abnormality. Neuroscientist. 2005;11:417–40. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- Herbert MR, et al. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52:588–96. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Herbert MR, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–26. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Howlin P, et al. Autism and developmental receptive language disorder--a follow-up comparison in early adult life. II: Social, behavioural, and psychiatric outcomes. J Child Psychol Psychiatry. 2000;41:561–78. doi: 10.1111/1469-7610.00643. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Neural Plasticity: the Effects of Environment on the Development of the Cerebral Cortex. Harvard Univ. Press; Cambridge: 2002. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Just MA, et al. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kamio Y, Toichi M. Dual access to semantics in autism: is pictorial access superior to verbal access? J Child Psychol Psychiatry. 2000;41:859–67. [PubMed] [Google Scholar]
- Kana RK, et al. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang AM, et al. An event-related fMRI study of implicit phrase-level syntactic and semantic processing. Neuroimage. 1999;10:555–61. doi: 10.1006/nimg.1999.0493. [DOI] [PubMed] [Google Scholar]
- Kleinhans N, et al. Executive functioning in Autistic Disorder and Asperger's Disorder: Flexibility, fluency, and inhibition. Developmental Neuropsychology. 2005;27:379–401. doi: 10.1207/s15326942dn2703_5. [DOI] [PubMed] [Google Scholar]
- Lord C, et al. The Autism Diagnostic Observation Schedule--Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, et al. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Manjiviona J, Prior M. Neuropsychological profiles of children with Asperger syndrome and autism. Autism. 1999;3:327–356. [Google Scholar]
- McCann J, Peppe S. Prosody in autism spectrum disorders: a critical review. Int J Lang Commun Disord. 2003;38:325–50. doi: 10.1080/1368282031000154204. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, et al. Neuropsychologic functioning in autism: profile of a complex information processing disorder. J Int Neuropsychol Soc. 1997;3:303–16. [PubMed] [Google Scholar]
- Muller R-A, et al. Linguistic theory and neuroimaging evidence: an fMRI study of Broca's area in lexical semantics. Neuropsychologia. 2003;41:1199–1207. doi: 10.1016/s0028-3932(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Muller RA, et al. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999a;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Muller RA, et al. Impairment of dentato-thalamo-cortical pathway in autistic men: language activation data from positron emission tomography. Neurosci Lett. 1998a;245:1–4. doi: 10.1016/s0304-3940(98)00151-7. [DOI] [PubMed] [Google Scholar]
- Muller RA, et al. Language organization in patients with early and late left-hemisphere lesion: a PET study. Neuropsychologia. 1999b;37:545–57. doi: 10.1016/s0028-3932(98)00109-2. [DOI] [PubMed] [Google Scholar]
- Muller RA, et al. Brain organization of language after early unilateral lesion: a PET study. Brain Lang. 1998b;62:422–51. doi: 10.1006/brln.1997.1931. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, et al. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc R Soc Lond B Biol Sci. 1996;263:989–95. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Paulesu E, et al. Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport. 1997;8:2011–7. doi: 10.1097/00001756-199705260-00042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, et al. FMRI of the prefrontal cortex during overt verbal fluency. Neuroreport. 1997;8:561–5. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Rojas DC, et al. Smaller left hemisphere planum temporale in adults with autistic disorder. Neurosci Lett. 2002;328:237–40. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Hamburger SD. Neuropsychological findings in high-functioning men with infantile autism, residual state. J Clin Exp Neuropsychol. 1988;10:201–21. doi: 10.1080/01688638808408236. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Hamburger SD. Neuropsychological divergence of high-level autism and severe dyslexia. J Autism Dev Disord. 1990;20:155–68. doi: 10.1007/BF02284715. [DOI] [PubMed] [Google Scholar]
- Seger CA, et al. Functional magnetic resonance imaging evidence for right-hemisphere involvement in processing unusual semantic relationships. Neuropsychology. 2000;14:361–9. doi: 10.1037//0894-4105.14.3.361. [DOI] [PubMed] [Google Scholar]
- Seghier ML, et al. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp. 2004;23:140–55. doi: 10.1002/hbm.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:303–14. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, et al. Difference of signal change by a language task on autistic patients using functional MRI. J Med Invest. 2004;51:59–62. doi: 10.2152/jmi.51.59. [DOI] [PubMed] [Google Scholar]
- Thomas M, Karmiloff-Smith A. Are developmental disorders like cases of adult brain damage? Implications from connectionist modelling. Behav Brain Sci. 2002;25:727–50. doi: 10.1017/s0140525x02000134. discussion 750-87. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kamio Y. Verbal association for simple common words in high-functioning autism. J Autism Dev Disord. 2001;31:483–90. doi: 10.1023/a:1012216925216. [DOI] [PubMed] [Google Scholar]
- Turner MA. Generating novel ideas: fluency performance in high-functioning and learning disabled individuals with autism. J Child Psychol Psychiatry. 1999;40:189–201. [PubMed] [Google Scholar]
- Volkmar FR, et al. Asperger's disorder. Am J Psychiatry. 2000;157:262–7. doi: 10.1176/appi.ajp.157.2.262. [DOI] [PubMed] [Google Scholar]
- Wang AT, et al. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–43. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, et al. Rate of head circumference growth as a function of autism diagnosis and history of autistic regression. J Child Neurol. 2007;22:1182–90. doi: 10.1177/0883073807306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M, et al. Delayed maturation of the frontal cortex in childhood autism. Am J Psychiatry. 1995;152:248–52. doi: 10.1176/ajp.152.2.248. [DOI] [PubMed] [Google Scholar]