Abstract
The normal mammary gland and invasive breast tumors are both complex ‘organs’ composed of multiple cell types as well as extracellular matrix (ECM) in three-dimensional (3D) space. Conventionally, both normal and malignant breast cells are studied in vitro as two-dimensional (2D) monolayers of epithelial cells, which results in the loss of structure and tissue function. Many laboratories are now investigating regulation of signaling function in the normal mammary gland using 3D cultures. However, it is important also to assay malignant breast cells ex vivo in a physiologically relevant environment to more closely mimic tumor architecture, signal transduction regulation and tumor behavior in vivo. Here we present the potential of these 3D models for drug testing, target validation and guidance of patient selection for clinical trials. We argue also that in order to get full insight into the biology of the normal and malignant breast, and to create in vivo-like models for therapeutic approaches in humans, we need to continue to create more complex heterotypic models to approach the full context the cells encounter in the human body.
Keywords: Three-dimensional cell cultures, tissue architecture, normal mammary gland, breast cancer, signal transduction, drug testing
1. Introduction
It is now becoming increasingly clear that cancer is not a single disease and that it is organ- and tissue-specific. Furthermore, even within a single organ such as the mammary gland, tumors are heterogeneous with respect to histology, gene expression and clinical outcome. In order to advance our understanding of the complex biology of breast cancer and eventually to improve clinical management of the disease, we need experimental model systems that recapitulate the in vivo functions, interactions and architecture of the mammary gland and breast tumors.
The mammary gland, like many glandular organs, is embedded in stroma, which is composed of mesenchymal cells, such as fibroblasts, adipocytes, immune cells, and extracellular matrix (ECM). Our laboratory postulated a long time ago that the ECM not only provides structural support but also signaling cues via transmembrane receptors, directing cytoskeletal and chromatin organization to maintain tissue integrity [1,2]. More than two decades ago, it was shown that collagen gels, which provide a 3D scaffold, allow epithelial cells of various tissues and origins to maintain some of their tissue structure and differentiated functions [3–6]. Building on these first observations, we developed 3D culture systems using either collagen gels [7,8] or, more importantly for epithelial cells, laminin-rich extracellular matrices (lrECM) to study tissue-specific functions of normal mouse mammary cells [9,10] as well as normal and malignant human cells [11]. In parallel with our experiments, others developed cultures for rat hepatocytes [12]. Since then, the in vivo-like properties provided by the 3D model systems have received broader appreciation and have been adopted for the study of diverse tissues and cells including skin [13], prostate [14], muscle [15], colon [16], bile duct [17], esophagus [18], adipocytes [19], fibroblasts [20,21] and embryonic stem cells [22,23].
In this review, we first depict how 3D cell culture models can be utilized to dissect signaling pathways that regulate function of normal and malignant mammary epithelial structures. We further describe the potential of these models for applied breast cancer research, namely the identification of prognostic and predictive profiles and therapeutic screening. Second, we discuss the future challenges we will have to face in order to bring the understanding of breast cancer and its treatment to full fruition.
2. The normal mammary gland and breast cancer
The normal human mammary gland consists of a branching ductal-lobular system. The lobules of the human breast are organized into 15–20 lobes which are drained by collecting ducts that converge at the nipple in a radial arrangement [24]. Each lobule in turn is made up of acini (also called alveoli) that form the functional secretory units of the mammary gland, the terminal duct lobular units (TDLUs). The acini and ducts have a central lumen and are lined by two cell layers, an inner layer of polarized luminal epithelial cells and an outer layer of myoepithelial cells. Surrounding this structure is a basement membrane (BM) separating the epithelium from the stroma (Fig. 1A). The latter is composed of varying amounts of fat, connective tissue, blood vessels, nerves, and lymphatics [24].
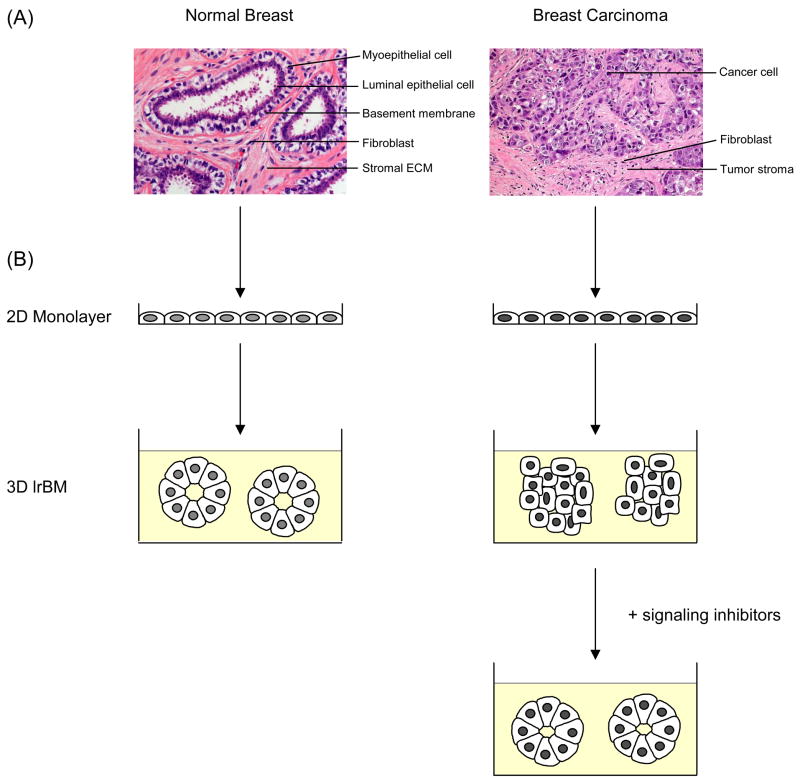
Fig. 1.
The normal and malignant breast. (A) Hematoxylin and eosin staining of tissue sections from a normal adult breast and an invasive ductal carcinoma. The normal mammary gland is a highly organized structure. The acini have a central lumen and are lined by an inner layer of luminal epithelial and an outer layer of myoepithelial cells. The bi-layer of epithelial cells is separated by a BM from the surrounding stroma, which is comprised of stromal ECM and stromal cells such as fibroblasts and adipocytes. Breast carcinomas have lost organized tissue architecture. Cancer-associated fibroblasts are the major cell type of the tumor stroma. (B) Cells from the normal and malignant mammary gland form indistinctive monolayers when plated on plastic substratum. (left) In 3D lrECM, normal mammary epithelial cells form spherical structures with a hollow lumen. (right) Breast cancer cells form disorganized tumor-like structures in 3D lrECM, which can be reverted to near-normal morphology by normalizing aberrant signaling pathways.
Almost all breast malignancies arise in the TDLU, regardless of the histological type of the tumor [25,26]. In general, carcinomas are characterized by the loss of epithelial polarity and tissue organization. Cancer cells that remain within the BM of the mammary ductal-lobular system are classified as benign in situ carcinomas. Once neoplastic cells rupture the BM and invade into the adjacent stroma, the tumor has become malignant (Fig. 1A). The most prominent changes in the cellular composition accompanying the progression from the normal mammary gland to invasive carcinoma are the loss of myoepithelial cells, the increase in myofibroblasts and immune cells in the stroma and enhanced vascularization [27–32]. Focal disruptions in the myoepithelial cell layer can already be observed in some cases of ductal carcinoma in situ (DCIS)[33], a precursor lesion of invasive cancer [34,35]. Myoepithelial cells isolated from DCIS also have been reported to show gene expression and epigenetic changes compared to myoepithelial cells isolated from normal breast tissue [36,37].
Invasive breast cancers exhibit a wide range of morphological types, molecular profiles and clinical behaviors [26]. The clinical heterogeneity of the disease is reflected by the fact that metastases can be detected at all stages of disease and over a long period of 10 years or more after initial diagnosis [38,39]. Histologically, invasive breast cancers are categorized into at least 18 different subtypes based on growth patterns and cytonuclear characteristics of the tumor cells [26]. A number of these histological subtypes show particular prognostic and clinical characteristics [26,40]. Aside from morphology, breast cancers are divided into two major groups based on whether or not the tumor cells express the nuclear estrogen receptor-α (ER). The ER status is used in clinical practice to predict the response to adjuvant anti-estrogen therapies, such as tamoxifen [41].
The analysis of gene expression using DNA microarrays led to a novel breast cancer classification. Within the group of ER-negative breast carcinomas, three distinct molecular subtypes (basal-like, HER2+ and normal breast-like) have been identified on the basis of their RNA molecular profiling. The ER-positive tumors could be subdivided into two subtypes (luminal A and luminal B)[42,43]. Recent work suggests that the intensively studied ‘basal-like’ tumors are not a homogeneous group but comprise a large phenotypic and prognostic spectrum [44–47]. The gene expression analyses performed in recent years highlight the complexity of the disease at molecular level, which might not be entirely surprising considering the histological and structural complexity of breast carcinomas. Besides cancer cells, invasive breast tumors comprise stromal cells, such as fibroblasts and immune cells, and ECM. The amount and composition of stromal cells and ECM varies greatly among tumors [26,40], a finding that has clinical importance. For example, invasive breast carcinomas can be classified based on the expression of ECM components, and these ECM classes were associated with survival and recurrence risk [48]. The specific characteristics of the breast cancer stroma may harbor a great body of prognostic and predictive information.
3. Modeling breast tissue-specific function in three dimensions
Non-malignant as well as malignant mammary cells have typically been studied as monolayer cultures on tissue culture plastic in the absence of proper ECM thereby losing their tissue-specific function and morphological organization [49]. It is, therefore, not surprising that many aspects of mammary biology and tumorigenesis are still not fully understood. Three-dimensional culture systems allow cells to organize into structures that mimic their in vivo architecture and are particularly useful for the investigation of gene functions and signaling pathways in a physiologically relevant context.
3.1. 3D culture models of human mammary cells
Besides providing structural support, the BM has been shown to be critical in regulating proliferation, organization and differentiation of the breast tissue [1,50]. The BM is a specialized form of ECM, and is composed mainly of laminin and type IV collagen (for review see [51]). By embedding cells in a 3D environment of lrECM, many aspects of the morphology and functions seen in vivo can be restored in culture [9–11,52]. Normal mammary epithelial cells and non-malignant cell lines form polarized spheroids (‘acini’) with a central lumen resembling the normal mammary gland acinus when grown in 3D lrECM [10,11] (Fig. 1B). Remarkably, mouse mammary epithelial cells cultured in 3D gels even respond to lactogenic hormones by producing and secreting milk proteins [8,10,52,53].
These data make it increasingly clear that 3D cultures can promote expression of tissue-specific functions by allowing cells to receive cues from their neighboring cells and the BM, which cannot be achieved when cells are plated on tissue culture plastic or other 2D substrata.
In addition to facilitating the study of non-malignant mammary cells these 3D models also allow the analysis of breast cancer cells in the more appropriate context. Morphologically, normal and malignant breast cells are indistinguishable when grown as monolayers (Fig. 1B). However, when cultured in a 3D lrECM, tumorigenic mammary cells can easily be discriminated from normal or non-malignant cells: instead of forming polarized acinar structures as they arrest growth [10], cancer cells continue to proliferate and form disorganized tumor-like structures [11,54–57] (Fig. 1B). The morphological differences observed between normal S1 and malignant T4-2 breast cells have been demonstrated to be due to aberrant integrin and growth-factor-receptor pathway activation in the cancer cells. The inhibition of either the β1-integrin, EGFR signaling, or related down-stream signaling pathways (MAPK and PI3K) causes disorganized tumor cells with multiple mutations to functionally and morphologically revert to growth-arrested acini [58–60](Fig. 1B). The attenuation of the aberrant signaling pathways in cancer cells grown in 3D lrECM also led to normalization of key signaling protein expression levels to those present in normal cells, a feedback inhibition that did not occur in 2D cultures [59,61]. This ‘reversion’ assay illustrates that signaling pathways are dramatically different in their regulation and integration when cells are propagated in a 3D environment [50,62].
Additional proteins have been identified to be central regulators of breast acinar architecture. Using these models, inhibition of the small GTPase Rap1 in T4-2 breast cancer cells was shown to restore the tissue polarity of the normal acinar architecture and reduced tumorigenicity of these cells in vivo [63]. In line with this, the re-expression of genes frequently lost in breast cancer, such as α-dystroglycan (α-DG), the carcinembryonic antigen-related cell adhesion molecule (CEACAM1), or the gap junction proteins hCx43 and hCx26, have been shown to allow breast cancer cells to form normal acinar-like structures in 3D cultures [64–66]. In contrast, the introduction of oncogenes or activated growth factor receptors into non-transformed mammary MCF10A cells, which form polarized acini when grown on 3D lrECM, altered acinar morphogenesis [67–69].
The integrity and polarity of epithelial cells is important for the expression of membrane receptors, such as coxsackie adenovirus receptor (CAR) [70]. When comparing CAR expression in normal mammary epithelial and spontaneous tumorigenic cells derived from a human breast cancer progression series [54,56,57], no difference in CAR protein levels were found when the cells were grown in 2D. In contrast, CAR protein expression was significantly reduced in non-malignant cells compared to malignant cells when grown in 3D [70]. Such observations indicate that the study of architecturally organized cells might help to identify previously unknown functions of genes or pathways. The activity of polo-like kinase 1, for example, which promotes the progression of cells through mitosis [71], was recently shown in our laboratory to be required for invasiveness of vimentin expressing cells [72].
Altogether, these data demonstrate convincingly that 3D culture models provide a physiologically relevant approach to dissect molecular mechanisms and signaling pathways in mammary epithelial cells ex vivo.
3.2. 3D models for breast cancer research and treatment
Cell lines derived from human primary tumors or metastases have been extensively used for the ex vivo and in vivo study of breast cancer. Because of the molecular heterogeneity of the disease, no individual cell line holds all the genomic and transcriptional abnormalities detected in human breast tumors, but it has been repeatedly shown that a panel of cell lines may indeed represent the spectrum of lesions observed in breast carcinoma [73–75]. Cell line studies resulted in the identification of biomarkers for prognosis and an overwhelming number of potential drug targets. However, only very few of these novel molecular markers and targets have been translated into clinical practice. To improve the success rate of the implementation of biomarkers and the development of candidate drugs, and to identify those breast cancer patients who may benefit most from a certain type of treatment, human tissue surrogates are needed. Three-dimensional culture systems, such as those used for the study of normal and malignant mammary epithelial cells, might be well-suited for predictive drug response screens and might guide patient selection for clinical trials.
In an attempt to overcome drug resistance and to augment the efficacy of breast cancer treatment, the combination of drugs, in particular of targeted therapeutics and cytotoxic agents, is under intense investigation ([76] and therein). The number of rational drug combinations is, however, too large for testing in clinical trials. Here also, 3D culture models provide an appropriate system for studying combination therapies in an in vivo-like environment to identify the most efficacious treatments.
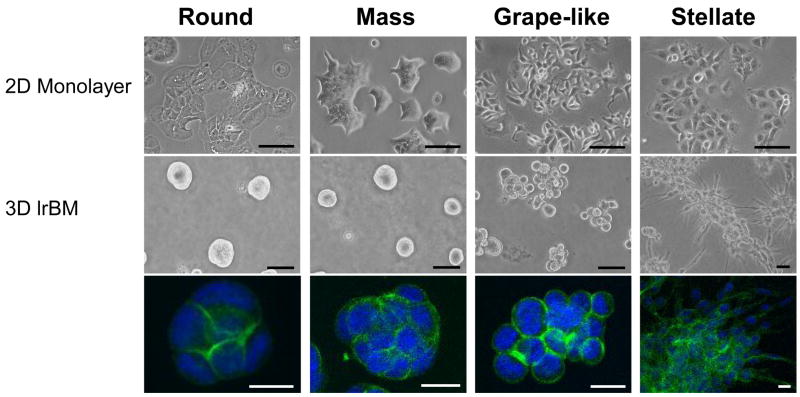
Our proposition is supported by data that both non-malignant and malignant mammary cells show significant differences in signal transduction genes when cultured in 3D environments [70,77]. Whereas a panel of 25 breast cancer cell lines was observed to form indistinctive monolayers when grown on 2D cultures, they showed characteristic morphologies when cultured on 3D lrECM (Fig. 2). These 3D morphologies have been categorized into four classes, referred to as Round, Mass, Stellate and Grape-like [77] (Fig. 2). The 3D morphological classes in turn correlated well with the underlying gene expression patterns and functions. The cell lines that fell into the ‘Stellate’ 3D morphology, for example, all lack E-cadherin, whereas the ones with ‘Grape-like’ morphology have elevated levels of HER2 [77], suggesting that the 3D morphology can also serve as a read-out for the behavior and function of carcinoma cells.
Fig. 2.
Morphologies of breast cancer cell lines cultured in 2D and 3D. (top) Images of 4 representative breast cancer cell lines cultured as 2D monolayer, (middle) and in 3D lrECM grouped by 3D morphological classification: Round, Mass, Grape-like and Stellate. (bottom) 3D cultures were stained for F-actin and nuclei were counterstained with DAPI. Scale bars: top panel, 100 μm; middle panel, 50 μm; bottom panel, 20 μm. Adapted from [77].
In addition, the 3D tissue architecture itself has been found to be fundamental to the behavior of cancer cells. When grown on tissue culture plastic, both non-malignant and malignant breast cells exhibited comparable sensitivity to apoptosis induced by different agents used for therapy of breast and other types of cancer [78]. When embedded in 3D lrECM, however, only the malignant cells were sensitive to apoptosis induction, whereas the acini formed by non-malignant cells were resistant. When the malignant cells were reverted from disorganized to polarized structures, they also showed apoptosis resistance in response to the chemotherapeutic agents tested. Interestingly, in a 3D microenvironment it is the correct polarization of the acini irrespective of the genetic make-up or growth rate of the non-malignant and the reverted malignant cells that accounts for resistance to apoptosis. This observation was traced to hemidesmosome formation, β4-integrin ligation and NFκB activation in the polarized structures [78]. Similarly, the breast cancer cell line MCF7 has been shown to be less responsive to the anti-estrogen tamoxifen when cultured on a 3D chitosan polymer scaffold compared to MCF7 cells grown on tissue culture plastic [79]. At a tamoxifen concentration of 10−6 M, there was a 50% growth inhibition of MCF7 cells in 2D, but only 25% in 3D cultures. The differential response was caused by a higher level of lactate production of MCF7 cells when grown in 3D compared to culture plastic similar to that observed for tumor cells in vivo [79]. This most probably reflects lower level of glucose uptake by transformed cells in 3D versus 2D [80]. These findings emphasize the benefits of studying cancer cell signaling or drug response in 3D tumor-like structures rather than in monolayer.
As discussed earlier in this review, tissue organization and proliferation are among the striking observable differences between non-malignant and their malignant counterparts. We have analyzed the transition of non-malignant breast epithelial cells from a disorganized, proliferating state to an organized growth-arrested state in 3D lrECM [81]. Microarray analysis of unpolarized dividing versus polarized acinus-like structures of two non-malignant mammary epithelial cell lines identified, as expected, a set of differentially expressed cell cycle and cell division genes. Importantly, those genes that were significantly down-regulated in the organized growth arrested acini compared to the proliferating cells, had prognostic value and could be applied to accurately discriminate between breast cancer patients with poor and good outcome [81].
Gene expression profiling of human breast tumors has been a powerful tool in the identification of molecular signatures predicting outcome in breast cancer patients more precisely than standard clinical and histological markers [42,82–84]. The discovery of expression profiles predicting drug response, however, remains a challenge and strongly depends on the study design, the drugs used, and the number of patients included. As a consequence, only a few studies have been able to identify clinically meaningful predictors to assign which individual patient is likely to benefit from a certain chemotherapeutic regimen (for reviews see [85,86]). In addition to primary tumor materials, cell lines cultured as 2D monolayers have been used to establish predictive response patterns [87,88]. The study of breast cancer cell lines in 3D culture models might generate even more powerful therapy response predictors, since the 3D architecture of tumor cells has been shown to have great impact on drug sensitivity [78].
While 3D lrECM cell culture models harbor great potential for the discovery of novel biomarkers, they also can be employed for functional studies of candidate genes or proteins and for the validation of potential breast cancer targets. The latter has been reported for two prospective drug targets, β1-integrin and tumor necrosis factor α-converting enzyme (TACE/ADAM17). The cell surface receptor β1-integrin mediates cell-extracellular matrix interactions [89]. This receptor has been recognized to play a role in invasion and metastasis [90,91], and increased β1-integrin expression has been correlated with poor survival in breast cancer patients [92]. In addition, and as illustrated earlier in this review, down-modulation of β1-integrin signaling in breast cancer cells results in reversion of the malignant phenotype by inducing growth arrest and restoration of tissue polarity when propagated in a 3D context [58,93]. Addition of β1-integrin inhibitory antibody, AIIB2, to already formed tumor colonies of several different breast cancer cell lines cultured on top of 3D lrECM gels resulted in a significant decrease in cell numbers and proliferation, and an increase in apoptosis [94]. In contrast, the acinar structures formed by non-malignant mammary epithelial cells did not respond to the antibody, again indicating the utility of 3D culture assays to discriminate non-malignant and malignant cell response to therapeutic agents [78]. Both observations could be confirmed in vivo: AIIB2 inhibited breast tumor growth in nude mice as a result of anti-proliferative and pro-apoptotic effects, whereas importantly no recognizable toxicity to the host was observed [94].
Similarly, TACE, a metalloproteinase, has emerged as a new therapeutic target in cancer [95]. TACE has been shown to be responsible for the shedding of several EGFR ligands, such as TGF-α and amphiregulin, which in turn bind and activate the receptor tyrosine kinase EGFR [96]. In breast cancer, EGFR is frequently overexpressed, leading to an increased downstream signaling, which has been implicated in cancer development and progression [97–99]. One strategy to inhibit receptor function is the use of small molecule tyrosine kinase inhibitors, binding to the ATP binding site of the tyrosine kinase domain of the receptor. Alternatively, EGFR signaling in breast cancer can be inhibited by blocking the activity of TACE, which regulates the bioavailability of EGFR ligands. The breast cancer cell line T4-2 has been found to be dependent on the activity of the EGFR pathway and to overexpress the EGFR ligands amphiregulin and TGF-α [100]. Comparable to the effects seen when targeting EGFR signaling, treatment of T4-2 cells grown in 3D lrECM with a small molecule inhibitor of TACE, TAPI-2, resulted in the reversion of the malignant phenotype of T4-2 cells into phenotypically normal mammary acinus-like colonies. The TACE-dependent shedding of amphiregulin and TGF-α was observed also in a number of additional breast cancer cells lines, and could be inhibited by TAPI-2 treatment, hence leading to downregulation of MAPK activity [100]. These findings suggest that besides targeting EGFR directly, the attenuation of the autocrine or paracrine activation of EGFR by inhibiting TACE might be an effective therapy for tumors of the breast and other tissues such as lung, which are driven by EGFR signaling. Collectively, these studies emphasize the value of 3D models over conventional cell culture approaches in the study of breast cancer as they harbor great potential for the implementation of therapeutic screening and drug target validation.
4. What will the future bring?
4.1. Development of heterotypic 3D cell culture models
Three-dimensional models such as those described in this review represent a proof of principle: by allowing tissue organization and BM signaling they mimic the phenotypic and morphological behavior of cells in vivo and better delineate normal from malignant cells than conventional tissue culture models. To even more faithfully recapitulate the histological complexity of the normal breast and breast cancers, and to help fill the remaining gap between cell culture and whole-animal systems, more intricate 3D models are required. This is because in addition to receiving cues from the microenvironment, the behavior of normal and malignant mammary cells is regulated by neighboring cells, stromal cells, soluble factors and physical forces [31,101].
The lrECM matrix currently used for 3D cell culture is extracted from a BM producing mouse tumor [102]. As with all natural products, this results in variations in the composition of ECM proteins and growth factors as well as the mechanical properties of the gels. This variation can challenge the reproducibility of experimental findings and the unraveling of the roles of specific ECM factors [103]. Synthetic scaffolds, however, provide well-defined and reproducible extracellular environments and have great potential for the study of complex physiological processes like breast tumorigenesis in culture [103–106]. These synthetic ECMs constitute the ideal foundation for building functional breast tissues ex vivo including multiple cell types.
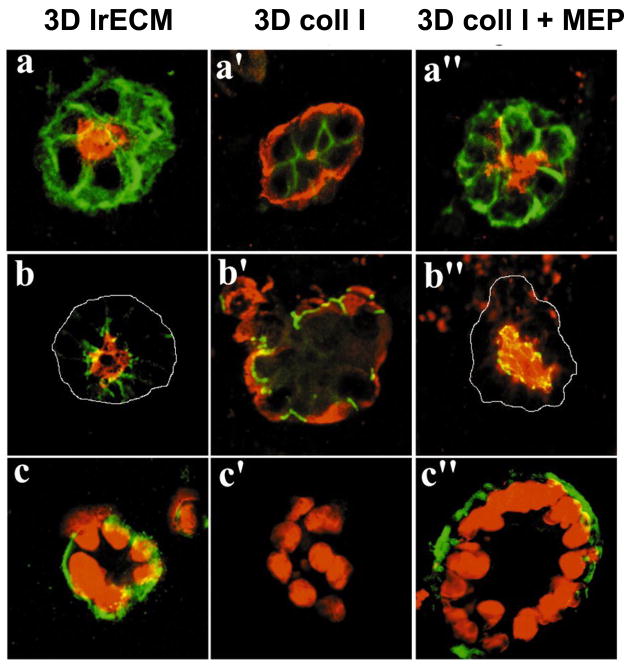
Even though it is known that the ductal-lobular system of the normal breast comprises a double-layered structure of inner luminal epithelial cells and outer myoepithelial cells, research has been focused mainly on luminal epithelial cells. This is probably due to the fact that most breast carcinomas are thought to be of luminal origin. The focus on myoepithelial cells has increased more recently because of their proposed natural tumor suppressive function [107,108]. A first step towards building a heterotypic breast cell model is the introduction of myoepithelial cells in the cell culture systems. It has been shown that the bilayered acinar structures can be recapitulated in 3D models by co-culturing luminal epithelial and myoepithelial cells [109,110]. Interestingly, unlike normal myoepithelial cells, tumor-derived myoepithelial cells appear to have lost their ability to confer correct polarity to epithelial cells [109], the crucial organizing principle in the mammary gland [50]. In vivo, normal myoepithelial cells produce laminin-1, a feature these cells might lose during malignant transformation of adjacent luminal cells. Thus, the inability of the ‘tumor myoepithelial cells’ to correct inside-out acini of normal epithelial cells in collagen gels has been shown to be a result of their low or nonfunctional laminin-1 expression [109] (Fig. 3).
Fig. 3.
Myoepithelial cells confer correct polarity to luminal epithelial cells. (a, b, c) In lrECM, luminal cells form acini with correct polarity, (a′, b′, c′) whereas in collagen I (coll I), they form inside-out acini with reversed polarity. (a″, b″, c″) By addition of human myoepithelial cells (MEP) the reversed polarity of luminal cells in collagen I is corrected. (a, a′, a″) Apical marker sialomucin (red) and basal marker epithelial specific antigen (green). (b, b′, b″) Sialomucin (red) and occludin (green), which is expressed at apical cell-cell contacts. (c, c′, c″) nuclear stain (red) and basal marker β4-integrin (green). Adapted from [109] with permission of the Company of Biologists.
Epithelial cells interact also via paracrine signaling with the adjacent stromal cells [111–114]. Fibroblasts are the major cell type of the stromal compartment and since the myoepithelial cells are largely lost in breast cancer, it has become apparent that breast cancer progression is largely influenced by the cross-talk between the epithelial tumor cells and stromal fibroblasts (for reviews see [115–117]). The importance of the paracrine functions of fibroblasts was illustrated by a gene expression signature of the response of fibroblasts to serum, which by itself was effective in accurately predicting metastasis and death in several different carcinoma types, but especially in breast cancer [118,119]. Even though cancer-associated fibroblasts (CAFs) have been identified as key determinants in the malignant progression of breast cancer, only few studies report the co-culture of mammary epithelial cells and organ specific fibroblasts in 3D environments. Co-cultivation of normal reduction mammoplasty fibroblasts with the non-malignant mammary epithelial cell line MCF10A on a reconstituted BM has been shown to result in suppressed epithelial cell proliferation [120,121]. Also in co-culture with the transformed MCF10AT cell line, the normal breast-associated fibroblasts had an inhibitory effect on MFC10AT cell growth [120]. CAFs, however, evoked ductal-alveolar morphogenesis of MCF10A cells [121]. Similarly, the cultivation of primary breast tumor cells with a mix of fibroblasts, vascular smooth muscle cells and pericytes in 3D collagen gels caused the tumor cells to spread and become invasive [122]. This effect on the breast carcinoma cells could be enhanced with greater concentrations of the stromal cells. Interestingly, the co-culture of fibroblasts with primary breast tumor cells or breast cancer cell lines was sufficient to form a structure of typical breast tumor morphology inside 3D collagen gels [122] and in spheroid cultures [123,124].
An additional stromal cell type that is abundant in the breast is the adipocyte. Co-cultivating primary non-malignant mammary epithelial cells or breast cancer cells with adipocytes in 3D collagen matrices resulted in differentiated epithelial structures embedded in nests of adipocytes [125,126]. The structural formation observed closely resembled human breast tissue in vivo. For a long time it was unclear whether adipocytes actually affect the behavior of breast carcinoma cells or whether they are innocent bystanders. It now has been shown that adipocytes interact with breast cancer cells in a paracrine manner and promote the growth of several ER-positive breast cancer cells lines but not that of ER-negative cells in the 3D co-culture model [126].
Until now, the heterotypic cell models described in the literature usually only include one additional cell type next to normal and malignant mammary epithelial cells. The final step towards achieving an engineered mammary gland or breast tumor, however, will be the incorporation of all the different stromal cell types, fibroblasts, immune cells, endothelial cells and adipocytes, together with epithelial cells in one culture model [105]. A major challenge in creating such a heterotypic cell system will be the different metabolic and nutritional requirements of the different cell types. Culture conditions will have to be defined in a way that allows each cell type to grow and maintain in a differentiated state in combination with the other cell types, which is a difficult task. Modeling a normal mammary gland involves an additional challenge since only the luminal epithelial and myoepithelial cells are in direct contact but physically separated from the stromal cells by the BM. Breast carcinomas on the other hand have lost their tissue organization and all cell types are intermingled. However, here also one has to act with caution to prevent the rapidly proliferating breast cancer cells from overgrowing the other slow cycling cells, such as endothelial cells. Once these obstacles have been overcome, designing a multicellular normal or cancerous breast ex vivo will allow many possibilities to study the heterotypic interactions between mammary epithelial or breast cancer cells and normal versus tumor-associated stromal cells.
4.2. The tumor microenvironment as drug target
Even if there is still much left to learn about the heterotypic epithelial-stromal interactions in normal and malignant tissues, we now recognize that cancer initiation and progression are the result of an evolving cross-talk between epithelial cells and the surrounding microenvironment (for reviews see [31,117,127–129]). Besides its contribution to tumorigenesis, tumor stroma might also be involved in drug resistance by affecting the bioavailability and efficacy of chemotherapeutic agents [130,131]. The appreciation of the importance of the microenvironment in cancer has begun to result in the development of drugs targeting either the tumor stroma cells or the tumor-host signaling processes. However, the perturbation of the tumor microenvironment is quite challenging. For example, a drug for systemic use needs to selectively target only the stromal component of the tumor and avoid endothelial, immune cells or fibroblasts in normal tissues elsewhere in the body. In addition, the heterotypic signaling between tumor and stromal cells is complex and balanced between pro- and anti-tumorigenic functions, as learned from the matrix metalloproteinases (MMPs) inhibitors. MMPs are promising drug targets for various cancer types based on their observed up-regulation and ability to remodel the ECM during invasion and metastasis (for reviews see [132–134]). The results from clinical trials, however, have been disappointing: broad MMP inhibitor treatment did not result in survival benefit and patients suffered from unexpected side effects [132]. This is because MMPs have many other signaling functions outside remodeling the tumor ECM including host-protective ones [134,135].
So far, the most successful therapeutic agents, which target a microenvironmental component in breast cancer, are aromatase inhibitors. The third generation inhibitors (anastrozole, letrozole and exemestane) effectively reduce the hazard of recurrence and have largely replaced tamoxifen in the adjuvant hormonal treatment for ER-positive breast cancer in postmenopausal women [136]. In contrast to tamoxifen, aromatase inhibitors target aromatase, a key enzyme in the biosynthesis of estradiol from androgens. The major producer of estrogen in breast carcinomas are the tumor-associated stromal cells, but the cancer cells themselves have been reported to have aromatase activity [137]. Another attractive target in the microenvironment is the tumor vasculature [138]. In order to grow beyond a certain size, both locally and at distant sites, a tumor has to recruit its own blood vessels for nutrient and oxygen supply and waste disposal. Angiogenesis, the process of neovascularization, has been repeatedly correlated with disease progression. In invasive breast carcinoma, microvessel density has been shown to be predictive of metastasis development and survival [139,140], as are the levels of the angiogenic growth factor VEGF [141]. A large number of antiangiogenic agents, which either target the growth factors or their receptors, have been developed and are currently in clinical trials. The humanized monoclonal antibody against the ligand VEGF-A, bevacizumab, is the most mature from a clinical standpoint. It is approved for the treatment of metastatic colorectal cancer in combination with fluorouracil and irinotecan [142]. In breast cancer, bevacizumab monotherapy has only minimal activity, however, in combination with capecitabine it has been shown to significantly increase the response rates in the metastatic setting [143]. The therapeutic benefit of combined antiangiogenic and cytotoxic drugs has been suggested to be due to the normalization of the tumor vasculature and the subsequent improved delivery of therapeutics [144]. Of note, several established therapies including tamoxifen and trastuzumab, a humanized monoclonal antibody against HER2, have antiangiogenic activity in addition to their effects in their specific targets [145,146].
There are numerous additional chemotherapeutic agents targeting either the breast cancer microenvironment or mediators of the tumor-stromal cell signaling in different preclinical or clinical stages, such as the nonsteroidal anti-inflammatory COX-2 inhibitors [147], or a blocking antibody against CSF-1, a chemoattractant for macrophages synthesized by cancer cells [148]. However, reviewing all targets and drugs under investigation would exceed the scope of this article [149].
So far, the results achieved in trials targeting the tumor microenvironment are encouraging but also pose new questions and challenges. Tumor and stroma cells form a complex functional entity, and each tumor-associated cell type has sophisticated roles in modulating epithelial cell behavior. To more successfully translate fundamental research findings into therapies in the clinic, a better understanding of the pro- and anti-tumorigenic effects of the host-stroma interactions is required and appropriate targets have to be identified. One important lesson is the need for an in-depth focus on the heterotypic cell signaling using culture systems with different cell types, such as the complex 3D models discussed above. In addition, clinical trials over the past years have taught us that the most effective treatments are those which combine targeted therapies with conventional cytotoxic agents. Again, the question remains how to combine such treatments optimally. Three-dimensional cultures of human breast tumors and other types of tumors have great potential in providing answers before proceeding into costly trials.
4.3. Breast tumor heterogeneity and the cancer stem cell as drug target
Breast cancers are histologically, molecularly and clinically heterogeneous. Not only is there a large variation regarding the nature of cell types between cancers, but even within a single tumor, a significant heterogeneity in phenotype and genotype can be observed [26,40,150]. Understanding the cause of this heterogeneity will be of importance for future therapeutic approaches.
Breast cancer is thought to initiate from a transforming event in a single cell, which subsequently acquires additional genetic changes over time [151,152]. The number of genes reported to be altered in breast cancer is large, yet the subset of these genes mutated in any given tumor and the overlap between different patients is rather small [26,75,153]. Morphogenesis studies using the non-malignant MCF10A cells in 3D culture systems demonstrated how specific cancer genes, such as Akt or ERBB2, might contribute to the phenotypic heterogeneity observed in breast cancer [67,68,154]. However, in most cases the presence of a given mutation can still not be uniquely associated with a specific histological or molecular breast cancer type.
A key to answering the question of breast cancer heterogeneity will be the identification of the cell or cells of origin, which are targets of transforming mutations, and additionally, the understanding of the origin of the hierarchy of the cells within the mammary gland [155]. It has been shown recently that two different populations of human mammary epithelial cells originating from the same patient, one of which was more myoepithelial-like than the other, after transformation gave rise to tumors of different histology and tumorigenicity when injected into mammary fat pads of immunocompromised mice [156]. It has been postulated that a subset of mammary stem or progenitor cells are targets of tumor-initiating genetic or epigenetic events. Depending on the kind of mutations the cells have sustained, these tumor-initiating cells would then have the ability to self-renew and differentiate accounting for the observed heterogeneity in breast cancer.
In rodents, a single mammary stem cell has been shown to give rise to a functional mammary gland [157,158]. Excitingly, a putative stem cell niche in the adult human breast also has been identified which gives rise to at least three lineage-restricted cell types outside the stem cell zone [155]. These findings may suggest that adult stem cells not only play a role in hematopoietic diseases but also in solid tumors such as breast cancer. A breast cancer subpopulation with the cell surface marker expression CD44+CD24−/low has been reported to efficiently produce tumors in immunodeficient mice containing all cell lineages of the original carcinoma whereas even large numbers of CD44−CD24+ cells could not [159]. Recently, increased ALDH1 activity has also been shown to identify a tumorigenic breast cancer cell population with the ability to self-renew and recapitulate the heterogeneity of the parental tumor when xenografted in mice [160]. Of note, only approximately 1% of the ALDH1-positive cells also had a CD44+CD24−/low phenotype [160]. These tumor-initiating cells are frequently referred to as ‘breast cancer stem cells’.
It is, however, still unknown whether transformed normal mammary stem or progenitor cells indeed form the origin of breast carcinomas or whether a particular subset of tumor cells acquires the capacity to de-differentiate and subsequently obtain stem cell-like properties such as self-renewal. Independent of this etiology issue, the implications for treatments would be profound if breast cancers would indeed be driven by a subpopulation of cells. Current therapies might not target the tumor-initiating cells effectively, as implied by the large number of patients who relapse after adjuvant chemotherapeutic and/or hormonal treatment [161]. The design of therapeutic agents specifically directed against tumor-initiating cells would theoretically result in a complete response, and if detected at early stage, would turn cancer into a curable disease limited to the primary organ. Could cancer stem cells represent druggable targets? At this time, we know far too little about the molecular features distinguishing a tumor-initiating cell from the bulk of tumor cells in order to be able to develop a ‘smart drug’. In addition, ATP binding cassette drug transporters, which are a major determinant in the resistance to chemotherapeutics, play a crucial role in the regulation of normal stem cells [162]. If cancer stem cells retain this feature, they will be challenging to treat.
The question of the cell of origin remains to be resolved. Is there only one uniform breast tumor-initiating cell or are we faced with heterogeneity at even the earliest stages? It has been suggested that the specialized microenvironment of the stem and progenitor cells, i.e. the niche, directs the behavior of this cell population [163,164]. The microenvironmental composition of the niche as well as the environmental and/or genetic changes leading to aberrant heterotypic signaling events could result in differences in the molecular make-up of the tumor-initiating cells in different patients from the start.
Acknowledgments
We would like to thank P.A. Kenny (Bissell laboratory) for critical reading of the manuscript, and J.L. Peterse (Netherlands Cancer Institute, Amsterdam, The Netherlands) for the photographs of Fig. 1. This work was supported by grants and a Distinguished Fellowship Award from the U.S. Department of Energy, Office of Biological and Environmental Research (DE-AC03 SF0098), and by the National Cancer Institute (RO1 CA064786) to M.J. Bissell with O.W. Petersen. B.Weigelt was supported by a postdoctoral fellowship of the Dutch Cancer Society (KWF).
Abbreviations
- BM
basement membrane
- ECM
extracellular matrix
- lr
laminin-rich
- TDLU
terminal ductal lobular unit
- 2D
two-dimensional
- 3D
three-dimensional
- ER
estrogen receptor-α
- HER2/ERBB2
human epidermal growth factor receptor 2
- EGFR
epidermal growth factor receptor
- MAPK
mitogen-activated protein kinase
- PI3K
phosphoinositide-3 kinase
- TACE
TNF-α converting enzyme
- MMP
matrix metalloproteinase
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of interest
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 2.Bissell MJ, Kenny PA, Radisky DC. Microenvironmental Regulators of Tissue Structure and Function Also Regulate Tumor Induction and Progression: The Role of Extracellular Matrix and Its Degrading Enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343–56. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsdale T, Bard J. Collagen subtrata for studies on cell behaviour. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay ED, Dodson JW. Secretion of collagen by corneal epithelium: I. Morphology of the Collagenous Products Produced by Isolated Epithelia Grown on Frozen-Killed Lens. J Cell Biol. 1973;57:190–213. doi: 10.1083/jcb.57.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes: Morphological and biochemical observations. Exp Cell Res. 1975;94:70–8. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- 6.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–28. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 7.Emerman JT, Bartley JC, Bissell MJ. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res. 1981;134:241–50. doi: 10.1016/0014-4827(81)90481-x. [DOI] [PubMed] [Google Scholar]
- 8.Lee EY-H, Lee WH, Kaetzei CS, Parry G, Bissell MJ. Interaction of Mouse Mammary Epithelial Cells with Collagen Substrata: Regulation of Casein Gene Expression and Secretion. Proc Natl Acad Sci USA. 1985;82:1419–23. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a Reconstituted Basement Membrane and Its Components on Casein Gene Expression and Secretion in Mouse Mammary Epithelial Cells. Proc Natl Acad Sci USA. 1987;84:136–40. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–35. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells. Proc Natl Acad Sci USA. 1992;89:9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79:801–12. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier F, Nesbit M, Hsu MY, Martin B, Van Belle P, Elder DE, et al. Human Melanoma Progression in Skin Reconstructs : Biological Significance of bFGF. Am J Pathol. 2000;156:193–200. doi: 10.1016/S0002-9440(10)64719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bello-DeOcampo D, Kleinman HK, Deocampo ND, Webber MM. Laminin-1 and alpha6beta1 integrin regulate acinar morphogenesis of normal and malignant human prostate epithelial cell. Prostate. 2001;46:142–53. doi: 10.1002/1097-0045(20010201)46:2<142::aid-pros1018>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Lao J, Chen BP, Li YS, Zhao Y, Chu J, et al. Genomic analysis of smooth muscle cells in three-dimensional collagen matrix. FASEB J. 2003;17:97–9. doi: 10.1096/fj.02-0256fje. [DOI] [PubMed] [Google Scholar]
- 16.Kalabis J, Patterson MJ, Enders GH, Marian B, Iozzo RV, Rogler G, et al. Stimulation of human colonic epithelial cells by leukemia inhibitory factor is dependent on collagen-embedded fibroblasts in organotypic culture. FASEB J. 2003;17:1115–7. doi: 10.1096/fj.02-0852fje. [DOI] [PubMed] [Google Scholar]
- 17.Mathis GA, Walls SA, Sirica AE. Biochemical Characteristics of Hyperplastic Rat Bile Ductular Epithelial Cells Cultured “on Top” and “Inside” Different Extracellular Matrix Substitutes. Cancer Res. 1988;48:6145–53. [PubMed] [Google Scholar]
- 18.Andl CD, Mizushima T, Nakagawa H, Oyama K, Herada H, Chruma K, et al. Epidermal Growth Factor Receptor Mediates Increased Cell Proliferation, Migration, and Aggregation in Esophageal Keratinocytes in Vitro and in Vivo. J Biol Chem. 2003;278:1824–30. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 19.Daya S, Loughlin AJ, MacQueen HA. Culture and differentiation of preadipocytes in two-dimensional and three-dimensional in vitro systems. Differentiation. 2007;75:360–70. doi: 10.1111/j.1432-0436.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 20.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking Cell-Matrix Adhesions to the Third Dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 21.Grinnell F, Ho CH, Tamariz E, Lee DJ, Skuta G. Dendritic Fibroblasts in Three-dimensional Collagen Matrices. Mol Biol Cell. 2003;14:384–95. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, et al. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157:1279–90. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–6. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osborne MP. Breast Anatomy and Development. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 1–14. [Google Scholar]
- 25.Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55:231–73. [PubMed] [Google Scholar]
- 26.Tavassoli FA, Devilee P. World Health Organization Classification of Tumours. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. [Google Scholar]
- 27.Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Munro Neville A, Rudland PS. Distribution of Myoepithelial Cells and Basement Membrane Proteins in the Normal Breast and in Benign and Malignant Breast Diseases. Cancer Res. 1982;42:4763–70. [PubMed] [Google Scholar]
- 28.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 29.Shekhar M, Pauley R, Heppner G. Host microenvironment in breast cancer development: Extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res. 2003;5:130–5. doi: 10.1186/bcr580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ. Myoepithelial Cells: Their Origin and Function in Breast Morphogenesis and Neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:261–72. doi: 10.1007/s10911-005-9586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 33.Man YG, Tai L, Barner R, Vang R, Saenger JS, Shekitka KM, et al. Cell clusters overlying focally disrupted mammary myoepithelial cell layers and adjacent cells within the same duct display different immunohistochemical and genetic features: implications for tumor progression and invasion. Breast Cancer Res. 2003;5:R231–41. doi: 10.1186/bcr653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst. 2004;96:906–20. doi: 10.1093/jnci/djh164. [DOI] [PubMed] [Google Scholar]
- 35.Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005;205:248–54. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- 36.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 38.Hellman S, Harris JR. Natural history of breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. Philadelphia: Lippincott, Williams & Wilkins; 2000. pp. 407–23. [Google Scholar]
- 39.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 40.Schnitt SJ, Guidi AJ. Pathology of invasive breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. Philadelphia: Lippincott, Williams & Wilkins; 2000. pp. 425–70. [Google Scholar]
- 41.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 Update of Recommendations for the Use of Tumor Markers in Breast Cancer. J Clin Oncol. 2007;25:5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 42.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 43.Hu Z, Fan C, Oh DS, Marron JS, He X, Qagish BF, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertucci F, Finetti P, Cervera N, Charafe-Jauffret E, Mamessier E, Adelaide J, et al. Gene Expression Profiling Shows Medullary Breast Cancer Is a Subgroup of Basal Breast Cancers. Cancer Res. 2006;66:4636–44. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 45.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincent-Salomon A, Gruel N, Lucchesi C, MacGrogan G, Dendale R, Sigal-Zafrani B, et al. Identification of typical medullary breast carcinoma as a genomic sub-group of basal-like carcinomas, a heterogeneous new molecular entity. Breast Cancer Res. 2007;9:R24. doi: 10.1186/bcr1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fadare O, Tavassoli FA. The phenotypic spectrum of basal-like breast cancers: a critical appraisal. Adv Anat Pathol. 2007;14:358–73. doi: 10.1097/PAP.0b013e31814b26fe. [DOI] [PubMed] [Google Scholar]
- 48.Bergamaschi A, Tagliabue E, Sorlie T, Naume B, Triulzi T, Orlandi R, et al. Extracellular matrix signature indentifies breast cancer subtypes with different clinical outcome. J Pathol. 2007;214:357–67. doi: 10.1002/path.2278. [DOI] [PubMed] [Google Scholar]
- 49.Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- 50.Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–46. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leblond CP, Inoue S. Structure, composition, and assembly of basement membrane. Am J Anat. 1989;185:367–90. doi: 10.1002/aja.1001850403. [DOI] [PubMed] [Google Scholar]
- 52.Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991;99:407–17. doi: 10.1242/jcs.99.2.407. [DOI] [PubMed] [Google Scholar]
- 53.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–55. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin Cancer Biol. 1995;6:174–84. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- 55.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Meth. 2007;4:359–65. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Briand P, Petersen OW, Van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev Biol. 1987;23:181–8. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- 57.Rizki A, Weaver VM, Lee SY, Rozenberg GI, Chin K, Myers CA, et al. A human breast cell model of pre-invasive to invasive transition. Cancer Res. 2008;68:1378–87. doi: 10.1158/0008-5472.CAN-07-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the Malignant Phenotype of Human Breast Cells in Three-Dimensional Culture and In Vivo by Integrin Blocking Antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta 1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–6. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, et al. Phenotypic Reversion or Death of Cancer Cells by Altering Signaling Pathways in Three-Dimensional Contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;15:753–62. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bissell MJ, Weaver VM, Lelievre SA, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757–63s. [PubMed] [Google Scholar]
- 63.Itoh M, Nelson CM, Myers CA, Bissell MJ. Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res. 2007;67:4759–66. doi: 10.1158/0008-5472.CAN-06-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirschi KK, Xu CE, Tsukamoto T, Sager R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 1996;7:861–70. [PubMed] [Google Scholar]
- 65.Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A Role for Dystroglycan in Epithelial Polarization: Loss of Function in Breast Tumor Cells. Cancer Res. 2002;62:7102–9. [PubMed] [Google Scholar]
- 66.Kirshner J, Chen CJ, Liu P, Huang J, Shively JE. CEACAM1-4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci USA. 2003;100:521–6. doi: 10.1073/pnas.232711199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–92. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The Role of Apoptosis in Creating and Maintaining Luminal Space within Normal and Oncogene-Expressing Mammary Acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 69.Wrobel CN, Debnath J, Lin E, Beausoleil S, Roussel MF, Brugge JS. Autocrine CSF-1R activation promotes Src-dependent disruption of mammary epithelial architecture. J Cell Biol. 2004;165:263–73. doi: 10.1083/jcb.200309102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anders M, Hansen R, Ding RX, Rauen KA, Bissell MJ, Korn WM. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc Natl Acad Sci USA. 2003;100:1943–48. doi: 10.1073/pnas.0337599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–76. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 72.Rizki A, Mott JD, Bissell MJ. Polo-like Kinase 1 Is Involved in Invasion through Extracellular Matrix. Cancer Res. 2007;67:11106–10. doi: 10.1158/0008-5472.CAN-07-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ross DT, Perou CM. A comparison of gene expression signatures from breast tumors and breast tissue derived cell lines. Dis Markers. 2001;17:99–109. doi: 10.1155/2001/850531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lacroix M, Leclercq G. Relevance of Breast Cancer Cell Lines as Models for Breast Tumours: An Update. Breast Cancer Res Treat. 2004;83:249–89. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 75.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waterhouse DN, Gelmon KA, Klasa R, Chi K, Huntsman D, Ramsay E, et al. Development and Assessment of Conventional and Targeted Drug Combinations for Use in the Treatment of Aggressive Breast Cancers. Current Cancer Drug Targets. 2006;6:455–89. doi: 10.2174/156800906778194586. [DOI] [PubMed] [Google Scholar]
- 77.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weaver VM, Lelièvre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhiman HK, Ray AR, Panda AK. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials. 2005;26:979–86. doi: 10.1016/j.biomaterials.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 80.Bissell MJ, Farson D, Tung AS. Cell shape and hexose transport in normal and virus-transformed cells in culture. J Supramol Struct. 1977;6:1–12. doi: 10.1002/jss.400060102. [DOI] [PubMed] [Google Scholar]
- 81.Fournier MV, Martin KJ, Kenny PA, Xhaja K, Bosch I, Yaswen P, et al. Gene Expression Signature in Organized and Growth-Arrested Mammary Acini Predicts Good Outcome in Breast Cancer. Cancer Res. 2006;66:7095–102. doi: 10.1158/0008-5472.CAN-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 83.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 85.Lønning PE, Knappskog S, Staalesen V, Chrisanthar R, Lillehaug JR. Breast cancer prognostication and prediction in the postgenomic era. Ann Oncol. 2007;18:1293–306. doi: 10.1093/annonc/mdm013. [DOI] [PubMed] [Google Scholar]
- 86.Sotiriou C, Piccart MJ. Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat Rev Cancer. 2007;7:545–53. doi: 10.1038/nrc2173. [DOI] [PubMed] [Google Scholar]
- 87.Potti A, Dressman HK, Bild A, Riederl RF, Chan G, Sayer R, et al. Genomic signatures to guide the use of chemotherapeutics. Nat Med. 2006;12:1294–300. doi: 10.1038/nm1491. [DOI] [PubMed] [Google Scholar]
- 88.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–7. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 89.Giancotti FG, Ruoslahti E. Integrin Signaling. Science. 1999;285:1028–33. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 90.Berry MG, Goode AW, Puddefoot JR, Vinson GP, Carpenter R. Integrin beta1-mediated invasion of human breast cancer cells: an ex vivo assay for invasiveness. Breast Cancer. 2003;10:214–9. doi: 10.1007/BF02966720. [DOI] [PubMed] [Google Scholar]
- 91.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 92.Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, et al. Increased beta1 Integrin Is Associated with Decreased Survival in Invasive Breast Cancer. Cancer Res. 2007;67:659–64. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- 93.Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol. 2004;164:603–12. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 Integrin Inhibitory Antibody Induces Apoptosis of Breast Cancer Cells, Inhibits Growth, and Distinguishes Malignant from Normal Phenotype in Three Dimensional Cultures and In vivo. Cancer Res. 2006;66:1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kenny PA. TACE: a new target in epidermal growth factor receptor dependent tumors. Differentiation. 2007;75:800–8. doi: 10.1111/j.1432-0436.2007.00198.x. [DOI] [PubMed] [Google Scholar]
- 96.Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, et al. Tumor Necrosis Factor-alpha Converting Enzyme (TACE) Regulates Epidermal Growth Factor Receptor Ligand Availability. J Biol Chem. 2002;277:12838–45. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 97.Chrysogelos SA, Yarden RI, Lauber AH, Murphy JM. Mechanisms of EGF receptor regulation in breast cancer cells. Breast Cancer Res Treat. 1994;31:227–36. doi: 10.1007/BF00666156. [DOI] [PubMed] [Google Scholar]
- 98.Chrysogelos SA, Dickson RB. EGF receptor expression, regulation, and function in breast cancer. Breast Cancer Res Treat. 1994;29:29–40. doi: 10.1007/BF00666179. [DOI] [PubMed] [Google Scholar]
- 99.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–59. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 100.Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007;117:337–45. doi: 10.1172/JCI29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 102Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparin sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–93. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 103.Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–45. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 104.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotech. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 105.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15:342–52. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 107.Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res. 1997;3:1949–58. [PubMed] [Google Scholar]
- 108.Sternlicht MA, Barsky SH. The myoepithelial defense: a host defense against cancer. Med Hypotheses. 1997;48:37–46. doi: 10.1016/s0306-9877(97)90022-0. [DOI] [PubMed] [Google Scholar]
- 109.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–30. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- 111.Cunha GR, Hom YK. Role of mesenchymal-epithelial interactions in mammary gland development. J Mammary Gland Biol Neoplasia. 1996;1:21–35. doi: 10.1007/BF02096300. [DOI] [PubMed] [Google Scholar]
- 112.Humphreys RC, Lydon J, O’Malley BW, Rosen JM. Mammary Gland Development Is Mediated by Both Stromal and Epithelial Progesterone Receptors. Mol Endocrinol. 1997;11:801–11. doi: 10.1210/mend.11.6.9891. [DOI] [PubMed] [Google Scholar]
- 113.Mueller SO, Clark JA, Myers PH, Korach KS. Mammary Gland Development in Adult Mice Requires Epithelial and Stromal Estrogen Receptor alpha. Endocrinology. 2002;143:2357–65. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]
- 114.Van Nguyen A, Pollard JW. Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol. 2002;247:11–25. doi: 10.1006/dbio.2002.0669. [DOI] [PubMed] [Google Scholar]
- 115.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;2001:1–169. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 116.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 118.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene Expression Signature of Fibroblast Serum Response Predicts Human Cancer Progression: Similarities between Tumors and Wounds PLoS. Biology. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chang HY, Nuyten DSA, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005;102:3738–43. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sadlonova A, Novak Z, Johnson MR, Bowe DB, Gault SR, Page GP, et al. Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro culture. Breast Cancer Res. 2005;7:R46–59. doi: 10.1186/bcr949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–6. [PubMed] [Google Scholar]
- 122.Rønnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–73. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kunz-Schughart LA, Heyder P, Schroeder J, Knuechel R. A heterologous 3-D coculture model of breast tumor cells and fibroblasts to study tumor-associated fibroblast differentiation. Exp Cell Res. 2001;266:74–86. doi: 10.1006/excr.2001.5210. [DOI] [PubMed] [Google Scholar]
- 124.Seidl P, Huettinger R, Knuechel R, Kunz-Schughart LA. Three-dimensional fibroblast-tumor cell interaction causes downregulation of RACK1 mRNA expression in breast cancer cells in vitro. Int J Cancer. 2002;102:129–36. doi: 10.1002/ijc.10675. [DOI] [PubMed] [Google Scholar]
- 125.Huss FR, Kratz G. Mammary epithelial cell and adipocyte co-culture in a 3-D matrix: the first step towards tissue-engineered human breast tissue. Cell Tissues Organs. 2001;169:361–7. doi: 10.1159/000047903. [DOI] [PubMed] [Google Scholar]
- 126.Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol. 2003;201:221–8. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- 127.Pupa SM, Menard S, Forti S, Tagliabue E. New insights into the role of extracellular matrix during tumor onset and progression. J Cell Physiol. 2002;192:259–67. doi: 10.1002/jcp.10142. [DOI] [PubMed] [Google Scholar]
- 128.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 129.Mueller MM, Fusenig NE. Friends or foes bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 130.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Advanced Drug Delivery Reviews. 2001;46:149–68. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 131.Kong HJ, Mooney DJ. Microenvironmental regulation of biomacromolecular therapies. Nat Rev Drug Discov. 2007;6:455–63. doi: 10.1038/nrd2309. [DOI] [PubMed] [Google Scholar]
- 132.Coussens LM, Fingleton B, Matrisian LM. Matrix Metalloproteinase Inhibitors and Cancer--Trials and Tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 133.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 134.Overall CM, Kleifeld O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 135.Martin M, Matrisian L. The other side of MMPs: Protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–24. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 136.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology Technology Assessment on the Use of Aromatase Inhibitors As Adjuvant Therapy for Postmenopausal Women With Hormone Receptor-Positive Breast Cancer: Status Report 2004. J Clin Oncol. 2005;23:619–29. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 137.Miki Y, Suzuki T, Sasano H. Controversies of aromatase localization in human breast cancer--Stromal versus parenchymal cells. J Steroid Biochem Mol Biol. 2007;106:97–101. doi: 10.1016/j.jsbmb.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 138.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 139.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 140.Weidner N, Folkman J, Pozza F, et al. Tumor Angiogenesis: A New Significant and Independent Prognostic Indicator in Early-Stage Breast Carcinoma. J Natl Cancer Inst. 1992;84:1875–87. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 141.Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, et al. Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst. 1997;89:139–47. doi: 10.1093/jnci/89.2.139. [DOI] [PubMed] [Google Scholar]
- 142.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 143.Miller KD, Chap LI, Holmes FA, Colbleigh MA, Marcom OK, Fehrenbacher L, et al. Randomized Phase III Trial of Capecitabine Compared With Bevacizumab Plus Capecitabine in Patients With Previously Treated Metastatic Breast Cancer. J Clin Oncol. 2005;23:792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 144.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 145.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: Herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–80. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 146.Garvin S, Dabrosin C. Tamoxifen Inhibits Secretion of Vascular Endothelial Growth Factor in Breast Cancer in Vivo. Cancer Res. 2003;63:8742–8. [PubMed] [Google Scholar]
- 147.Mazhar D, Ang R, Waxman J. COX inhibitors and breast cancer. Br J Cancer. 2006;94:346–50. doi: 10.1038/sj.bjc.6602942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, Schaefer R, et al. Colony-Stimulating Factor-1 Blockade by Antisense Oligonucleotides and Small Interfering RNAs Suppresses Growth of Human Mammary Tumor Xenografts in Mice. Cancer Res. 2004;64:5378–84. doi: 10.1158/0008-5472.CAN-04-0961. [DOI] [PubMed] [Google Scholar]
- 149.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–74. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Teixeira MR, Pandis N, Heim S. Cytogenetic clues to breast carcinogenesis. Genes Chromosomes Cancer. 2002;33:1–16. doi: 10.1002/gcc.1206. [DOI] [PubMed] [Google Scholar]
- 151.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–8. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 152.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 153.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The Consensus Coding Sequences of Human Breast and Colorectal Cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 154.Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol. 2003;163:315–26. doi: 10.1083/jcb.200304159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, et al. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ince TA, Richardson AL, Bell GW, Saitoh M, Godar S, Karnoub AE, et al. Transformation of Different Human Breast Epithelial Cell Types Leads to Distinct Tumor Phenotypes. Cancer Cell. 2007;12:160–70. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 157.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 158.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 159.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–17. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 162.Bunting KD. ABC Transporters as Phenotypic Markers and Functional Regulators of Stem Cells. Stem Cells. 2002;20:11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 163.Bissell MJ, LaBarge MA. Context, tissue plasticity, and cancer: Are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.LaBarge MA, Petersen OW, Bissell MJ. Of microenvironments and mammary stem cells. Stem Cell Rev. 2007;3:137–46. doi: 10.1007/s12015-007-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]