Abstract
Hiatus hernia refers to conditions in which elements of the abdominal cavity, most commonly the stomach, herniate through the esophageal hiatus into the mediastinum. With the most common type (type I or sliding hiatus hernia) this is associated with laxity of the phrenoesophageal membrane and the gastric cardia herniates. Sliding hiatus hernia is readily diagnosed by barium swallow radiography, endoscopy, or manometry when greater than 2 cm in axial span. However, the mobility of the esophagogastric junction precludes the reliable detection of more subtle disruption by endoscopy or radiography. Detecting lesser degrees of axial separation between the lower esophageal sphincter and crural diaphragm can only be reliably accomplished with high resolution manometry, a technique that permits real time localization of these esophagogastric junction components without swallow or distention related artifact.
Keywords: Hiatus hernia, high resolution manometry, endoscopy radiography
Introduction
The esophageal hiatal orifice is an eliptically shaped opening through the diaphragm with its long axis in the sagittal plane through which the esophagus and vagus nerves gain access to the abdomen. In general terms, hiatus hernia refers to herniation of elements of the abdominal cavity through the esophageal hiatus of the diaphragm and into the mediastinum. Of the openings through the diaphragm, it is only the esophageal hiatus that is vulnerable to visceral herniation because it faces directly into the abdominal cavity and, hence, is directly subjected to the pressure stresses between the two cavities. Notably, the esophagus does not tightly fill the hiatus because it needs to expand to accommodate luminal contents. Thus, the integrity of the hiatus depends upon the structures bridging the gap between the esophagus and the surrounding crural diaphragm making the detailed anatomy of this area of prime importance.
Anatomy of the Hiatus and Gastroesophageal Junction
Although there is some anatomic variability, the most common anatomic pattern is for the hiatus to be formed by elements of the right diaphragmatic crus [1]. The crura arise from tendinous fibers emerging from the anterior longitudinal ligament over the upper lumbar vertebrae (Figure 1). The crura pass upward in close contact with the vertebral bodies for most of their course and incline forward only as they arch around the esophagus [1]. Upon emerging from the tendinous origin of the right crus, the muscle fibers form two ribbon-like bundles separated by connective tissue. The dorsal bundle forms the left limb of the right crus (thoracic aspect) and the ventral bundle becomes the right limb (abdominal aspect) of the right crus. As they approach the hiatus, the muscle fibers diverge and cross each other in a scissor-like fashion with the ventral bundle passing to the upper right region and the dorsal bundle passing to the upper left region. The lateral fibers of each hiatal limb insert directly into the central tendon of the diaphragm but the medial fibers, which form the hiatal margins, incline toward the midline and decussate with each other in a trellis-like fashion in front of the esophagus [1].
Figure 1.
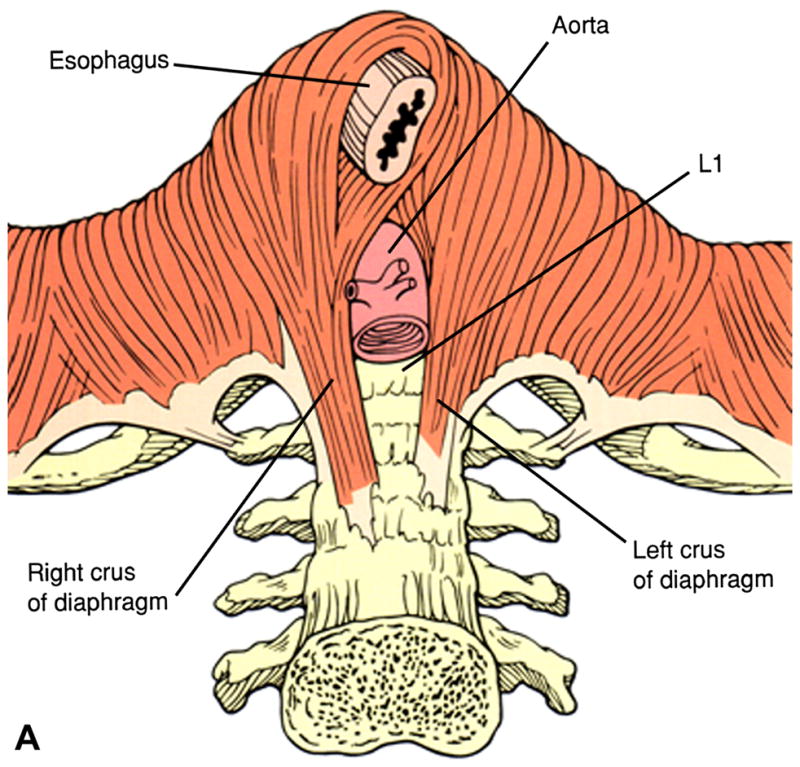
The most common anatomy of the diaphragmatic hiatus in which the muscular elements of the crural diaphragm derive from the right diaphragmatic crus. The right crus arises from the anterior longitudinal ligament overlying the lumbar vertebrae. Once muscular elements emerge from the tendon, two flat muscular bands form that cross each other in scissor-like fashion, form the walls of the hiatus, and decussate with each other anterior to the esophagus. Modified from Jaffee BM, Surgery of the esophagus. In Orlando RC Ed. Atlas of Esophageal Diseases, Second Edition. pp 223–242.
Under normal circumstances, the esophagus is anchored to the diaphragm such that the stomach cannot be displaced through the hiatus into the mediastinum. The main restraining structures are the phrenoesophageal ligaments, alternatively referred to as the phrenoesophageal membrane, and an aggregation of posterior structures including the vagus nerve and radicles of the left gastric vein and artery [2, 3]. The phrenoesophageal membrane is formed from the fascia transversalis on the under surface of the diaphragm and, to a lesser degree, fused elements of the endothoracic fascia. This elastic membrane inserts circumferentially into the esophageal musculature, close to the squamocolumnar junction, and extends for about a centimeter above the gastroesophageal junction [5]. Thus, the axial position of the squamocolumnar junction is normally within or slightly distal to the diaphragmatic hiatus and surrounded by the crural diaphragm [6]. In addition to its role of maintaining fixation of the esophagogastric region to the diaphragm, the phrenoesophageal membrane also closes the potential space between the esophagus and the diaphragm making it a key structure to consider in the pathogenesis of hiatus hernia. With age, the amount of elastic tissue in the phrenoesophageal membrane progressively declines, increasing its laxity and increasing the risk for developing hiatal hernias [6, 7].
Types of Hiatal Hernia
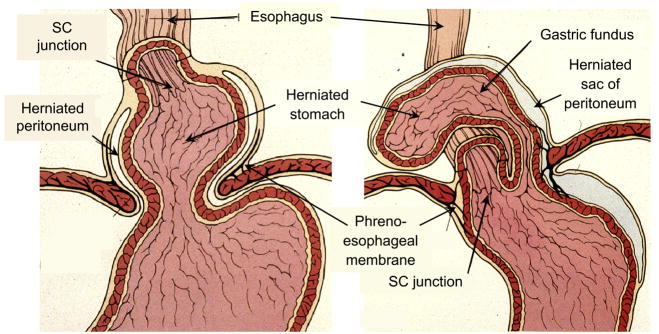
With hiatus hernia elements of the abdominal cavity, most commonly the stomach, are displaced through the esophageal hiatus of the diaphragm into the mediastinum. The most comprehensive classification scheme recognizes 4 types of hiatal hernia. With type I, or sliding hiatal hernia, there is a widening of the muscular hiatal tunnel and circumferential laxity of the phrenoesophageal membrane, allowing a portion of the gastric cardia to herniate upward (Figure 2). With a well developed hernia, the esophageal hiatus abuts directly on the transverse membrane of the central tendon of the diaphragm and the anterior hiatal muscles are absent or reduced to a few atrophic strands [1]. The hiatus itself is no longer a sagittal slit but a rounded opening whose transverse diameter approximates the sagittal diameter in size. This change in caliber of the hiatus is most apparent during distention [8]. Associated with the widening of the hiatal orifice, the phrenoesophageal membrane becomes attenuated and inconspicuous in comparison to its normal prominence. However, although thinned, the phrenoesophageal membrane remains intact and the associated herniated gastric cardia is contained within the posterior mediastinum. Type I hiatus hernias are by far the most common type. The major significance of type I hernias is in their association with reflux disease. They are also the most difficult to objectively define and, hence, the major focus of controversy in diagnosis.
Figure 2.
Distinction between a sliding hiatal hernia (type I) and paraesophageal hernia (type II). With type I hernia the leading edge is the gastric cardia while with type two it is the gastric fundus. The SCJ maintains its native position in the paraesophageal hernia while it is displaced upward with the sliding hernia. Modified from Jaffee BM, Surgery of the esophagus. In Orlando RC Ed. Atlas of Esophageal Diseases, Second Edition. pp 223–242.
The less common types of hiatus hernia, Types II, III and IV are all varieties of “paraesophageal hernias.” Taken together, these account for, at most, 5–15% of all hiatal hernias [9]. Although these hernias may also be associated with significant gastroesophageal reflux their main clinical significance lies in the potential for mechanical complications. A type II hernia results from a localized defect in the phrenoesophageal membrane while the gastroesophageal junction remains fixed to the preaortic fascia and the median arcuate ligament (Figure 2) [9]. The gastric fundus then serves as the leading point of herniation. Type III hernias have elements of both types I and II hernias. With progressive enlargement of the hernia through the hiatus, the phrenoesophageal membrane stretches, displacing the gastroesophageal junction above the diaphragm, thereby adding a sliding element to the type II hernia. Type IV hiatus hernia is associated with a large defect in the phrenoesophageal membrane, allowing other organs, such as colon, spleen, pancreas and small intestine to enter the hernia sac.
Although the etiology of paraesophageal hernias is usually unclear, they are a recognized complication of surgical dissection of the hiatus as occurs during antireflux procedures, esophagomyotomy, or partial gastrectomy. Many patients with a type II hernia are either asymptomatic or have only vague, intermittent symptoms. When present, symptoms are generally related to ischemia or either partial or complete obstruction. The most common symptoms are epigastric or substernal pain, postprandial fullness, substernal fullness, nausea, and retching. An upright radiograph of the thorax may be diagnostic, revealing a retrocardiac air-fluid level within a paraesophageal hernia or intrathoracic stomach. Barium contrast studies are almost always diagnostic and attention should focus on the position of the EGJ in order to differentiate type II and III hernias.
The natural history of a type II hernia is progressive enlargement so that the entire stomach eventually herniates, with the pylorus juxtaposed to the gastric cardia, forming an upside-down, intrathoracic stomach. Either as cause or effect, paraesophageal hernias are associated with abnormal laxity of structures normally preventing displacement of the stomach; the gastrosplenic and gastrocolic ligaments. As the hernia enlarges, the greater curvature of the stomach rolls up into the thorax. Because the stomach is fixed at the gastroesophageal junction, the herniated stomach tends to rotate around its longitudinal axis resulting in an organoaxial volvulus. Gastric volvulus may lead to acute gastric obstruction, incarceration, and perforation [9].
Mobility of the Esophagogastric Junction
Following from the anatomical relationships described above, the definition of a type I hiatus hernia is dependent on the anatomic relationship of the distal esophagus, hiatus, and stomach. However, it is of cardinal importance to recognize that this relationship is not static. Rather, it is altered with contraction of the longitudinal layer of the esophageal muscularis propria because this is associated with esophageal shortening and the distal end must elevate with shortening. With this elevation, the phrenoesophageal membrane is stretched and the EGJ transiently elevates above the hiatus. Thus, “physiological herniation” occurs during primary peristalsis, secondary peristalsis, esophageal distention, and transient LES relaxation since in each case the gastric cardia tents through the diaphragmatic hiatus [10–12]. In fact, it is this profound mobility of the EGJ that makes it so difficult to standardize the assessment and measurement of a type I hiatus hernia. Furthermore, since much of the axial motion of the EGJ occurs in the setting of a closed esophageal lumen, it is not readily detectable using imaging modalities such as endoscopy or barium contrast radiography that require an open, distended lumen to assess EGJ position. There lies a substantial part of the difficulty in the assessment of type I hiatus hernia; the assessment itself is greatly influenced by the method of measurement. What is demonstrable radiographically is different from what is demonstrable endoscopically or manometrically. One way to demonstrate this limitation is to mark the position of the squamocolumnar junction with an endoclip and then use digital imaging techniques to track the motion of the endoclip relative to fixed anatomical landmarks such as the vertebrae during standardized protocols.
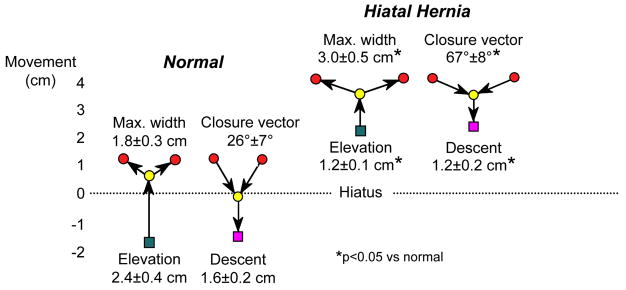
The endoclip method has been used to quantify EGJ mobility in the setting of primary peristalsis [10, 13], secondary peristalsis [11], transient LES relaxation [12] and pH electrode placement [14]. With each of these conditions, longitudinal muscle contraction leads to esophageal shortening which leads to stretch of the phrenoesophageal membrane and displacement of the EGJ through the hiatus. However, there is no opposing muscle to the longitudinal muscle of the esophagus to re-establish its resting length. Rather, with relaxation of the esophageal longitudinal muscle, it is the elastic recoil of the phrenoesophageal membrane that is then responsible for pulling the squamocolumnar junction back to its normal position. From this description, one might anticipate that swallow related EGJ motion would be attenuated with type I hiatus hernia, a condition associated with atrophy and laxity of the phrenoesophageal ligament. Figure 3 illustrates exactly that. Whereas normal controls exhibited an average of almost 2.5 cm of axial motion during the swallow sequence, the comparable measurement was only 1.2 cm in a group of patients with a sliding hiatus hernia [13]. Also note in Figure 3 how axial much EGJ motion occurs prior to luminal opening and subsequent to luminal closure; this motion would not be detectable during endoscopy or standard fluoroscopy.
Figure 3.
Axial mobility of the EGJ during swallow in normal controls and patients with type I hiatus hernia demonstrated by fluoroscopically tracking the movement of endoscopically place endoclips at the squamocolumnar junction. Clip movements are referenced to a point on the vertebral column. The green square represents the position of the SCJ prior to swallow, almost 2 cm distal to the center of the hiatus in the normal controls and 2 cm above it in the case of the hernia patients. With the initiation of swallow, the clip elevates to the point indicated by the yellow circle before luminal opening occurs. The red circles then indicate the maximal diameter of opening achieved, significantly wider in the case of the hernia patients, consistent with the reduced compliance demonstrable in these patients. During closure the angle of descent is steeper with the normals than with the hernia patients and the final position (magenta square) is essentially the same as prior to the swallow. Laxity and loss of elasticity of the phrenoesophageal membrane is suggested by two observations: 1) the attenuated axial movement of the SCJ in the hernia patients and 2) the flattening of the closure vector in the hernia patients suggesting diminished elastic recoil as the longitudinal muscle of the esophagus relaxes post-swallow. Modified from Kahrilas, PJ, Wu, S, Lin, S, et al. Attenuation of esophageal shortening during peristalsis with hiatus hernia. Gastroenterology 1995;109:1818.
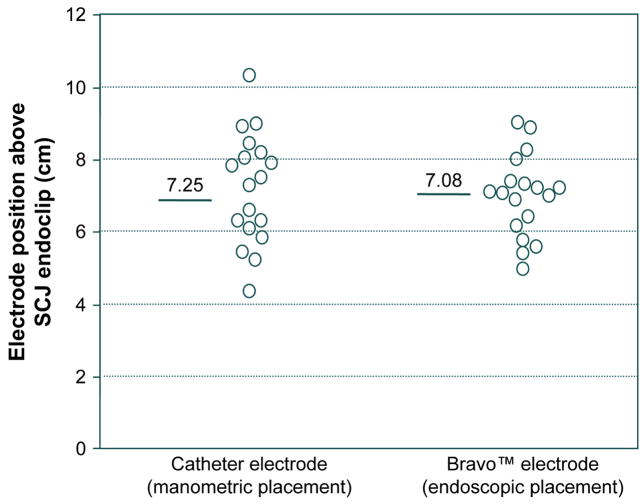
Another illustration of the mobility of the EGJ is found in the assessment of the accuracy of placement of pH electrodes for ambulatory esophageal pH monitoring studies [14]. By convention these are placed 5 cm proximal to the upper margin of the LES when using a catheter type of electrode or 6 cm proximal to the SCJ when using the Bravo™ capsule type. Thus, the measurement must be made using either a manometry catheter or an endoscope, each of which might be expected to stimulate some degree of contractile activity in the esophagus. Evident in Figure 4, the accuracy of placement when assessed with fluoroscopy and an endoclip is only roughly ±2 cm with either method. Furthermore, in each case the average electrode position is about 1 cm proximal to the intended position, which would be anticipated if the measurement process triggered this amount of shortening.
Figure 4.
Accuracy and variability in manometric and endoscopic pH electrode placement assessed by fluoroscopic imaging of final placement in reference to an endoclip placed at the SCJ. In all cases, the intended placement was 6 cm proximal to the SCJ. Data for the 18 subjects all of whom had both pH electrodes in place concurrently. Both intra-subject and inter-subject variability were greater with the manometric placement. Presumably, much of the inaccuracy in placement with both electrodes was attributable to shortening of the esophageal during the assessment of SCJ or LES position attributable to endoscopy or manometry. Modified from Pandolfino JE, Schreiner MA, Lee TJ, et al. Comparison of the Bravo™ Wireless and Digitrapper™ Catheter-based pH Monitoring Systems for Measuring Esophageal Acid Exposure. Am J Gastroenterol 2005;100:1466–1476.
In summary, the EGJ is a mobile structure with its mobility dependent upon contractile activity of the longitudinal muscle of the distal esophagus and the integrity/elastic properties of the phrenoesophageal membrane. This mobility complicates the detection and measurement of type I hiatus hernia because, ultimately, a sliding hiatus hernia is defined by the position of the EGJ relative to the diaphragmatic hiatus. Minimal perturbations such as swallowing, esophageal distention, or esophageal instrumentation are associated with esophageal shortening (and hence EGJ-hiatus disassociation) in the range of 2 cm. Hence, one would anticipate this magnitude of inherent error in measurement modalities dependent on these perturbations.
The Radiographic Assessment of Sliding Hiatus Hernia
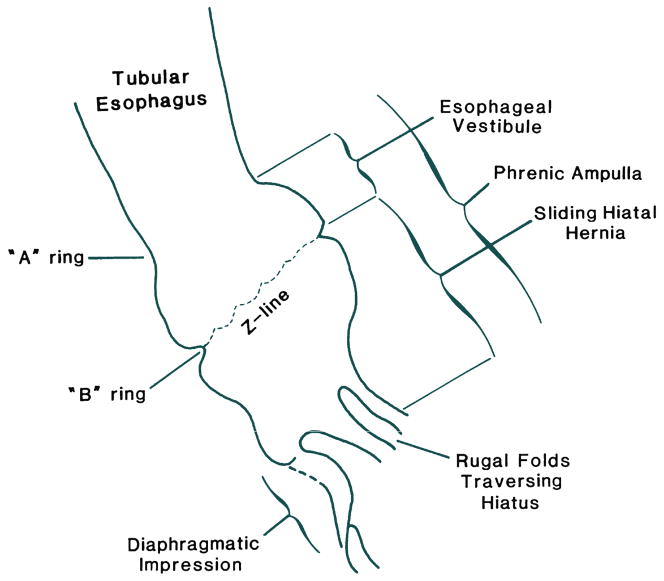
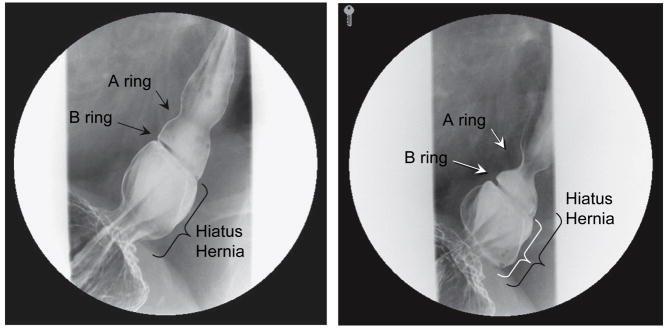
The radiographic demonstration of a sliding hiatus hernia is usually done in the setting of a barium swallow examination. However, in order to demonstrate the relative positions of the EGJ and the diaphragmatic hiatus, the positions of these structures must be visible radiograpically, which means the esophagus must be distended with consequent shortening and displacement of the EGJ as discussed above. Recognition of this confounding effect led to the 2 cm rule wherein there must be more than 2 cm separation between the B ring and the diaphragmatic hiatus before being considered a sliding hiatus hernia (Figure 5) [15]. Lesser magnitudes of separation are attributed to physiologic herniation.
Figure 5.
Anatomical features of a sliding hiatus hernia viewed radiographically during swallowing. The A ring is a muscular ring visible during swallowing which demarcates the superior margin of the LES. Physiologically, the A ring corresponds to the highest pressure zone within the LES. The B ring at the squamocolumnar junction is present in only about 15% of individuals and allows for accurate division of the phrenic ampulla into the esophageal vestibule (A ring to B ring) and the sliding hiatus hernia (B ring to the subdiaphragmatic stomach). By convention the distinction between normal and hiatus hernia is a ≥ 2cm separation between the B ring and the hiatus. Rugal folds traversing the hiatus support the conviction that a portion of the stomach is supradiaphragmatic. From Kahrilas, PJ. Hiatus hernia causes reflux: fact or fiction? Gullet 1993;3(Suppl):21, with permission.
Another limitation of fluoroscopy is diagrammed in Figure 5. Sliding hiatus hernia is defined as a greater than 2 cm separation between the B ring and the diaphragmatic hiatus. However, a B ring, located at the SCJ, is only demonstrable in about 15% of individuals. Hence, the confusing array of terminology in Figure 5. Again, not all of the structures illustrated in Figure 3 are always demonstrable radiographically. Commonly, an A ring, but not a B ring is evident in which case the limits of the measurement defining hiatus hernia become arbitrary. In the absence of a B ring, the globular structure seen radiographically that forms above the diaphragm and beneath the tubular esophagus during deglutition is more accurately termed the phrenic ampulla. In such cases, the demonstration of rugal folds traversing the diaphragm is used as the defining criterion. Alternatively, a B ring but not an A ring may be evident in which case it is easy to apply the ≥2 cm B ring to hiatus criterion for defining a sliding hiatus hernia. Parenthetically, in instances in which the B ring is of such prominence that the luminal diameter is less than 13 mm it is termed a Schatzki ring.
Another limitation of the radiographic assessment of the size of a type I hiatus hernia is illustrated in Figure 6. The two panels in Figure 6 are sequential images in the same individual taken during the course of a single swallow. The size estimate of the hernia will be about 1 cm different depending upon whether it is made from the image at the left of Figure 6, taken early in the peristaltic sequence or the image on the right, taken near its termination. And then, of course, if the measurement could be taken between swallows, the size estimate would be even less. Furthermore, being a part of the diaphragm, the hiatus is both at the focal point of the complex pressure dynamics of respiration and exposed to the positive abdominal to mediastinal pressure gradient that acts to extrude the abdominal contents into the chest. Hence, it is not surprising that with the addition of abdominal compression during barium swallow imaging, the frequency of identification of type I hiatus hernia dramatically increased to 55% in a report of 955 patients studied [16].
Figure 6.
Radiographs taken sequentially during a swallow of a patient with a small axial hiatal hernia, a well developed A ring, and a well developed B ring evident. In such cases the criterion for defining hiatus hernia is that the measurement from the B ring to the diaphragmatic hiatus exceed 2 cm. This distance is indicated by the black bracket in the image on the left, obtained early in the swallow sequence, and the white bracket in the image on the right, obtained late in the swallow sequence. The black bracket from the left image is superimposed on the right making the point that size estimate of a sliding hiatus hernia will vary depending on when in the swallow sequence the measurement is made.
In summary, largely because of the inherent subjectivity in the radiographic criteria for defining type I hiatal hernia, estimates of prevalence vary enormously, from 10% to 80% of the adult population in North America [9]. In marginal instances, type I hiatal hernia is simply an exaggeration of the normal phrenic ampulla making its identification entirely dependent on measurement technique. Only when a sliding hiatal hernia enlarges further, such that >2 cm of gastric pouch is herniated upward, is its presence obvious because gastric folds are evident traversing the diaphragm both during swallow-induced shortening and at rest. Thus in practical terms, unless a strict protocol for measurement is tightly adhered to, the identification of type I hernias less than 3 cm in size with barium swallow radiography is unreliable. Furthermore because of a lack of standardization in the convention of when the size measurement of a type I hernia is taken with respect to deglutitive esophageal shortening, the magnitude of the size estimate has an inherent 2 cm error.
The Endoscopic Assessment of Sliding Hiatus Hernia
In general, there has been little uniformity or rigor applied to the assessment of sliding hiatus hernia with endoscopy. Sliding hiatus hernia is diagnosed when the apparent separation between the squamocolumnar junction and the diaphragmatic impression is greater than 2 cm as measured using the hash marks on the endoscope (spaced 5 cm apart) relative to the incisors (usually obscured by an opaque bite block). The accuracy and reproducibility of such measurements has not been tested, but are conceptually vulnerable to the same limitations as measurements made during barium swallow radiography. Added confounding influences are Barrett’s metaplasia that makes it more difficult to ascertain the location of the native SCJ, an extremely patulous hiatus that makes it difficult to precisely localize the crural diaphragm, and excess insufflation of the stomach that might exaggerate the apparent size of the hernia. The variability in endoscopic interpretation of signs consistent with reflux (including hiatus hernia) was recently the subject of an experiment in which 120 endoscopists were asked to interpret the identical 2-minute video of an upper endoscopy. Half of the participants were given a patient history consistent with reflux and the other half a patient history of epigastic pain; 42% of the group given the reflux history reported endoscopic findings consistent with reflux as opposed to only 12% given a history of epigastric pain [17].
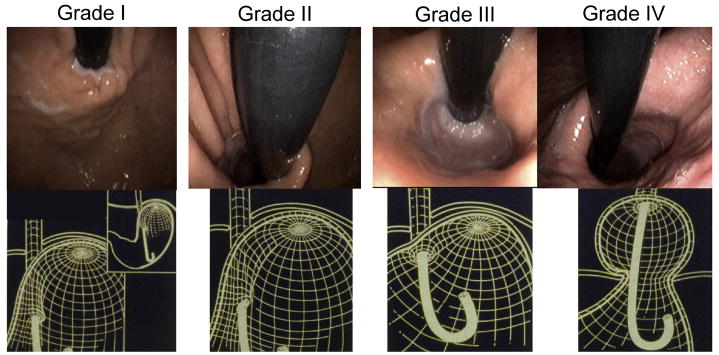
Another approach to the endoscopic grading of sliding hiatus hernia is to assess the appearance of the EGJ from a retroflexed position and to incorporate an assessment of hiatal integrity along with the assessment of axial displacement. The progression from normal anatomy to type I hernia was well illustrated in an analysis of “flap valve” integrity as a predictor of reflux symptoms (Figure 7) [18]. That analysis concluded that both reflux symptoms and EGJ competence (assessed in a post mortem experiment) were directly correlated to flap valve grade. Note that in Figure 7, only the grade IV flap valve constitutes a sliding hiatal hernia as the SCJ is still at or below the level of the hiatus in types I, II, and III. Differentiating among these types depends upon a subjective assessment of cardia integrity, the reproducibility of which has yet to be demonstrated. An attempt at objectifying that assessment utilized image analysis software to quantify the circumference of the gastric cardia using the size of the endoscope traversing the EGJ in the same image as a reference to correct for magnification [19]. That analysis of 273 endoscopies found a direct relationship between the magnitude of the cardia circumference as viewed in retroflexion and the presence (and severity) of GERD.
Figure 7.
Endoscopic appearance and corresponding three-dimensional representation of the progressive anatomic disruption of the gastroesophageal junction as occurs with development of a Type I hiatus hernia. In the grade I configuration, a ridge of muscular tissue is closely approximated to the shaft of the retroflexed endoscope. With a grade II configuration the ridge of tissue is slightly less well defined and there has been slight orad displacement of the squamocolumnar junction along with widening of the angle of His. In the grade III appearance the ridge of tissue at the gastric entryway is barely present and there is often incomplete luminal closure around the endoscope. Note, however, that this is not a hiatal hernia because the SCJ is not displaced axially in the endoscopic photograph. With grade IV deformity, no muscular ridge is present at the gastric entry. The gastroesophageal area stays open all the time, and squamous epithelium of the distal esophagus can be seen from the retroflexed endoscopic view. A hiatus hernia is always present with grade IV deformity. Modified from Hill, LD, Kraemer, SJM, Aye, RW, et al. Laparoscopic Hill repair. Contemporary Surgery, 1994;44:1.
In summary, there has been little study of the sensitivity or reproducibility of the endoscopic grading and measurement of sliding hiatus hernia. What information does exist suggests that endoscopy suffers from similar limitations to barium swallow radiography but is probably even more subjective because of additional confounding factors. Bits of recent data suggest that endoscopy may provide valuable information regarding the appearance of the EGJ during retroflexed imaging but this requires further study. Thus in practical terms, unless a strict protocol for measurement is tightly adhered to, the identification of type I hernias less than 3 cm in size with endoscopy is unreliable. Furthermore because of a lack of standardization in the convention of when the size measurement of a type I hernia is taken with respect to entry, exit, and the extent of gastric distention, the magnitude of the size estimate has an inherent 2 cm error.
The Manometric Assessment of sliding Hiatal Hernia
Manometric landmarks of the EGJ are different than either endoscopic or radiographic landmarks. The most notable features are: 1) that intragastric pressure is greater than intraesophageal pressure, especially during inspiration, 2) that the high pressure zone of the EGJ has both tonic (LES) and phasic (crural diaphragm) components, and 3) that respiration causes both intraluminal pressure changes and relative movement between pressure sensors and structural components of the EGJ. Thus, as one withdraws a catheter across the EGJ from the stomach, inspiration is associated with pressure augmentation when below the diaphragm and a fall in pressure when above it. The location at which this shifts is referred to as the pressure inversion point and, in the simplest case, this is the level of the crural diaphragm. However, great variability exists among individuals in: 1) the magnitude of LES pressure, 2) the magnitude of pressure augmentation associated with crural diaphragm contraction, 3) the magnitude of difference between intragastric and intraesophageal pressure and, most importantly 5) axial separation between the LES and the crural diaphragm. Hence, an inspiratory decrease in pressure can be indicative of a supradiaphragmatic location or it can result from movement within a high pressure zone from a locus of higher pressure to a locus of lower pressure. Thus, the pressure inversion point, an essential landmark in the definition of a sliding hiatal hernia, can mean more than one thing and is an inherently unreliable measurement.
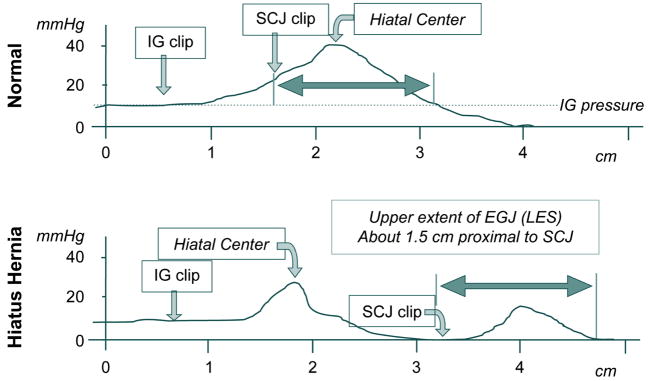
Although not practical for routine clinical assessment, endoclip studies can be used to circumvent most of the problems alluded to above. With such studies, the locations and pressure contributions of the LES and crural diaphragm can be localized within the EGJ pressure signature. Figure 8 illustrates two examples of such studies obtained from a normal individual and a subject with a sliding hernia [5]. The pull-throughs were done under fluoroscopy using a motorized puller to calibrate position, a manometric catheter with radio-opaque markings to correlate pressure locus with fluoroscopic landmarks, and endoclips located at the intragastric extreme of the EGJ and at the SCJ. The tracings were obtained during suspended respiration. It was thus possible to precisely correlate intraluminal pressure with the distal margin of the EGJ, the center of the hiatus, and the SCJ. Note that in the normal individual pressure effects of the diaphragm and LES are superimposed and indistinguishable while in the individual with a sliding hernia they are 2.5 cm apart allowing for each component to be individually characterized. Also note the variability in location of the drop from intragastric to intraesophageal pressure. In the normal individual, the pressure drop occurs proximal to the LES whereas in this hernia patient, the drop occurs within the hernia between the hiatus and the LES.
Figure 8.
Examples of manometric pull-through tracings with single (top) and double (bottom) peak axial pressure profiles. In each case, an endoclip was placed at the intragastric (IG) aspect of the EGJ and at the SCJ before the subject underwent a pull through under fluoroscopy with a manometric catheter that also had radio-opaque markers at the pressure recording sites. This allowed for correlation between the clipped anatomic landmarks, the pressure profile, and the fluoroscopic landmarks localized on the pull through tracings. Note the 2 cm separation between the hiatal center and the intragastric clip in the normal; this corresponds to the “submerged segment” of the EGJ. Also note that the proximal aspect of the EGJ pressure profile extends 1.5 cm proximal to the EGJ, corresponding to position of the A ring often evident in barium swallow radiography. Data from: Kahrilas PJ, Lin S, Chen J, Manka M. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut 1999;44:483–489.
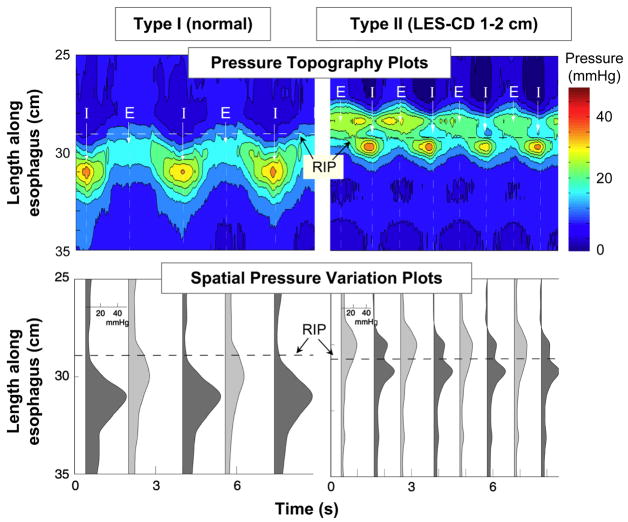
Although the pull-through tracings in Figure 8 are very useful in demonstrating the correlation between anatomy and intraluminal pressure, they represent only one instant in time during suspended respiration. Thus, the dynamics of the respiratory effect on EGJ cannot be easily appreciated. In order to see the whole picture, these pull-through recordings would have to be achieved in real time, during normal respiration. Such is the potential of high resolution manometry with the application of topographic plotting methods. By utilizing many closely spaced manometric pressure sensors and interpolating between adjacent sensors, the axial pressure profile with the EGJ can be visualized in real time. Evident in Figure 9, this facilitates the localization and quantification of the crural diaphragm contraction within the EGJ [20, 21]. In the normal individual (type I) the crural diaphragm effect is directly superimposed on the LES resulting in substantial pressure augmentation during respiration, the extremes of which are illustrated in the spatial pressure variation plots in the lower panels. One even appreciates that the LES and CD are tethered together by the downward displacement of the EGJ high pressure zone with inspiration.
Figure 9.
High resolution manometry examples of EGJ pressure morphology subtypes primarily distinguished by the extent of lower esophageal sphincter-crural diaphragm (LES-CD) separation. The upper plot in each panel is a pressure topography representation of the pressure changes spanning from the distal esophagus, across the EGJ, and into the proximal stomach during several respiratory cycles. The pressure scale is shown at the right. The lower plots illustrates a series of spatial pressure variation plots at the instants of peak inspiration (dark gray) and expiration (light gray) corresponding to the times marked I and E on the upper panels with pressure magnitudeon the x-axis and axial location along the y-axis. The location of the respiratory inversion point (RIP) is shown by the horizontal dashed line. Type I is characterized by complete overlap of the CD and the LES with a single pressure peak in the spatial pressure variation plots during both inspiration and expiration. The RIP lies at the proximal margin of the EGJ. Type II is characterized by minimal, but discernible, LES-CD separation making for a double peaked spatial pressure variation plot, but the nadir pressure between the peaks was still greater than gastric pressure. The RIP is within the EGJ at the proximal margin of the CD. Modified from: Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High resolution manometry of the EGJ: and analysis of crural diaphragm function in GERD. Am J Gastroenterol 2007;102:1056–1063.
The right panels of Figure 9 illustrate an example of low grade disruption of the EGJ (type II) such that there is quantifiable separation between the crural diaphragm and the LES, but the magnitude of this separation is insufficient to constitute a sliding hernia because the pressure minimum between peaks (lower panel) remains above gastric pressure and luminal closure is maintained along the entire length from above the LES to below the crural diaphragm. Of all of the methods for assessing sliding hiatus hernia, high resolution manometry is the only one capable of reliably detecting this condition, the intermediate stage between normal and overt sliding hiatus hernia.
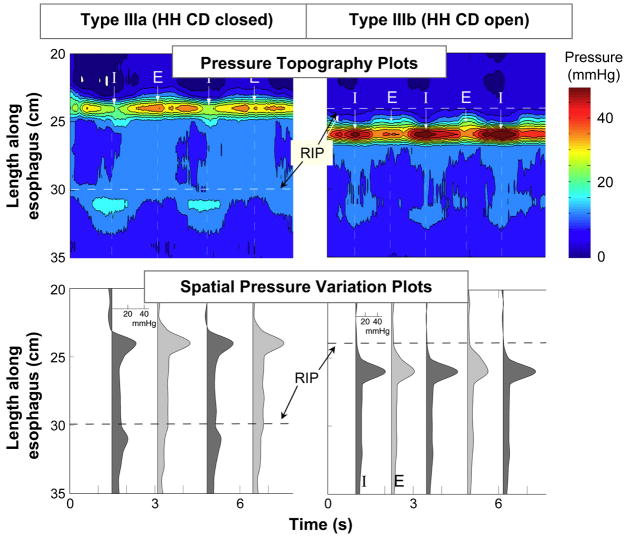
Progressive disruption of the EGJ results in further separation of the crural diaphragm and LES and an overt sliding hiatus hernia (Figure 10). When this separation exceeds about 2 cm, the pressure minimum between peaks in the spatial pressure variation plots (lower panels) is at or below gastric pressure. Also note the laxity of the fixation between the LES and the diaphragm. No longer does the LES pressure band exhibit downward displacement with inspiration. The distinction between a type IIIa and type IIIb hernia is in the position of the respiratory inversion point. The respiratory inversion point (RIP) was defined as the axial position along the EGJ at which the inspiratory EGJ pressure became less than the expiratory EGJ pressure. Conceptually, this is the position at which the external EGJ environment switches from intra-abdominal to intra-mediastinal pressure. With type IIIa this is still at the proximal boundary of the crural diaphragm whereas with a type IIIb hernia, the hiatus is so patulous as to never seal off the hernia pouch from the stomach with consequent migration of the RIP to the proximal margin of the LES.
Figure 10.
Same layout as Figure 10. EGJ type IIIa was defined when LES-CD separation was >2 cm at inspiration. This is the high resolution manometry signature of hiatus hernia. Two subtypes were discernible, IIIa and IIIb, with the distinction being that the respiratory inversion point was proximal to the CD with IIIa and proximal to the LES in IIIb. The shift in respiratory inversion point is likely indicative of a grossly patulous hiatus, open throughout the respiratory cycle. Minimal EGJ pressure increase reflecting CD contraction is observed during inspiration with either type. Modified from: Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High resolution manometry of the EGJ: and analysis of crural diaphragm function in GERD. Am J Gastroenterol 2007;102:1056–1063.
In summary, high resolution manometry objectifies the assessment of sliding hiatus hernia. For the first time, it offers a means to complete the continuum from normal to overt sliding hernia by detecting intermediate grades of EGJ disruption. It also offers a means for prolonged observation permitting the assessment of intermittent herniation in some individuals [22]. Using high resolution manometry with topographic plotting, three major subtypes of EGJ pressure morphology are demonstrable (Figures 9 and 10): type I with the crural diaphragm completely superimposed on the LES, type II with 1–2 cm separation between the two, and type III with greater than 2 cm separation. In a recent analysis, it was noted that EGJ type III was rarely found in asymptomatic controls or functional heartburn patients but was a frequent finding in GERD patients demonstrating the clinical significance to this diagnostic approach [21].
Summary
In conclusion, sliding hiatus hernia can be seen as one end of the spectrum of disruption of the EGJ in which there is widening of the diaphragmatic hiatus and axial displacement between the LES and crural diaphragm of sufficient magnitude that a pouch of stomach can be appreciated between the two, be it with endoscopy, radiography or manometry. However, sliding hiatus hernia is not an all or none phenomenon. Lesser degrees of disruption may be evident as simple dilatation of the hiatus without axial displacement between EGJ components or as lesser degrees of separation. Diagnosis of these more subtle abnormalities is challenging and likely at the root of the historic variability in reported incidence of sliding hiatus hernia. High resolution manometry stands to dramatically improve on this as it offers for the first time a means to localize the LES and crural diaphragm within the EGJ in real time, for prolonged periods, and without swallow or distention related artifact. Hence the accuracy of diagnosing sliding hiatus hernia greatly improves.
Practice Points
Type I (sliding) hiatus hernia results from laxity and loss of elasticity of the phrenoesophageal ligament
Endoscopy and radiography are relatively insensitive in the detection of small type I hiatus hernias because the exams trigger esophageal shortening and physiological herniation
High resolution manometry with pressure topography plotting allows for precise localization and quantification of the individual physiological elements of the esophagogastric junction with minimal perturbation
Research Agenda
The relationship between reflux disease and the detailed pressure morphology of the esophagogastric junction needs to be re-examined in light of the enhanced capabilities of high resolution manometry
Acknowledgments
This work was supported by R01 DC00646 (PJK) from the Public Health Service
Abbreviations
- HRM
High-resolution manometry
- LES
lower esophageal sphincter
- EGJ
esophagogastric junction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marchand P. The anatomy of esophageal hiatus of the diaphragm and the pathogenesis of hiatus herniation. Thorac Surg. 1959;37:81–92. [PubMed] [Google Scholar]
- 2.Barrett NR. Discussion on hiatus hernia. Proc Roy Soc Med. 1932;122:736–796. [Google Scholar]
- 3.Schatzki R. Die hernien des hiatus oesophageus. Deutsches Arch f klin med. 1932;173:85–103. [Google Scholar]
- 4.Low A. A note on the crura of the diaphragm and the muscle of Treitsz. J Anatomy Lond. 1907;42:93–96. [PMC free article] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Lin S, Chen J, Manka M. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut. 1999;44:483–489. doi: 10.1136/gut.44.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf BS. Sliding hiatal hernia: the need for redefinition. Am J Roentgenol. 1973;117:231–247. doi: 10.2214/ajr.117.2.231. [DOI] [PubMed] [Google Scholar]
- 7.Friedland GW. Historical review of the changing concepts of lower esophageal anatomy: 430B.C - 1977. Am J Roentgoenol. 1978;131:373–388. doi: 10.2214/ajr.131.3.373. [DOI] [PubMed] [Google Scholar]
- 8.Pandolfino JE, Shi G, Trueworthy B, Kahrilas PJ. Esophagogastric junction opening during relaxation distinguishes non-hernia reflux patients, hernia patients and normals. Gastroenterology. 2003;125:1018–1024. doi: 10.1016/s0016-5085(03)01210-1. [DOI] [PubMed] [Google Scholar]
- 9.Skinner DB. Hernias (hiatal, traumatic, and congenital) In: Berk JE, editor. Gastroenterology. Vol. 4. W. B. Saunders; Philadelphia: 1985. pp. 705–716. Chapter 53. [Google Scholar]
- 10.Pouderoux P, Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of esophageal shortening during esophageal peristalsis. Gastroenterology. 1997;112:1147–1154. doi: 10.1016/s0016-5085(97)70125-2. [DOI] [PubMed] [Google Scholar]
- 11.Shi G, Pandolfino JE, Joehl RJ, Brasseur JG, Kahrilas PJ. Distinct patterns of esophageal shortening during primary peristalsis, secondary peristalsis, and transient lower esophageal sphincter relaxations. Neurogastroenterol Mot. 2002;14:505–512. doi: 10.1046/j.1365-2982.2002.00351.x. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Zhang Q, Ghosh S, Han A, Boniquit C, Kahrilas PJ. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology. 2006;131:1725–1733. doi: 10.1053/j.gastro.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Kahrilas PJ, Wu S, Lin S, et al. Attenuation of esophageal shortening during peristalsis with hiatus hernia. Gastroenterology. 1995;109:1818–1825. doi: 10.1016/0016-5085(95)90748-3. [DOI] [PubMed] [Google Scholar]
- 14.Pandolfino JE, Schreiner MA, Lee TJ, Zang Q, Boniquit C, Kahrilas PJ. Comparison of the bravo wireless and digitrapper catheter-based pH monitoring systems for measuring esophageal acid exposure. Am J Gastro. 2005;100:1466–476. doi: 10.1111/j.1572-0241.2005.41719.x. [DOI] [PubMed] [Google Scholar]
- 15.Ott DJ, Gelfand DW, Chen YM, et al. Predictive relationship of hiatal hernia to reflux esophagitis. Gastrointest Rad. 1985;10:317–320. doi: 10.1007/BF01893120. [DOI] [PubMed] [Google Scholar]
- 16.Stilson WL, et al. Hiatal hernia and gastroesophageal reflux. A clinical analysis of more than 1000 cases. Radiology. 1969;93:1323–1327. doi: 10.1148/93.6.1323. [DOI] [PubMed] [Google Scholar]
- 17.Bytzer P. Information bias in endoscopic assessment. Am J Gastroenterol. 2007;102:1585–7. doi: 10.1111/j.1572-0241.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- 18.Hill LD, Kozarek RA, Stefan JM, et al. The gastroesophageal flap valve: in vitro and in vivo observations. G astrointest Endosc. 1996;44:541–547. doi: 10.1016/s0016-5107(96)70006-8. [DOI] [PubMed] [Google Scholar]
- 19.Seltman AK, Kahrilas PJ, Chang EY, Mori M, Hunter JG, Jobe BA. Endoscopic measurement of cardia circumference as an indicator of GERD. Gastrointest Endosc. 2006;63:22–31. doi: 10.1016/j.gie.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Pandolfino JE, Kim H, Ghosh S, Clarke JO, Zhang Q, Kahrilas PJ. High-Resolution Manometry of the EGJ: An Analysis of Crural Diaphragm Function in GERD. Am J of Gastro. 2007;102:1056–1063. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 21.Pandolfino JE, Kim H, Ghosh S, Clarke JO, Zhang Q, Kahrilas PJ. High-Resolution Manometry of the EGJ: An Analysis of Crural Diaphragm Function in GERD. Am J of Gastroenterol. 2007;102:1056–1063. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 22.Bredenoord AJ, Weusten BL, Timmer R, Smout AJ. Intermittent spatial separation of diaphragm and lower esophageal sphincter favors acidic and weakly acidic reflux. Gastroenterology. 2006;130:334–340. doi: 10.1053/j.gastro.2005.10.053. [DOI] [PubMed] [Google Scholar]