Abstract
Many natural actions require the coordination of two different kinds of movements. How are targets chosen under these circumstances: do central commands instruct different movement systems in parallel, or does the execution of one movement activate a serial chain that automatically chooses targets for the other movement? We examined a natural eye tracking action that consists of orienting saccades and tracking smooth pursuit eye movements, and found strong physiological evidence for a serial strategy. Monkeys chose freely between two identical spots that appeared at different sites in the visual field and moved in orthogonal directions. If a saccade was evoked to one of the moving targets by microstimulation in either the frontal eye field (FEF) or the superior colliculus (SC), then the same target was automatically chosen for pursuit. Our results imply that the neural signals responsible for saccade execution can also act as an internal command of target choice for other movement systems.
People make choices at every waking moment; perhaps the most basic and ubiquitous of these choices is where to look. Several times a second, primates express their choice of where to look with rapid, saccadic eye movements, which align gaze with different objects in the visual world. Saccadic movements in isolation are commonly used to examine the neural processes underlying choices and decisions1,2. In natural movements, however, when saccades are coordinated with smooth pursuit eye movements, the situation is both richer and more interesting. The choice to track a moving target smoothly is almost always accompanied by a saccade to point the eye at the target. Indeed, most natural movements involve coordination of different kinds of movement; for example, different parts of the body such as eye and hand, or different movement systems such as orienting and tracking. In natural coordinated movements, are targets for different components of the motor act chosen in parallel by a single overarching choice system? Or might the execution of one kind of movement, such as an orienting movement, exert serial control over target choice for another kind of movement, such as a tracking movement? We used saccades and smooth pursuit eye movements to discriminate these two (not necessarily mutually exclusive) possibilities and came to the conclusion that this particular coordinated movement uses serial target choice.
Pursuit and saccadic movements provide a fortuitous combination for examination of target choice for coordinated movements because they achieve the common goal of pointing the fovea at a moving target using fundamentally, though not entirely, different neural circuits3,4. Pursuit movements are slow rotations of the eye that serve to minimize the motion of images across the retina. Saccades are rapid shifts in eye position that serve to eliminate the difference between the position of the eye and that of the chosen target. Several studies have examined how saccades5-7 and smooth pursuit eye movements8-10 each accomplish target selection, but little is known about how they do so together.
We have previously shown11 that target choice for pursuit and saccades is temporally linked when monkeys freely choose to track either of two identical moving targets. Before the first saccade, pursuit eye velocity is determined by a vector average of the response to each target presented alone12. Immediately after a targeting saccade, pursuit is in the direction of the saccade target11. Because of latencies in the visuomotor pathways, the post-saccadic eye velocity must be driven by pre-saccadic visual inputs. Thus, the pursuit target choice that is linked to saccades must be attributed to modulation of the visuomotor drive by an internal command signal. Here we directly tested the hypothesis that the saccadic system provides this internal command signal by studying pursuit target choice after electrically evoked saccades. Our data show that evoked saccades are sufficient to select targets for pursuit, and thus target choice can be a serial process for naturally coordinated movements.
RESULTS
Saccades were evoked by electrical microstimulation at 34 sites in the saccadic portion of the FEF and at 12 sites in the SC. Applying microstimulation with short pulse trains (Methods) elicits fixed-vector saccades at short latencies13-15 (Fig. 1a). After determining the direction and amplitude of the evoked saccade at one site, we arranged the trajectories of two targets as follows (Fig. 1b): the ‘stim target’ moved away from the position of fixation with a trajectory that would intersect the endpoint of the elicited saccade. The ‘non-stim target’ started from an equal eccentricity but moved in an orthogonal direction, with a trajectory that took it far from the endpoint of the elicited saccade.
Fig. 1.
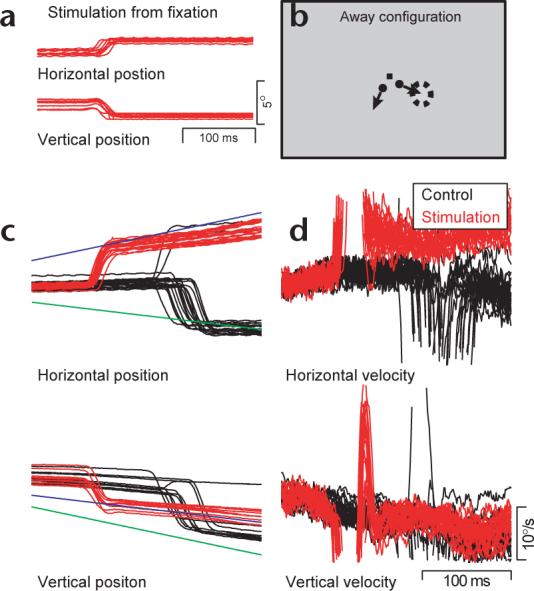
Example of saccade-induced target choice for pursuit at a single stimulation site. (a) Eye position traces from trials in which microstimulation was applied during fixation show evoked saccadic eye movement. (b) Target configurations were specifically tailored for the evoked saccade from this site such that one target (stim target) crossed the endpoint of the evoked saccade (dashed circle), and the other target (non-stim target) moved in an orthogonal direction. Eye position (c) and velocity (d) are shown for the away configuration. Blue and green traces are stim target trajectory and non-stim target trajectory, respectively. Black and red traces are eye movement records from control and stimulation trials, respectively. Upward deflections indicate rightward (for horizontal traces) or upward (for vertical traces) eye position (c) or velocity (d).
We tested the hypothesis that saccade execution selects targets for pursuit. First we evoked a targeting saccade with microstimulation before the monkey would naturally make a saccade (Methods), and then we compared the pursuit target choice after evoked saccades with that after natural targeting saccades in control trials. According to our hypothesis, the eye velocity after an evoked saccade should exhibit target choice that is as specific for the stim target as that after a natural saccade. If our hypothesis is incorrect, pursuit target choice should not be modified by the evoked saccade, and eye velocity should be vector averaging after the saccade. We designed our experiments to test (i) the extent to which the evoked saccade caused pursuit target choice and (ii) the degree to which pursuit target choice was the same as that after the natural targeting saccades made by monkeys in two-target trials without stimulation of the FEF (see Supplementary Data online for animations of example data).
Consider first an example of the eye movements recorded at one stimulation site for targets that moved away from the position of fixation (Fig. 1c-d). The eye position record from two-target control trials without microstimulation (Fig. 1c, black traces) shows that the monkey almost always made a saccade to the non-stim target (n = 38) rather than to the stim target (n = 2); average saccade latency was 245.3 ms relative to target motion onset. In contrast, stimulation of the FEF 117 ms after the onset of target motion (Fig. 1c, red traces) evoked saccades down and to the right, which pointed the eye toward the stim target every time with an average latency of 34.9 ms after microstimulation (151.9 ms relative to target motion onset). The saccades evoked in stimulation trials pointed the eye as close to the stim target as did the natural saccades in the control trials: the absolute post-saccadic position error was 0.52° and 1.3° for evoked and natural saccades, respectively.
Eye speed was enhanced after evoked saccades and was strongly biased toward the direction of motion of the stim target (Fig. 1d). In this example, eye speed after the evoked saccade was greater than at the same time in the control trials (12.93°/s versus 7.72°/s, Student's t-test, P < 0.001) and slightly larger than after the natural targeting saccades (11.53°/s, Student's t-test, P = 0.03). The direction of pursuit after the evoked saccade was 321.8°, which is close to the direction of tracking to the stim target presented alone (328.3°). It is also close to the direction of eye velocity after natural targeting saccades to the stim target (314.1°), but far from the direction of tracking to the non-stim target presented alone (262.4°). Thus, pursuit showed target choice for the stim target.
From the data in Fig. 1, where the direction of the evoked saccade and the direction of target motion are identical, we cannot tell whether the microstimulation-evoked saccade enhanced the response to the target motion (target choice), or had a direct effect on smooth eye velocity. To disassociate these two possibilities, we had the targets move toward the point of fixation so that the saccade and the target motions would be in opposite directions (Fig. 2a). As before, the eye speed after the evoked saccade was greater than that in control trials at the same time (Fig. 2c, 7.96°/s versus 3.99°/s, Student's t-test, P < 0.001). The direction of post-saccadic eye velocity averaged 178.28°, in good agreement with the direction of pursuit to the stim target presented alone (159.56°) and poor agreement with the direction of pursuit to the non-stim target (231.1°). We conclude that the effects of saccades on the direction and speed of post-saccadic pursuit indeed reflect target choice, at least for this stimulation site in the FEF.
Fig. 2.
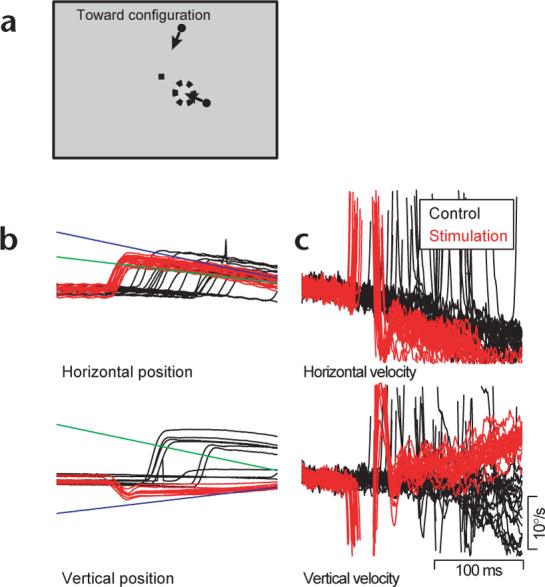
Saccade-induced target choice for pursuit is selective for the direction of motion of the target and not the direction of the saccade. At the same stimulation site as in Figure 1, we tested target motion towards the initial fixation position (a) in the opposite direction as the evoked saccade, thus disassociating the two directions. Eye and target position (b) and velocity (c) traces follow the same convention as in Fig. 1.
We used both an experiment-by-experiment analysis and a trial-by-trial analysis to evaluate the generality of saccade-induced pursuit target choice for a total of 109 different experiments conducted at 34 stimulation sites in the FEF in two monkeys (Methods). In the experiment-by-experiment analysis, eye velocity immediately after the evoked saccade was larger than eye velocity at the same time in control trials in 94% of the experiments (102 of 109); the difference was statistically significant in 89% of those 102 experiments (P < 0.05, Student's t-test). In 82% of the 109 experiments, the direction of post-saccadic pursuit for stimulation trials was closer to that evoked by the stim target alone than was the direction of the pre-saccadic eye velocity in control trials at the same time: the difference between post-saccadic and control direction was statistically significant in 82% of those (Watson-Williams test, P < 0.05).
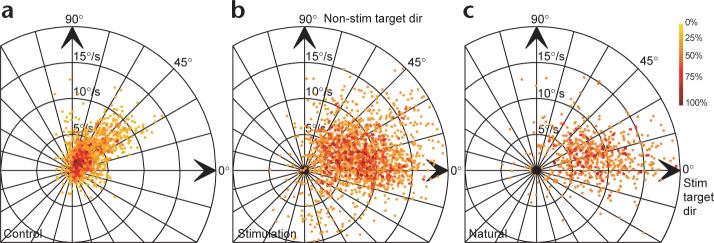
In the trial-by-trial analysis, we considered each trial as an independent event and pooled all the data for all sites in both monkeys. We created polar plots of eye velocity after evoked saccades in stimulation trials (Fig. 3b), of pre-saccadic eye velocity the same time in control trials (Fig. 3a) and of post-saccadic eye velocity for the natural saccades in control trials (Fig. 3c; see fig. legend for details). There was a substantial difference between eye velocity immediately after the evoked saccade (Fig. 3b), which was selective for the stim target, and pre-saccadic eye velocity at the same time after the onset of target motion in control trials Fig. 3a), which was vector averaging. Eye velocity immediately after both evoked (Fig. 3b) and natural (Fig. 3c) saccades was similarly target-selecting.
Fig. 3.
Trial-by-trial analysis of target choice for pursuit by saccades. Each point shows the direction and speed of smooth eye velocity from a single trial. Different graphs plot data measured after evoked saccades (b), after natural saccades (c), and at the same time in control trials as after evoked saccades on stimulation trials (a). Points have been rotated and flipped as necessary so that the direction of pursuit to the stim target presented alone is rightward (0°, stim target dir) and the direction of the non-stim target is upward (non-stim target dir). Points have been colored according to how densely packed on the graph they are: 100% density refers to the maximum density for each graph. Two and thirteen points plotted off the axis of the graphs in (b) and (c), respectively, and were therefore omitted.
The eye velocity after evoked saccades (Fig. 3b and red ellipse in Fig. 4) had a similar mean and standard ellipse compared to eye velocity after natural saccades (Fig. 3c and blue ellipse in Fig. 4), but not compared with eye velocity at the same time in control trials (Fig. 3a and black ellipse in Fig. 4). Mean eye speed in the stimulation trials (8.63°/s) was much greater than in the control trials (3.60°/s, Student's t-test, P < 0.001), but was statistically indistinguishable from mean eye speed after natural targeting saccades (8.86°/s, Student's t-test, P = 0.19). Further, the mean direction of smooth eye velocity after evoked saccades (14.97°) was closer to the stim target direction of zero degrees than it was to the vector average direction in control trials (41.41°, Watson-Williams test, P < 0.001), and was the same as that after natural targeting saccades (15.31°, Watson-Williams test, P = 0.81). Finally, the size of the standard ellipses shows that the variability after natural saccades was comparable to that after evoked saccades ( σmajor = 4.08 versus 4.28, σminor = 3.49 versus 2.28), but distinct from that of the control data (Fig. 4, black).
Fig. 4.
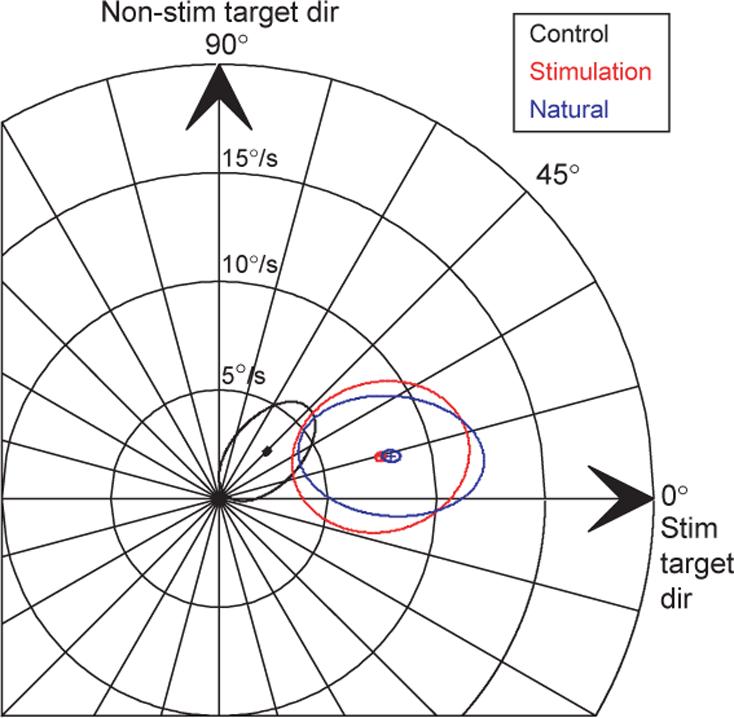
Target choice for pursuit by saccades, averaged across all trials. Small ellipses mark the 95% confidence ellipses around the mean and large ellipses indicate the standard ellipse. Different colors indicate control eye velocity (black), eye velocity after stimulation evoked saccades (red), and eye velocity after natural targeting saccades (blue). Ellipses were computed after rotating and flipping the points as described in Fig. 3.
We took two steps to ensure that the pursuit eye velocity immediately after a saccade was not confounded with eye velocity resulting from the saccade itself. First, saccadic velocity was either in the same direction (Fig. 1) or the opposite direction (Fig. 2) relative to the smooth motion of the stim target. Separate analysis of these trials showed that saccades caused similar target selection for both directions of motion. For the data in Fig. 3, the average pursuit velocities after the evoked saccade for target motion in the same versus opposite direction relative to the saccade were 8.5°/s and 8.7°/s; the average directions were 17.6° and 12.5° from the stim target direction. The slight difference in direction of pursuit (5.1°, Watson-Williams test, P < 0.01) for targets that moved in the same versus opposite direction as the saccades was also present, though not statistically significant, for natural targeting saccades (16.5° versus 14.5°, Watson-Williams test, P = 0.63).
Second, we verified that target-selecting pursuit after an evoked saccade was not a transient phenomenon by using choice probability16,17 to quantify the degree of pursuit target choice11. We analyzed the first 50 ms of post-saccadic eye velocity because this interval is unequivocally before the time when post-saccadic image motion first affects smooth eye movement18. Choice probability quantifies how well the direction of the saccade can be predicted based on the smooth eye velocity after the saccade. During the first 50 ms after both the evoked and natural saccades Fig. 5), choice probability was close to the value of 1, as expected for target-selecting pursuit. The choice probability was higher after the natural versus stimulation-evoked targeting saccades, reflecting the slightly lower mean eye velocity and greater variability after evoked saccades. As expected for vector averaging pursuit, choice probability was close to 0.5 when eye velocity was measured from control trials over the same interval relative to target motion onset as was used to obtain post-saccadic eye velocities from stimulation trials.
Fig. 5.
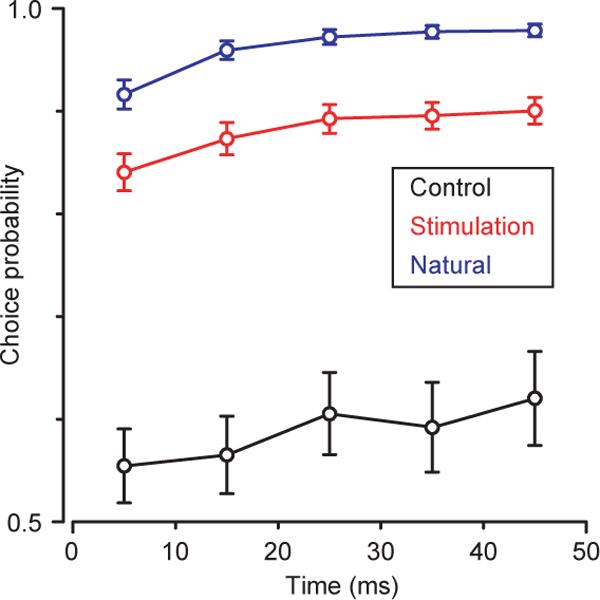
Target choice for pursuit by saccades is not a transient phenomenon. Choice probability is plotted as a function of time after the end of stimulation evoked saccades (red) and natural targeting saccades (blue) and at the same time in control trials (black) as for stimulation evoked saccades. Bootstrapping was used to estimate 95% confidence intervals.
These data are consistent with the idea that signals related to execution of the evoked saccade effectively choose the stim target for pursuit by enhancing the smooth eye movement selectively for the visual motion provided by the stim target. If the pursuit system had already chosen a target before we stimulated, however, the signals could have nonspecifically enhanced the eye velocity that had already been dictated by the nascent choice of the pursuit system. To probe for an effect of the monkey's natural choice on the pursuit target choice evoked by microstimulation, we examined data from trial configurations in which monkeys had a strong preference to make saccades to the stim or non-stim target in control trials (Methods). We then compared the direction of eye velocity after evoked saccades separately for these two groups of trials. For experiments in which the monkeys naturally chose the non-stim target or the stim target, the direction of post-saccadic eye velocity averaged 17.8° (n = 941) and 13.3° (n = 257). Both of these directions were much closer to the stim target direction (0°) than to the vector average direction of control eye velocity at the same time (41.1°). Therefore, the monkey's pre-existing target choice biases were largely, but incompletely, superceded by the choices imposed after evoked saccades.
To test whether pursuit target choice could be biased without overt saccadic eye movements, we adjusted the frequency of stimulation to just below the point where saccades could be evoked reliably. Representative traces from one site in the FEF show that when we used suprathreshold stimulation, the evoked saccade caused the pursuit system to select a target moving to the left, so that post-saccadic eye velocity was strongly leftward (Fig. 6a, red traces). Subthreshold stimulation (167 instead of 333 Hz) did not evoke saccades and caused no discernable change in eye velocity (Fig. 6a, cyan traces). Summary data from seven sites in both monkeys were used to compare pursuit target choice after subthreshold (111−333 Hz) versus suprathreshold (200−500 Hz) stimulation (Fig. 6b). Eye velocity after evoked saccades (red ellipse) had enhanced speed (8.6°/s) relative to the same time after the onset of target motion in the trials with subthreshold stimulation (3.1°/s) and relative to the controls without microstimulation (3.1°/s). The direction of post-saccadic pursuit after evoked saccades was 10.8° from the direction of the stim target direction, compared to 32° and 36.3° at the same time after the onset of target motion in the non-stimulation controls and the trials with subthreshold stimulation. The latter two measurements were statistically indistinguishable (Hotelling's T2 test, P = 0.13). Thus, pursuit target selection is all-or-none, depending on the execution of a saccade.
Fig. 6.
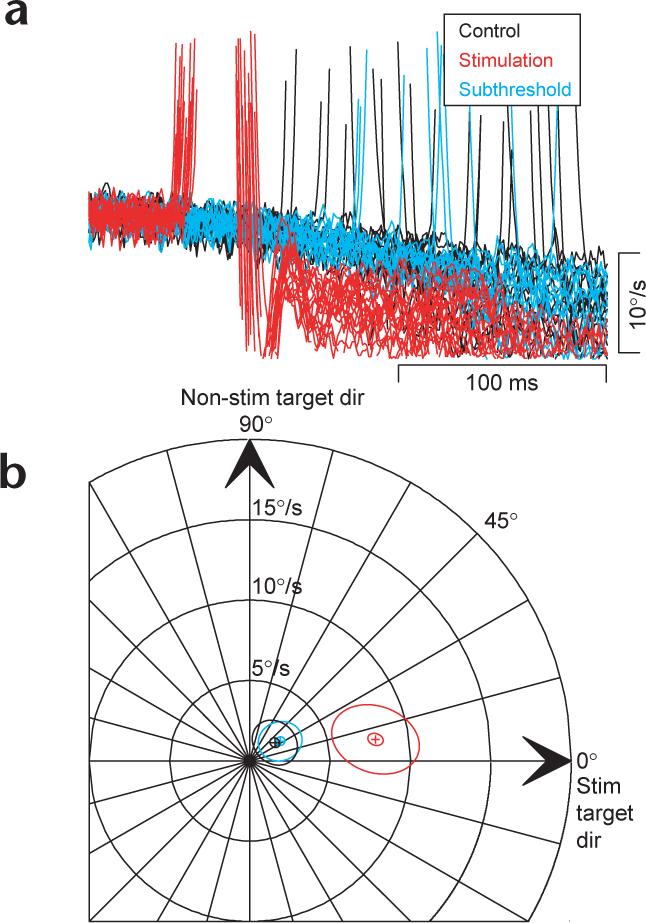
Subthreshold stimulation of the FEF does not enhance pursuit. (a) Horizontal eye velocity is plotted for one site where suprathreshold stimulation (red traces) caused pursuit target selection. When stimulation frequency was reduced to just below the rate at which saccades are elicited (cyan traces), it did not change pursuit compared to control (black traces) trials. (b) Summary data for all seven subthreshold sites. Pursuit velocity in subthreshold trials (cyan) analyzed at the same time relative to target onset as suprathreshold trials (red) do not differ significantly from control non-stimulation trials (black).
By conducting these same experiments with suprathreshold stimulation of the SC, we showed that the target-selecting effect of evoked saccades is a more general consequence of saccade execution, and not specific to microstimulation of the FEF. Stimulation of a site in the SC elicited saccades from fixation that were nearly purely rightward (horizontal amplitude 8.7°, vertical amplitude −1.8°) and the motion of the stim target was leftward. Immediately after the evoked saccade, there was a strong leftward component with a speed of 13.0°/s and in a nearly leftward direction (183.1°; Fig. 7a, red traces). In control (no stimulation) trials, the eye speed at the same time averaged 0.6°/s-too small to have a meaningful direction (Fig. 7a, black traces).
Fig. 7.
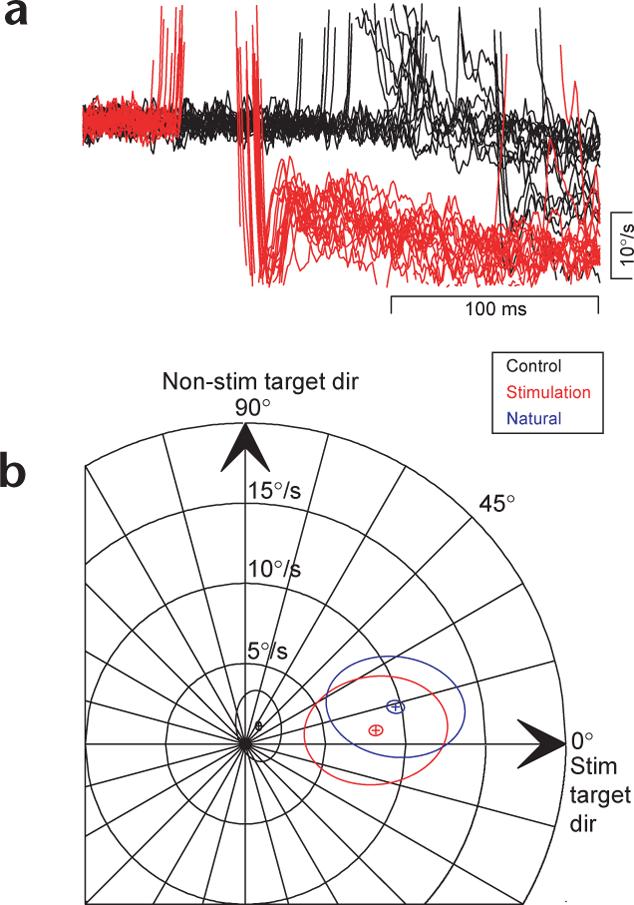
Saccades elicited from the SC also cause target selection for pursuit. (a) Horizontal eye velocity trace showing stimulation effect (red) as compared to controls (black). (b) Summary data, same conventions as Figs. 6 and 4.
Target choice for pursuit was a reliable consequence of saccades evoked by microstimulation in the SC. The eye velocity after evoked saccades was enhanced in speed and was nearly in the direction of the stim target (Fig. 7b, red ellipse; mean speed 9.0°/s, mean direction 4.6°). In contrast, we observed nearly perfect vector averaging pursuit (Fig. 7b, black ellipse, mean speed 2.4°/s, mean direction 43.7°) at the same time in control trials where stimulation was not applied. As before, the smooth eye velocity after saccades evoked from the SC was qualitatively similar to that after natural targeting saccades (Fig. 7b, blue ellipse, mean speed 10.2°/s, mean direction 15.0°).
We tested the effects of SC-evoked saccades on stim target motions in both directions: toward and away from the point of fixation. Post-saccadic eye speed was always enhanced; its direction depended on the direction of target motion and not on the direction of the saccade. Pursuit after evoked saccades to targets moving toward versus away from the position of fixation had mean speeds of 10.1°/s and 7.2°/s and average directions of 6.2° and 1.0° from the stim target direction. For both directions of target motion, these averages were significantly different from the direction and speed measured at the same time in control trials (Hotelling's T2 test, P < 0.0001). We conclude that saccades evoked by microstimulation from the SC select targets for pursuit just as effectively as do saccades evoked from the FEF.
DISCUSSION
We have shown that the signals involved in the execution of saccades guide target choice for the pursuit eye movement system. When electrical stimulation in the brain was used to evoke saccades to moving targets at a time when monkeys would normally make nonspecific vector averaging pursuit, pursuit became immediately selective for the target at the endpoint of the evoked saccade. The target-selective effect of saccades was linked to the direction of target motion rather than to saccade direction, ruling out the possibility that it results from either a simple mechanical facilitation of the eye in the orbit or a low-level motor phenomenon. Further, target selection for pursuit had an all-or-none dependence on the execution of the saccade and did not occur after stimulation that was below the threshold for evoking saccades, at least in the FEF.
Multiple consequences of saccadic eye movements
Signals related to saccadic eye movements have long been thought to have neural consequences other than moving the eyes. Many have argued that the brain discriminates the visual consequences of self motion from displacement in the outside world by consulting motor outflow, alternatively termed ‘effort of will’19, ‘corollary discharge’20 or ‘efference copy’21. Indeed, psychophysical studies have documented peri-saccadic changes in perception that also have been attributed to motor outflow from saccadic eye movements22. Finally, a number of studies have linked saccadic eye movements and spatially specific, enhanced perceptual processing23-29.
Our results indicate an additional role for saccadic motor outflow in target selection for other kinds of movements. A priori, the linkage of target choice for saccades and pursuit11 could result either from a serial linkage in which saccade execution causes target selection for pursuit or from parallel choice commands exerted simultaneously on both systems, or from some combination of both. Our results using electrical microstimulation of the FEF and SC provide physiological evidence demonstrating the existence of a powerful serial linkage. Pursuit target choice after electrically evoked saccades was as complete as after natural saccades, supporting the conclusion that this serial mechanism predominates under natural conditions without microstimulation11. However, monkeys can use explicit cues to choose pursuit targets without making a saccade8,30, raising the possibility that parallel mechanisms may also contribute.
Serial target selection makes teleological sense. Saccades and pursuit normally occur together when an object is tracked, and serial target selection would capitalize on this natural linkage of the two movements. Further, saccades are our primary visuomotor mechanism for orienting overt attention to stimuli of interest. Execution of a saccade is the clearest expression the motor system can give of the importance of an object: signals related to the execution of saccades as orienting movements would afford the most reliable and conservative basis for choosing the same target for other movements. Indeed, natural reach and grasp movements are preceded by saccades to the point of manual contact31, and it seems plausible that saccades could play a similar role in influencing the selection of targets for manual motor systems.
Saccades cause target choice for pursuit that appears rapidly, immediately after the end of the saccade. In our experiments, the most sensitive foveal part of the retina was pointed towards the stim target after (but not before) a saccade, suggesting that pursuit target choice could result from more powerful visual inputs from the fovea. However, many studies32 have argued that smooth eye velocity after a saccade is driven by visual motion signals present before the saccade, because of the visuomotor processing delays of the pursuit system. Furthermore, changes in target motion during a saccade have been shown to have their first effect on pursuit eye velocity more than 50 ms after the end of the saccade18. Thus, post-saccadic visual inputs from the fovea cannot affect pursuit eye velocity in the analysis period we used. Instead, post-saccadic pursuit target choice must reflect a selective modulation of the visual signals present before the saccade.
Candidate neural mechanisms for pursuit target choice
When a monkey is presented with two identical targets moving in different directions without any cues about which target he should track to receive a reward, pre-saccadic pursuit is in a direction that corresponds to the vector average of the response to each target alone8,12. Pursuit target choice reflects a shift from the initial vector averaging to a winner-take-all behavior: pursuit must then be driven selectively by the visual inputs from the chosen target, even if the other target is still present.
Our data show that saccade commands have access to the process of converting vector averaging pursuit into the winner-take-all behavior that reflects pursuit target choice. We conceptualize this process as a modifiable visual aperture through which the pursuit system views the world33. Before the pursuit system has chosen a target, it views the visual field through a large spatial aperture and generates smooth eye movements that represent a compromise among the different targets that are moving through the field. Once pursuit has chosen a target, the spatial aperture closes around that target and the visual inputs within the smaller aperture are processed with a higher gain. The aperture can be controlled either from covert attentional mechanisms such as those used when an animal is cued which target to track by target color or position9,30,34,35, or by overt attentional mechanisms such as visuomotor orientation through saccades.
If target choice for pursuit can be affected by the same covert and overt mechanisms that give rise to enhanced spatial processing in perceptual tasks, then it is worth considering whether the neural correlates of spatial attention could participate in target choice for pursuit. The visual inputs for pursuit arise from the middle temporal visual area (MT) and the medial superior temporal area (MST)36. Attention causes the responses of neurons in both MT and MST to be stronger for an attended stimulus than for a non-attended stimulus10,37-39. However, attentional modulation has not been documented in the rapid time frame that would be required to affect pursuit movements in our task. Moreover, the median magnitude of attentional modulation seems too modest: 40% modulation is the strongest documented when only one of the stimuli falls inside the receptive field of the neuron39 (other studies10,38 report attentional modulations of less than 16%).
Neurons in the parietal cortex remap their receptive fields in anticipation of a saccade that will bring a visual stimulus onto its receptive field40. Remapping, if it occurs in the motion processing pathways associated with pursuit, could be a mechanism of saccade-induced target choice for pursuit. For example, our results would be expected if the saccadic motor plan caused directional responses to begin before or during the saccade in visual motion neurons with foveal receptive fields.
Possible neural loci for pursuit target choice
One of the striking aspects of our results is that target choice was equally effective after saccades evoked from the SC and the FEF. One explanation is that stimulation of either of these sites activates the entire saccadic system, including cortical areas that control target choice for pursuit. Another explanation is that target choice for pursuit occurs in subcortical neural circuitry, close to the motor output of the pursuit system. Conceptually, two independent pursuit motor plans could emanate from the cortex and vector averaging could occur as a low-level brainstem mechanism. Saccadic signals, originating from the cortex or the SC, could signal the appropriate target and rapidly switch the combination of the two plans from vector averaging to winner-take-all behavior. Support for this idea comes from the finding that vector averaging occurs late in the pursuit system, after the sites of both pursuit learning41 and on-line gain control42. Our data cannot discriminate whether pursuit target selection occurs on motor or sensory signals, or both.
Our results suggest a scenario very different from the idea that classically defined saccadic areas provide a position error signal to drive pursuit43,44. Position error refers to the difference between eye position and target position and has a weak influence on steady-state, but not initial, pursuit movements45. Position error cannot control the initiation of pursuit, because the position error from a target can be in a different direction from target motion. For example, when the target starts to the left and moves to the right, as in our ‘toward’ configuration, position error will be in the wrong direction to drive pursuit. We propose instead that the saccadic system signals the spatial location of the target motion information that should control pursuit. This effect could be mediated by neurons in the SC that signal the target of a pursuit movement46 or a saccade5 in advance of movement initiation.
Microstimulation as an exogenous command for pursuit
Microstimulation has been used effectively to manipulate decision making by injecting signals into cortical areas that carry relevant sensory information47,48. While our experiments also use microstimulation as a tool for examining behavioral choices, there are major differences. The saccadic regions of the FEF and the SC do not transmit visual motion signals that are primarily responsible for pursuit movements; stimulating in these areas does not provide a command for smooth eye velocity. Instead, stimulation influences the choice of behavioral output by manipulating an internal signal related to saccade execution, which in turn signals the location of the target in the visual field. Our results suggest that microstimulation of the FEF and the SC during our task is able to directly access the selection mechanism controlling target choice for smooth pursuit eye movements.
METHODS
Subjects and equipment
Two male rhesus monkeys (6 and 12 kg) were used in experiments approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco. All experimental procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Behavioral training, general experimental approaches and surgical procedures have been described previously11. All surgical procedures were conducted using sterile surgical techniques under general anesthesia (isoflurane). Analgesics (buprenorphine HCl 0.01−0.03 mg/kg and ketorolac 7.5−15 mg) were provided during post-surgical recovery. Eye position was monitored using the scleral search coil technique, while the head position was maintained fixed using an implanted head post. Eye velocity was provided by analog differentiation of the position signal using an analog double-pole filter that differentiated signals at frequencies below 100 Hz and rejected signals at higher frequencies (−40 db/decade). This filter was chosen based on discussion in ref. 49. Eye position and velocity signals were digitized at 1 kHz and stored for further analysis on a UNIX based Alpha workstation.
Visual stimuli
Perceptually identical white spots of light 0.5° in diameter and a small red LED were back-projected onto a tangent screen located 114 cm from the monkey's eyes. The positions of the spots were controlled by a mirror galvanometer system driven by digital-to-analog converters that were updated at a rate of 1 kHz. All trials began with the appearance of a red spot at the center of the screen, which monkeys were required to fixate within 2.0° for at least 700−1000 ms. For the pursuit trials, one or two white spots would start at eccentric positions and immediately begin moving either towards or away from the fixation position. Target speed was 25°/s at three of the stimulation sites and 20°/s at all others. In two-target trials, monkeys were not given any cue as to which target they should track or when to make a saccade. Fixation requirements were lax during the initial target motion to allow the monkeys to adopt whatever choice behavior they naturally preferred. Once monkeys chose a target with a saccade, the other target was extinguished and they were required to pursue the selected target with an accuracy of 3.5° until it had traversed a total of 15° of visual angle or had come within a degree of the border of the tangent screen (18° in any direction from the center). The target then stepped another 1° in the same direction, stopped, and remained visible for 450 ms; the monkey was required to fixate within 3.5°. To extinguish the non-chosen target, the end of saccades were detected online as the time when horizontal and vertical eye velocity both dropped below 50°/s after either exceeded 50°/s. We discarded those trials in which the automated procedure had extinguished a target before the onset of a saccade.
Trial sets were customized for each stimulation site. First, we estimated the amplitude, direction and latency of the saccades elicited from fixation (Fig. 1a). Then, we chose a suitable combination of starting eccentricity for a stim target that moved along the direction of the saccade vector and a stimulation onset time so that the target would cross the endpoint of the evoked saccade after pursuit initiation but before the monkey would naturally make a saccade. In practice, the distance from eye position at the end of the evoked saccade to the stim target ranged from 0.4° to 3.5° (mean 1.3°) for different sites. Finally, we chose three non-stim target trajectories that started at points rotated 90°, 180° and 270° in visual space and moved in directions either opposite or orthogonal to the stim target. Thus, a full experiment contained four single-target trials that presented the motion of each target alone; six control two-target trials consisting of all possible pairs of stim and non-stim targets, without stimulation of the FEF or the SC; three stimulation two-target trials consisting of the stim target paired with each of the non-stim targets, with stimulation of the FEF or the SC; and a fixation trial in which stimulation was applied. Control two-target trials in which neither target was the stim target were run at a higher frequency than the other two-target trials, to balance the number of trials that did and did not include the stim target. Trials were interleaved randomly in an order that was shuffled each time the monkey completed the list.
At 23 stimulation sites, we ran two blocks of trials in which the stim target moved first toward and then away from the position of fixation. At 11 additional sites, we tested only stim target motion toward the position of fixation. Each block of trials paired the stim target motion with the orthogonal motion of two different non-stim targets, so that we obtained data for 114 different combinations of stim and non-stim target (23 sites × 2 directions of motion × 2 non-stim targets + 11 sites × 2 non-stim targets). Each separate combination of stim and non-stim target is an ‘experiment’. Five experiments were not analyzed because the configuration of target position and motion dictated by the stimulation site did not require saccades in the control single target conditions. This left 109 experiments for analysis.
Data analysis
The beginning and end of saccades were marked on the velocity traces by visual inspection using a custom-built application. All further analysis was done in Matlab 5.3 (Mathworks, Natick, Massachusetts). For each trial, we averaged pre- and post-saccadic eye velocity in 10-ms intervals, and reported post-saccadic eye velocity as that in the first interval after the end of the first targeting saccade. Choice probabilities were calculated according to methods we have previously reported11. Briefly, saccadic and pursuit weights were calculated for each trial to quantify the degree to which a saccade or smooth eye velocity can be described as targeting versus vector averaging. For both the saccade weight and the pursuit weight, we determined the value of w that best fitted the following equation to the data:
For saccadic weights, and are vectors representing the position of the two spots at the end of the saccade, and is a vector representing the position of the eye when spots and were both displayed. For pursuit weights, and are vectors representing the pre- or post-saccadic eye velocity (averaged over a 10-ms window) averaged across all single spot trials where one spot or the other is presented. is a vector representing the velocity of the eye for each trial when spots and were both displayed. Targets were pseudo-randomly assigned to be either and on a trial-by-trial basis. Weights take on a value of 0.5 for perfect vector averaging versus 0 or 1 for perfect target choice for one or the other target.
Choice probabilities were calculated as the area under a receiver operating characteristic (ROC) curve17 compiled from two distributions of pursuit weights associated with trials where the saccade weight was greater than 0.5 or less than 0.5; 95% confidence intervals were calculated by bootstrapping. Natural biases in pursuit target choice were quantified by calculating the percent of saccade weights less than 0.5 (saccades directed towards the stim target) in control two-target trials with no stimulation. Experiments in which this percentage was greater than 75% or less than 25% were considered to show a strong natural preference for the stim or non-stim target, respectively.
Electrophysiology
We studied sites in the anterior bank of the arcuate sulcus where saccadic eye movements were evoked by microstimulation with currents less than or equal to 50 μA using pulse trains lasting 50−70 ms. Each train consisted of 250−500 Hz bimodal pulses of duration 0.2 ms. We adjusted stimulus current to a level that reliably elicited saccades on virtually every pursuit trial (range 30−75 μA, median 45 μA). Saccades varied in amplitude (0.9°−14.4°), but were all evoked at short latency (31.6 ± 12.6 ms). Stimulation sites were anterior to the area along the posterior bank of the arcuate sulcus where microstimulation evoked smooth pursuit movements50.
We studied 12 sites in the SC in one of the monkeys used in the FEF experiments. To approach the SC perpendicular to its surface in such a way that the visual and motor maps are aligned, we angled the recording cylinder so that our electrode approached the SC from the posterior at an angle 28° back from vertical. The SC was identified by recording single and multiple units which responded selectively during a delayed saccade task. On every penetration, we first identified the superficial layers of the SC by the predominant response of units to the visual presentation of the saccade stimulus without any saccade-related burst. We measured receptive fields to confirm that neurons were responding to stimuli in restricted parts of the visual field. We identified the intermediate layers of the SC by the appearance of saccade-related bursts, generally to saccades of the same metrics as the best visual stimulus we tested in the superficial layers, and by the ability to evoke saccades at short latencies (19.2 ± 5.7 ms) with low currents13 (current range 15−35 μA, median 25 μA). We used pulse trains of 70 ms and 500 Hz; bimodal pulse duration was 0.3 ms.
Acknowledgments
We are grateful to P. Glimcher, M. Shadlen and the Lisberger lab for helpful discussions, and to A. Doupe and I. Chou for comments on an earlier version of the paper. We also thank J. Horton for surgical assistance, K. MacLeod, E. Montgomery and S. Tokiyama for surgical, animal and technical assistance, M. Meneses for animal husbandry, K. McGary for electronics, L. Bocskai for machining, S. Ruffner for computer programming, D. Kleinhesselink for network management and E. Molyneaux for administrative support. Research was supported by the Howard Hughes Medical Institute, National Eye Institute grant EY03878, and a Burroughs Welcome Fund training grant in Quantitative Biology (J.L.G.).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Glimcher PW. Making choices: the neurophysiology of visual-saccadic decision making. Trends Neurosci. 2001;24:654–659. doi: 10.1016/s0166-2236(00)01932-9. [DOI] [PubMed] [Google Scholar]
- 2.Schall JD. Neural basis of deciding, choosing and acting. Nat. Rev. Neurosci. 2001;2:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- 3.Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci. Res. 1991;11:79–107. doi: 10.1016/0168-0102(91)90048-4. [DOI] [PubMed] [Google Scholar]
- 4.Wurtz RH, Goldberg ME. The Neurobiology of Saccadic Eye Movements. Elsevier; New York; 1989. [Google Scholar]
- 5.Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature. 1992;355:542–545. doi: 10.1038/355542a0. [DOI] [PubMed] [Google Scholar]
- 6.Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- 7.Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J. Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrera VP. Task-dependent modulation of the sensorimotor transformation for smooth pursuit eye movements. J. Neurophysiol. 2000;84:2725–2738. doi: 10.1152/jn.2000.84.6.2725. [DOI] [PubMed] [Google Scholar]
- 9.Krauzlis RJ, Zivotofsky AZ, Miles FA. Target selection for pursuit and saccadic eye movements in humans. J. Cogn. Neurosci. 1999;11:641–649. doi: 10.1162/089892999563706. [DOI] [PubMed] [Google Scholar]
- 10.Recanzone GH, Wurtz RH. Effects of attention on MT and MST neuronal activity during pursuit initiation. J. Neurophysiol. 2000;83:777–790. doi: 10.1152/jn.2000.83.2.777. [DOI] [PubMed] [Google Scholar]
- 11.Gardner JL, Lisberger SG. Linked target selection for saccadic and smooth pursuit eye movements. J. Neurosci. 2001;21:2075–2084. doi: 10.1523/JNEUROSCI.21-06-02075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisberger SG, Ferrera VP. Vector averaging for smooth pursuit eye movements initiated by two moving targets in monkeys. J. Neurosci. 1997;17:7490–7502. doi: 10.1523/JNEUROSCI.17-19-07490.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- 14.Robinson DA, Fuchs AF. Eye movements evoked by stimulation of frontal eye fields. J. Neurophysiol. 1969;32:637–648. doi: 10.1152/jn.1969.32.5.637. [DOI] [PubMed] [Google Scholar]
- 15.Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- 16.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley; New York: 1966. [Google Scholar]
- 18.Chou IH, Lisberger SG. Spatial generalization of learning in smooth pursuit eye movements: implications for the coordinate frame and sites of learning. J. Neurosci. 2002;22:4728–4739. doi: 10.1523/JNEUROSCI.22-11-04728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Helmholtz H. In: Helmholtz's Treatise on Physiological Optics. Southall JPC, editor. The Optical Society of America; Rochester, New York: 1867/1924. [Google Scholar]
- 20.Sperry R. Neural basis of the spontaneous optokinetic response produced by visual inversion. J. Comp. Physiol. Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- 21.Von Holst E, Mittelstaedt H. Das reafferenzprinzip. Wechselwirkung zwischen zentralnervensystem and peripherie. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- 22.Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci. 2001;24:113–121. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd M, Findlay JM, Hockey RJ. The relationship between eye movements and spatial attention. Q. J. Exp. Psychol. 1986;38A:475–491. doi: 10.1080/14640748608401609. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept. Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- 25.Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- 26.Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- 27.Sheliga BM, Riggio L, Rizzolatti G. Orienting of attention and eye movements. Exp. Brain Res. 1994;98:507–522. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- 28.Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- 29.Moore T, Fallah M. Control of eye movements and spatial attention. Proc. Natl. Acad. Sci. USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Recanzone GH, Wurtz RH. Shift in smooth pursuit initiation and MT and MST neuronal activity under different stimulus conditions. J. Neurophysiol. 1999;82:1710–1727. doi: 10.1152/jn.1999.82.4.1710. [DOI] [PubMed] [Google Scholar]
- 31.Johansson RS, Göran W, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J. Neurosci. 2001;21:6917–6932. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu. Rev. Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- 33.Miles FA, Schwarz U, Busettini C. In: Representations of Vision: Trends and Tacit Assumptions in Vision Research. Gorea A, editor. Cambridge University Press; 1991. pp. 185–199. [Google Scholar]
- 34.Kowler E, Steen JVD, Tamminga EP, Collewijn H. Voluntary selection of the target for smooth eye movement in the presence of superimposed, full-field stationary and moving stimuli. Vision Res. 1984;24:1789–1798. doi: 10.1016/0042-6989(84)90010-5. [DOI] [PubMed] [Google Scholar]
- 35.Ferrera VP, Lisberger SG. Attention and target selection for smooth pursuit eye movements. J. Neurosci. 1995;15:7472–7484. doi: 10.1523/JNEUROSCI.15-11-07472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J. Neurophysiol. 1989;62:31–47. doi: 10.1152/jn.1989.62.1.31. [DOI] [PubMed] [Google Scholar]
- 37.Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 38.Seidemann E, Newsome WT. Effect of spatial attention on the responses of area MT neurons. J. Neurophysiol. 1999;81:1783–1794. doi: 10.1152/jn.1999.81.4.1783. [DOI] [PubMed] [Google Scholar]
- 39.Treue S, Maunsell JH. Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J. Neurosci. 1999;19:7591–7602. doi: 10.1523/JNEUROSCI.19-17-07591.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duhamel J-R, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 41.Kahlon M, Lisberger SG. Vector averaging occurs downstream from learning in smooth pursuit eye movements of monkeys. J. Neurosci. 1999;19:9039–9053. doi: 10.1523/JNEUROSCI.19-20-09039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka M, Lisberger SG. Role of arcuate frontal pursuit area of monkeys in smooth pursuit eye movements, II. Relation to vector averaging pursuit of two-target stimuli. J. Neurophysiol. 2002;87:2700–2714. doi: 10.1152/jn.2002.87.6.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krauzlis RJ, Basso MA, Wurtz RH. Shared motor error for multiple eye movements. Science. 1997;276:1693–1695. doi: 10.1126/science.276.5319.1693. [DOI] [PubMed] [Google Scholar]
- 44.Missal M, de Brouwer S, Lefevre P, Olivier E. Activity of mesencephalic vertical burst neurons during saccades and smooth pursuit. J. Neurophysiol. 2000;83:2080–2092. doi: 10.1152/jn.2000.83.4.2080. [DOI] [PubMed] [Google Scholar]
- 45.Morris EJ, Lisberger SG. Different responses to small visual errors during initiation and maintenance of smooth-pursuit eye movements in monkeys. J. Neurophysiol. 1987;58:1351–1369. doi: 10.1152/jn.1987.58.6.1351. [DOI] [PubMed] [Google Scholar]
- 46.Krauzlis RJ, Dill N. Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron. doi: 10.1016/s0896-6273(02)00756-0. in press. [DOI] [PubMed] [Google Scholar]
- 47.Salzman CD, Britten KH, Newsome WT. Cortical microstimulation influences perceptual judgements of motion direction. Nature. 1990;346:174–177. doi: 10.1038/346174a0. [DOI] [PubMed] [Google Scholar]
- 48.Romo R, Hernandez A, Zainos A, Salinas E. Somatosensory discrimination based on cortical microstimulation. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- 49.Lisberger SG. Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J. Neurophysiol. 1998;79:1918–1930. doi: 10.1152/jn.1998.79.4.1918. [DOI] [PubMed] [Google Scholar]
- 50.Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J. Neurophysiol. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]