Abstract
Ultrasonography was used to measure pennation angle and electromyography (EMG) to record muscle activity of the human tibialis anterior (TA), lateral gastrocnemius (LG), medial gastrocnemius (MG), and soleus (SOL) muscles during graded isometric ankle plantar and dorsiflexion contractions done on a Biodex dynamometer. Data from eight male and eight female subjects were collected in increments of approximately 25% of maximum voluntary contraction (MVC) ranging from rest to MVC. A significant positive linear relationship (p < 0.05) between normalized EMG and pennation angle for all muscles was observed when subject specific pennation angles at rest and MVC were included in the analysis. These were included to account for gender differences and inter-subject variability in pennation angle. The coefficient of determination, R2, ranged between 0.76 for the TA to 0.87 for the SOL. The EMG-pennation angle relationships have ramifications for use in EMG-driven models of muscle force. The regression equations can be used to characterize fiber pennation angle more accurately and to determine how it changes with contraction intensity, thus providing improved estimates of muscle force when using musculoskeletal models.
Keywords: ultrasound, regression, optimal pennation angle, sex differences
Introduction
Fiber pennation angle is an important functional characteristic of muscle (Fukashiro et al., 2006). Muscles with large pennation angles such as the soleus allow more fibers to be arranged in parallel within a given cross sectional area, thereby increasing a muscle’s force generating potential. Fiber pennation angle varies with muscle contraction intensity and fiber length (Maganaris et al., 1998; Maganaris, 2003; Narici et al., 1996), and thus such changes should be considered when using musculoskeletal models to estimate muscle force.
Changes in pennation angle during contraction have been predicted by planimetric models assuming parallel fibers and constant muscle thickness (Scott and Winter, 1991). Results from recent studies using real-time ultrasonography question these inherent assumptions of the planimetric model (Hodges et al., 2003, Maganaris et al., 1998, Muramatsu et al., 2002). For example, Maganaris and colleagues (1998) showed that a planimetric model underestimated pennation angle at MVC by 12° and 14° for the lateral gastrocnemius and soleus compared to in vivo measures recorded using ultrasonography. They approximated that errors of this magnitude would underestimate plantar flexion force by approximately 10% which is significant when considering the Achilles tendon undergoes tensile loading in excess of 2000 Newtons during relatively low effort tasks such as walking (Finni et al., 1998; Komi et al., 1992).
Ultrasound is a helpful tool for those developing musculoskeletal models because subject specific architecture (e.g., pennation angle) can be measured and used as inputs rather than relying upon literature-based values that may not be appropriate for a particular subject. Since muscle force increases with increasing muscle activation and because pennation angle changes with increasing force, we hypothesize that there is a relationship between muscle activation and fiber pennation angle. Identifying such a relationship would benefit musculoskeletal models by providing a more accurate representation of fiber pennation angle and how it changes with contraction, especially for muscles that are highly pennate.
Hodges et al. (2003) investigated the relationship between muscle geometry and contraction intensity for several muscles, including the TA. Pennation angle for the TA increased significantly with EMG up until approximately 20% of maximum voluntary contraction (MVC). Although the relationship was highly significant with an R2 value of 0.92, the small sample size (4 men and 1 woman) precludes extrapolation of the EMG-pennation angle relationship to a broader group of subjects and across sexes. Moreover, from a modeling consideration it is usually of more interest to include as many of the prime movers spanning a joint rather than an isolated muscle.
In this study we asked if pennation angle for the primary ankle plantar and dorsiflexor muscles (TA, LG, MG and SOL) could be predicted from EMG-based measures of muscle activation during isometric contractions. Of specific interest was to establish regression equations that could be used to predict pennation angle at %MVC typical of functional tasks such as walking (i.e., 25% – 75%). We hypothesized that pennation angle would increase with EMG, and the strength of the relationship would be related to the change in pennation angle from rest to MVC.
Methods
Sixteen adult subjects (8 male and 8 female) with no history of musculoskeletal injury participated in this study. The male and females averaged 24 years of age with standard deviations (SD) of 3.6 and 4.2 years respectively. The average male height was 178 centimeters (SD = 6.6), and 166 centimeters (SD = 5.4) for the females. On average the men weighed 82.0 kg (SD = 11.0) and the females were 66.4 kg (SD = 8.4). All subjects provided written informed consent prior to participation and the study was approved by the Human Subjects Review Board of the University of Delaware.
Muscle activity and ultrasound images for the TA, LG, MG and SOL for the right and left legs were tested at ankle joint angles that placed the muscles near their optimal fiber length. This was approximated using a lower extremity biomechanical model of the leg (Delp et al., 1990). The TA was tested in approximately 30° of plantar flexion and the MG, LG, and SOL in 20° of dorsiflexion. A Biodex System 3 dynamometer (Biodex, Shirley, NY) was used to set the ankle angle and monitor the joint moment. All testing was done with the knee fully extended. Subjects wore sneakers and the foot was securely strapped to the foot plate of the ankle attachment. The head of the dynamometer was adjusted so the axis of rotation approximated the sagittal plane ankle axis passing through the lateral malleolus. The thigh was secured using straps attached to the chair to minimize leg movement and the subject was seated with the hip in slight extension to reduce strain on the hamstrings. The sampling frequency of the Biodex was set at 1000 Hz.
Muscle activity for the TA, LG, and MG was recorded using surface electrodes with a 2 cm inter-electrode spacing (Norotrode 20, Myotronics-Noromed, Inc., WA). Muscle activity for the SOL was recorded using intramuscular electrodes to minimize cross talk from adjacent muscles. The wires were inserted distal and medial to the muscle belly of the gastrocnemius. Electrode placement was standardized for each subject as described elsewhere (Perotto, 1994). The EMG data were sampled at 1000 Hz. The electrodes were connected to a differential preamplifier that had a gain of 1000 and included a two-pole 30–10,000 Hz bandpass filter. The signals were further processed in hardware (Frequency Devices, Haverhill, MA) by amplifying and low pass filtering the signal with an 8-pole Butterworth filter at a cut-off frequency of 500 Hz.
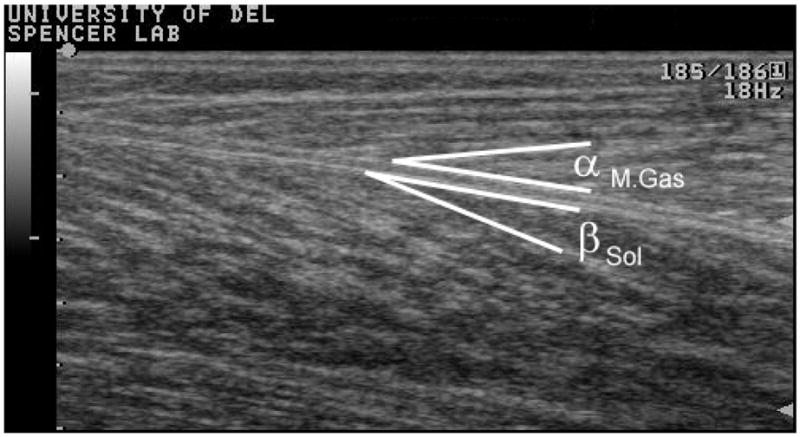
A variable frequency 60 mm linear transducer (Aloka-5000, Tokyo, Japan) was used to record the ultrasound images. The transducer was placed just inferior to the surface electrode placements of the TA, LG, and MG. Muscle architecture does not change significantly over the length of these muscles (Maganaris et al., 1998; Maganaris and Baltzopoulos, 1999). The insertion of the superficial fibers into the central aponeurosis was used to define the pennation angle for the TA. The insertion of the fascicles into the deep aponeurosis between the SOL and the LG and MG was used to define pennation angle for these muscles (see Figure 1). All images were initially scanned at 10 MHz with a B-mode setting and adjusted to 7.5 MHz for subjects with thicker calf muscles to better image the SOL.
Figure 1.
Two trials at rest and MVC were collected for the TA, LG and MG while simultaneously recording EMG, moment, and ultrasound images. The SOL was imaged deep to the LG and MG trials. Five seconds of data were collected for each trial to ensure the joint moment reached a plateau before saving the ultrasound image. Subjects then performed isometric contractions at approximately 25%, 50% and 75% of their maximum plantar and dorsiflexion moments. Two trials were collected at each level of effort with a 1 minute rest provided between trials. Pennation angle and EMG for the two trials were averaged, while the average of four trials was used for the Sol (i.e., two trials deep to the MG and two deep to the LG). A visual reference (i.e., % maximum moment) was displayed on the Biodex monitor to assist with targeting. Subjects were encouraged to do their best to match the target moment. Data for the TA were collected first, followed by the plantar flexor trials.
The root mean square (RMS) EMG amplitude was measured for a one second period in the middle of each contraction when the moment was visually confirmed to be stable (Hodges et al., 2003). The baseline channel voltage for each muscle determined during rest was subtracted from each of the trials to represent the active change in muscle activity. The RMS EMG signal was then normalized to the maximum RMS EMG from the larger of the two MVC trials, and this quantity, normalized EMG was used as a measure of muscle activation during contraction. Normalized EMG ranged between 0 and 1.0.Exploratory curve fitting revealed a linear trend between normalized EMG and pennation angle when pennation angle at rest and MVC were included in the analysis. These were included to account for large gender differences and inter-subject variability in pennation angle (Manal et al., 2006). Separate linear regressions were conducted for each muscle with a p-value less than 0.05 considered statistically significant. Pennation angles estimated using the regression equations were plotted relative to the pennation angles measured using ultrasound. In addition, we applied the regression equations using subject specific EMG and average values for pennation angle at rest and MVC. This was done to evaluate the efficacy of the equations when subject specific pennation angles at rest and MVC are not available.
Results
The reliability of our ultrasound measurements has previously been reported (Manal et al., 2006) and compares favorably with values reported in the literature (Fukunaga et al., 1997; Maganaris et al., 1998). The coefficient of variation for scanning a site multiple times and computing the pennation angle for each scan was 8.5%. The repeatability of the pennation angle computed from the same image had a coefficient of variation of 4.2%.
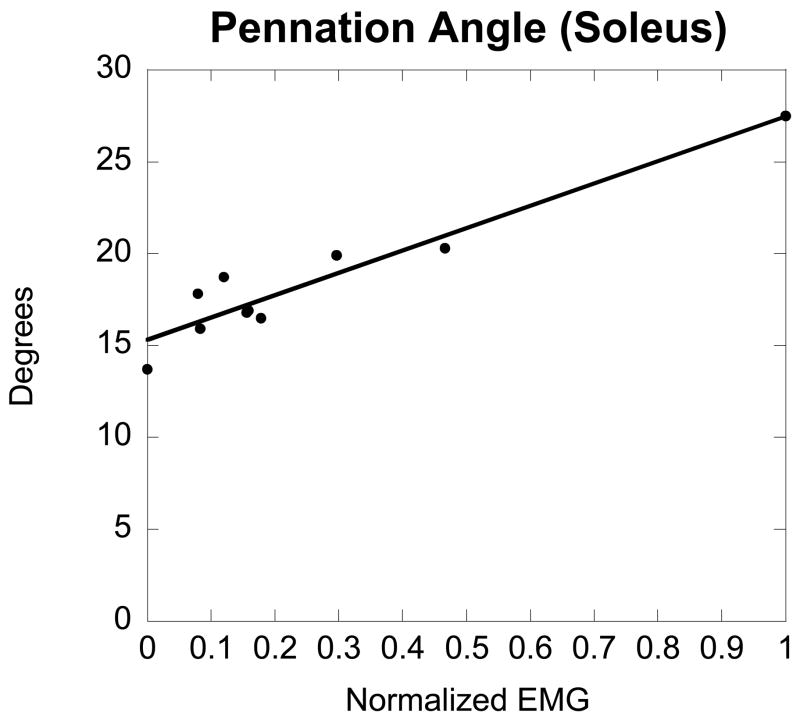
Exemplar data depicting the linear relationship between soleus EMG and pennation angle for a female subject are shown in Figure 2. It is important to note that the slope and fit of the line does vary from subject to subject because of inter-subject variability and gender differences in pennation angle (Table 1). For these reasons, pennation angle at rest and MVC were included in the analysis so that subject specific values may be predicted for subjects with similar normalized EMG.
Figure 2.
Table 1.
| Tibialis Anterior | Lateral Gastrocnemius | Medial Gastrocnemius | Soleus | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Rest | 9.4 (1.8)
[7.0 – 14.0] |
8.7 (1.0)
[7.0 – 10.5] |
14.1 (4.8)
[9.0 – 27.0] |
11.8 (3.2)
[7.0 – 18.0] |
18.6 (2.9)
[14.0 – 24.0] |
15.8 (1.8)
[12.0 – 18.0] |
20.0 (5.8)
[14.5 – 37.0] |
15.2 (3.8)
[8.5 – 21.5] |
| MVC | 14.3 (2.2)
[11.0 – 18.5] |
12.1 (1.4)
[9.5 – 14.5] |
22.4 (8.7)
[15.5 – 45.0] |
16.7 (4.7)
[11.5 – 27.5] |
34.6 (6.6)
[24.0 – 42.5] |
27.4 (5.5)
[22.0 – 41.5] |
39.7 (7.3)
[26.5 – 53.5] |
27.1 (7.4)
[14.0 – 37.5] |
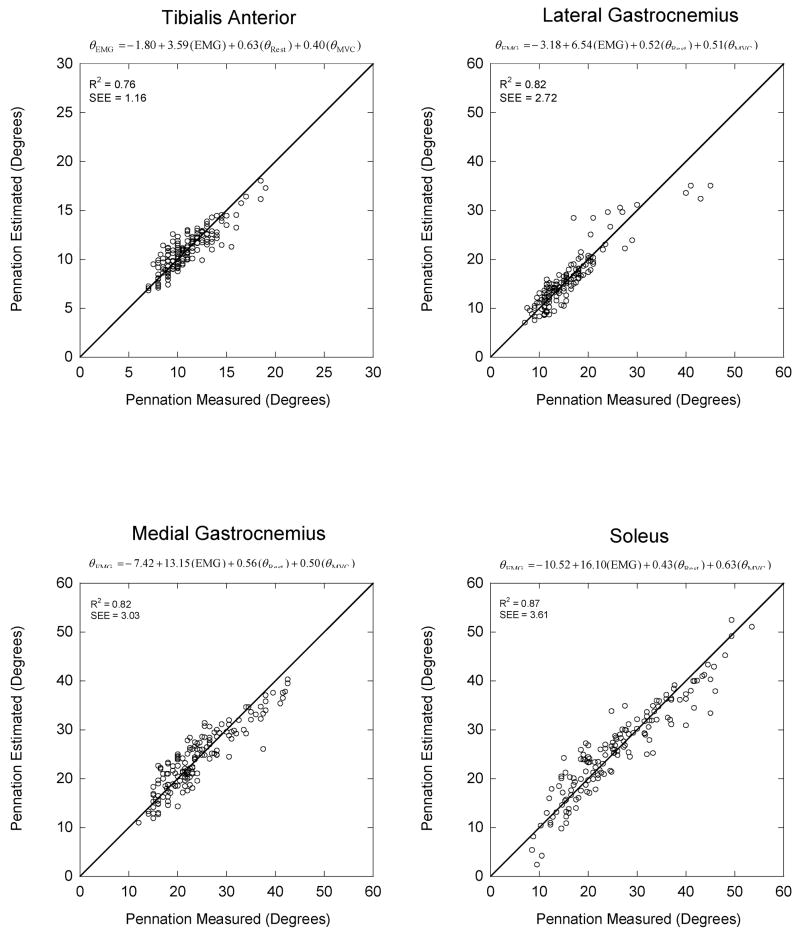
The magnitude of the difference between the linear regression pennation angles and the values measured with ultrasound for all subjects is illustrated in Figure 3. The scattering of data points about the diagonal line depicts the magnitude of the error. The slope for each regression equation was statistically significant (p <0.05) with R2 values of 0.76, 0.82, 0.82, and 0.87 for the TA, LG, MG, and SOL, respectively. The SOL had the largest slope, 16.10, indicating it underwent the largest change in pennation angle with increasing EMG. The largest difference between the ultrasound measured and the regression estimated pennation angle for the TA was 4.2°, with values of −11.5°, 11.4° and 11.6° for the LG, MG and SOL respectively. The average difference for all subjects and across contraction intensities was considerably smaller, ranging between 1° for the TA to almost 3° for the soleus (Table 2).
Figure 3.
Table 2.
| Tibialis Anterior | Lateral Gastrocnemius | Medial Gastrocnemius | Soleus | |
|---|---|---|---|---|
| Subject Specific | 0.88 (0.74) | 1.87 (1.93) | 2.40 (1.79) | 2.74 (2.28) |
| Group Average | 1.39 (1.16) | 3.97 (3.93) | 3.80 (3.10) | 5.60 (3.68) |
We also applied the regression equations using subject specific EMG and group average pennation angles reported in Table 1. Not surprisingly, differences between regression estimated and ultrasound measure pennation angles were greater than when using subject specific values at rest and MVC. The largest difference for all muscles was 5.6° for the SOL when averaged across subjects and contraction intensities (Table 2).
Discussion
Muscle fiber length and pennation angle changes with muscle contraction intensity (Maganaris et al., 1998; Maganaris, 2003; Narici et al., 1996). Contractile effort is reflected in the magnitude of the electromyogram (Buchanan et al., 2004), and therefore the EMG signal may be a predictor of fiber length and pennation angle change. The overarching finding of our study was that the relationship between EMG and pennation angle was fairly linear and statistically significant for all muscles tested (p < 0.05 ) when pennation angle at rest and MVC were included in the analysis. The SOL exhibited the strongest relationship with an R2 value of 0.87, while the TA was the weakest (R2 = 0.76).
The only other study to report pennation angle change as a function of muscle activity noted a highly significant exponential relationship for the TA (Hodges et al., 2003). In contrast, we observed a roughly linear increase in pennation angle with increasing EMG. Certain methodological differences may be related, in part, to differences between our study and the findings of Hodges et al., (2003). Firstly, we collected data with the ankle in 30° of plantar flexion (where the TA is near optimal fiber length) while they collected data with the ankle in a neutral position. Consequently, fiber length was different between studies which may have influenced the EMG-pennation angle relationship. Another difference was they collected data at finer increments of %MVC than we did. Given the reported non-linearity was at low levels of MVC and because of the step-size of our increments (i.e., 25%), we do not have the resolution to accurately characterize the relationship between EMG and pennation angle at low levels of MVC. Interestingly, the relationship they report limits increasing pennation angle for efforts above approximately 50% MVC. In contrast, the linear relationship we noted and data presented by Ito and colleagues (1998) both indicate pennation angle increases with contraction effort greater than 50% MVC.
The precise relationship between EMG and pennation angle at efforts below 20% MVC may be irrelevant from a modeling standpoint. This is because the TA pennation angle is small and therefore even a relatively large change of several degrees would have a negligible effect on the resulting force. Of greater interest from a modeling perspective would be to identify a significant and clinically relevant EMG-pennation angle relationship for ankle muscles with large cross sectional areas and pennation angles; the major force generators. For example, the cross-sectional area and pennation angle for the medial gastrocnemius and soleus are larger than the lateral gastrocnemius and the tibialis anterior (Friedrich and Brand, 1990; Wickiewicz et al., 1983; Manal et al., 2006) and therefore identifying a relationship between EMG and pennation angle for these muscles may have greater clinical significance. The EMG-pennation angle relationship for the MG and SOL was linear, statistically significant (p < 0.05) and both had R2 values greater than 0.8 indicating that more than 80% of the variance in pennation angle could be explained from normalized EMG and subject specific pennation angle at rest and MVC. The significance of the regression equations reported in this paper is that they may provide more anatomically appropriate pennation angles than cadaver-based estimates. For example, Bogey and colleagues (2005) used pennation angles originally reported by Wickiewicz et al., (1983) in their model of the ankle. Pennation angle for the LG ranged between 5° and 10° for the three cadavers tested. This is in stark contrast to findings by Kawakami et al. (1998) who reported in vivo ultrasound pennation angles of 31±6° for the LG at MVC, considerably different than the smaller values used by Bogey et al., (2005). The average pennation angle for the male subjects in our study was 22°; identical to the in vivo value reported by Maganaris (2003). Differences between cadaver and in vivo measures of muscle architecture for the triceps surae were studied by Martin and colleagues (2001). They concluded that: “when developing models of skeletal muscle based on cadaveric studies, the architectural differences between live and cadaveric tissue should be taken into consideration” (Martin et al., 2001, p. 429). The regression equations reported in our study can be used to account for some of these differences, even when subject specific values for pennation angle at rest and MVC are not available by using average values reported in Table 1.
The ability to predict pennation angle from EMG has several practical applications. Firstly, it is time consuming to record pennation angle across a full range of contraction intensities and for multiple muscles. Pennation angle can be approximated using the regression equations; requiring only 2 scans per muscle (i.e., rest & MVC), and therefore does not add significantly to the time required for data collection. Moreover, for those without access to ultrasound, average values reported in Table 1 can be used to estimate pennation angle, albeit with greater error as noted in Table 2. The error however may still be less than when using cadaver based values from the literature. Because muscle geometry changes with muscle contraction (Otten, 1988), it may be possible to predict EMG by inverting the EMG-pennation angle relationship for deep lying muscles such as soleus which are not readily sampled using surface EMG.
A limitation of our study is that we cannot necessarily use the regression equations for pennation angle and EMG to other joint angles because contraction produces greater changes in architectural parameters when a muscle contracts at a shorter length (Herbert et al., 2002). That is, the regression equations are specific to isometric contractions for the joint angles tested. To be applicable over a range of motion this relationship would have to be confirmed at several joint angles. The feasibility of using the relationship in a dynamic case is unclear because pennation angle changes with joint angle in passive as well as activated states. It is also important to note the regression equation for the soleus was based on EMG recorded from wire electrodes. Because we do not know how cross talk changes with increases in % MVC it is not know how well the regression equation would work with EMG recorded using surface electrodes. Nonetheless, it is evident that there is a strong relationship between muscle activity measured by EMG and muscle fiber pennation angle at the joint angles tested for the subjects in our study.
We also applied the equations to data for two additional healthy adult subjects (1 male & 1 female). This was done to check how well the equations worked with novel data and to evaluate if pennation angle could be predicted at low levels of force. Data for these subjects were collected at finer increments (4% MVC) at levels below 25% MVC and then at 25%, 50%, 75% and 100% MVC. The biggest errors occurred when the forces were small however the errors were of a similar magnitude as when fitting the data to the original 16 subjects. The correlation between the predicted and the ultrasound measured pennation angles for these subjects were 0.48, 0.72, 0.89 and 0.90 for the TA, LG, MG and Sol respectively. These findings are promising, especially for the MG and soleus, and suggest that the equations may generalize to healthy adult subjects. Additional subjects are needed to critically evaluate whether this is indeed the case.
The results of this study establish the regression equations to predict pennation angle near optimal fiber length for the TA, LG, MG and SOL muscles from EMG. Incorporating this relationship into a model may better represent the architecture of these large muscles which dominate ankle plantar flexion. The equations are easy to implement and can improve musculoskeletal modeling force estimates for the primary plantar and dorsiflexors.
Acknowledgments
The authors wish to thank Joseph Gardinier for his assistance with data collection. This work supported, in part, by NIH grants R01-AR46386, R01-HD38582, R01-AR48212 and P20-RR16458.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bogey RA, Perry J, Gitter AJ. An EMG-to-force processing approach for determining ankle muscle forces during normal human gait. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2005;13(3):302–310. doi: 10.1109/TNSRE.2005.851768. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: Estimation of muscle forces and joint moments and movements from measurements of neural command. Journal of Applied Biomechanics. 2004;20:367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Transactions on Biomedical Engineering. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- Finni T, Komi PV, Lukkariniemi J. Achilles tendon loading during walking: Application of a novel optic fiber technique. European Journal of Applied Physiology and Occupational Physiology. 1998;77:289–291. doi: 10.1007/s004210050335. [DOI] [PubMed] [Google Scholar]
- Friederich JA, Brand RA. Muscle fiber architecture in the human lower limb. Journal of Biomechanics. 1990;23:91–95. doi: 10.1016/0021-9290(90)90373-b. [DOI] [PubMed] [Google Scholar]
- Fukashiro S, Hay DC, Nagano A. Biomechanical behavior of muscle-tendon complex during dynamic human movements. Journal of Applied Biomechanics. 2006;22:131–147. doi: 10.1123/jab.22.2.131. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Kawakami Y, Kuno S, Funato K, Fukashiro S. Muscle architecture and function in humans. Journal of Biomechanics. 1997;30:457–463. doi: 10.1016/s0021-9290(96)00171-6. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Moseley AM, Butler JE, Gandevia SC. Change in length of relaxed muscle fascicles and tendons with knee and ankle movement in humans. Journal of Physiology. 2002;539:637–645. doi: 10.1113/jphysiol.2001.012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW, Pengel LH, Herbert RD, Gandevia SC. Measurement of muscle contraction with ultrasound imaging. Muscle & Nerve. 2003;27:682–692. doi: 10.1002/mus.10375. [DOI] [PubMed] [Google Scholar]
- Ito M, Kawakami Y, Ichinose Y, Fukashiro S, Fukunaga T. Nonisometric behavior of fascicles during isometric contractions of a human muscle. Journal of Applied Physiology. 1998;85:1230–1235. doi: 10.1152/jappl.1998.85.4.1230. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. Journal of Applied Physiology. 1998;85:398–404. doi: 10.1152/jappl.1998.85.2.398. [DOI] [PubMed] [Google Scholar]
- Komi PV, Fukashiro S, Jarvinen M. Biomechanical loading of achilles tendon during normal locomotion. Clinical Journal of Sports Medicine. 1992;11:521–531. [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. In vivo measurements of the triceps surae complex architecture in man: Implications for muscle function. Journal of Physiology. 1998;512(Pt 2):603–614. doi: 10.1111/j.1469-7793.1998.603be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V. Predictability of in vivo changes in pennation angle of human tibialis anterior muscle from rest to maximum isometric dorsiflexion. European Journal of Applied Physiology and Occupational Physiology. 1999;79:294–297. doi: 10.1007/s004210050510. [DOI] [PubMed] [Google Scholar]
- Maganaris CN. Force-length characteristics of the in vivo human gastrocnemius muscle. Clinical Anatomy. 2003;16:215–223. doi: 10.1002/ca.10064. [DOI] [PubMed] [Google Scholar]
- Manal K, Roberts DP, Buchanan TS. Optimal pennation angle of the primary ankle dorsi and plantarflexors: variations with sex, contraction intensity and limb. Journal of Applied Biomechanics. 2006;22:255–263. doi: 10.1123/jab.22.4.255. [DOI] [PubMed] [Google Scholar]
- Martin DC, Medri MK, Chow RS, Oxorn V, Leekam RN, Agur AM, McKee NH. Comparing human skeletal muscle architectural parameters of cadavers with in vivo ultrasonographic measurements. Journal of Anatomy. 2001;199:429–434. doi: 10.1046/j.1469-7580.2001.19940429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T, Muraoka T, Kawakami Y, Shibayama A, Fukunaga T. In vivo determination of fascicle curvature in contracting human skeletal muscles. Journal of Applied Physiology. 2002;92:129–134. doi: 10.1152/jappl.2002.92.1.129. [DOI] [PubMed] [Google Scholar]
- Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. Journal of Physiology. 1996;496(Pt 1):287–297. doi: 10.1113/jphysiol.1996.sp021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten E. Concepts and models of functional architecture in skeletal muscle. Exercise and Sport Sciences Reviews. 1988;16:89–137. [PubMed] [Google Scholar]
- Perotto AO. Anatomical guide for the electromyographer: The limbs and trunk. Springfield, IL: 1994. [Google Scholar]
- Scott SH, Winter DA. A comparison of three muscle pennation assumptions and their effect on isometric and isotonic force. Journal of Biomechanics. 1991;24:163–167. doi: 10.1016/0021-9290(91)90361-p. [DOI] [PubMed] [Google Scholar]
- Wickiewicz TL, Roy RR, Powell PL, Edgerton VR. Muscle architecture of the human lower limb. Clinical Orthopaedics and Related Research. 1983:275–283. [PubMed] [Google Scholar]
- Yamaguchi GT, Sawa AGU, Moran DW, Fessler MJ, Winters JM. A Survey of Human Musculotendon Actuator Parameters. In: Winters JM, Woo SL-Y, editors. Multiple Muscle Systems: Biomechanics and Movement Organization. Springer-Verlag; New York: 1990. [Google Scholar]