SUMMARY
The dynamin family of GTPases regulate mitochondrial fission and fusion processes and have been implicated in controlling the release of caspase activators from mitochondria during apoptosis. Here we report that profusion genes fzo-1 and eat-3, or the profission gene drp-1, are not required for apoptosis activation in C. elegans. However minor proapoptotic roles for drp-1 and fis-2, a homolog of human Fis1, are revealed in sensitized genetic backgrounds. drp-1 and fis-2 function independent of one another and the Bcl-2 homolog CED-9, and downstream of the CED-3 caspase, to promote elimination of mitochondria in dying cells, an event that could facilitate cell death execution. Interestingly, CED-3 can cleave DRP-1, which appears to be important for DRP-1’s proapoptotic function but not its mitochondria fission function. Our findings demonstrate that mitochondria dynamics do not regulate apoptosis activation in C. elegans and reveal distinct roles for drp-1 and fis-2 as mediators of cell death execution downstream of caspase activation.
INTRODUCTION
Genetic studies in C. elegans have led to the identification of a central killing pathway that is conserved between nematodes and humans (Horvitz, 1999). In cells destined to undergo apoptosis, the BH3-only proapoptotic protein EGL-1 is upregulated and binds to CED-9, an anti-apoptotic Bcl-2 homolog, resulting in the disassociation of CED-4, a mammalian Apaf-1 homolog, from the CED-4/CED-9 complex tethered on the surface of mitochondria (Horvitz, 1999). CED-4 subsequently oligomerizes and activates the CED-3 caspase zymogen (Yan et al., 2006), which then orchestrates numerous cell disassembly and cleanup processes, including fragmentation of chromosomal DNA (Parrish et al., 2001; Wang et al., 2002), and removal of cell corpses (Reddien and Horvitz, 2004).
The cell death activation process in mammals appears to be far more complex and involves release of proapoptotic factors from the intermembrane space of mitochondria. For example, Bcl-2 family proteins impinge on the structure of mitochondria, causing dramatic changes in mitochondria size, cristae structure, and ultimately increased permeability of the outer mitochondrial membrane to caspase activators such as cytochrome c and Smac/Diablo (Antignani and Youle, 2006; Cereghetti and Scorrano, 2006). In addition, dynamin family GTPases such as DRP1, which is required for mitochondrial fission, and OPA1 and MFN1/FZO1, which control inner and outer mitochondrial membrane fusion events, respectively, have been reported to mediate some of the structural rearrangements of mitochondria observed during apoptosis (Cereghetti and Scorrano, 2006; Chan, 2006). Downregulation of MFN1/FZO1 and concurrent activation of DRP1 was shown to cause dramatic fragmentation of the mitochondrial network during apoptosis (Frank et al., 2001; Karbowski et al., 2004; Karbowski et al., 2002), whereas disruption of OPA1 oligomers leads to remodeling of inner membrane cristae (Cipolat et al., 2006; Frezza et al., 2006). These changes in mitochondrial network and structure, in some cellular contexts (Frank et al., 2001; Frezza et al., 2006; Lee et al., 2004), but not in others (Delivani et al., 2006; Estaquier and Arnoult, 2007; Parone et al., 2006), appear to affect the release of cytochrome c from the mitochondrial intermembrane space and subsequent caspase activation. Fis1, which may recruit DRP1 to the outer mitochondrial membrane (Okamoto and Shaw, 2005), has also been suggested to mediate mitochondrial fission and apoptosis signaling in yeast (Fannjiang et al., 2004; Mozdy et al., 2000) and in mammals (Alirol et al., 2006; James et al., 2003; Lee et al., 2004; Parone et al., 2006). Recently, C. elegans drp-1 was reported to promote ced-9-dependent mitochondrial fission and apoptosis, possibly by releasing caspase activating factors from mitochondria (Jagasia et al., 2005), leading to the hypothesis that apoptotic mitochondrial fission is an evolutionarily conserved aspect of caspase activation. However, this hypothesis has yet to be substantiated by genetic analysis. As a result, the exact roles of mitochondrial fission and fusion processes in apoptosis, and their positions in the cell death pathway with respect to caspase activation and Bcl-2 family proteins, remain unclear (Parone and Martinou, 2006).
Here, we report systematic genetic and cell biological characterization of the contribution of mitochondrial fission and fusion processes to programmed cell death in C. elegans. Surprisingly, we found that loss of the mitochondrial dynamin genes, drp-1, fzo-1, and eat-3 (an OPA1 homolog), does not affect the activation or the kinetics of programmed cell death in C. elegans, even though loss of drp-1 blocks mitochondrial fission, and loss of fzo-1 or eat-3 prevents mitochondrial fusion and causes excessive mitochondrial fission. However, minor and independent roles for drp-1 and fis-2 (a Fis1 homolog) downstream of CED-3 activation in promoting mitochondrial elimination and cell death execution were uncovered in sensitized genetic backgrounds. Furthermore, we find that DRP-1 can be cleaved by CED-3 in vitro, and such cleavage appears to be important for DRP-1’s proapoptotic function in vivo, but not its mitochondrial fission function, suggesting that the pro-apoptotic function of DRP-1 can be separated from its mitochondrial fission function. Our results argue against a conserved, caspase activating role for mitochondrial dynamins and highlight distinct roles for drp-1 and fis-2 downstream of caspase activation.
RESULTS
drp-1, fzo-1 and eat-3 regulate mitochondrial fission and fusion in C. elegans
The C. elegans genome contains three genes, fzo-1, eat-3, and drp-1, which encode orthologs of MFN1/FZO1, OPA1, and DRP1, respectively. Two homologs of Fis1, fis-1 and fis-2, exist in the worm and they share a similar degree of sequence homology to the human and yeast Fis1 proteins (Figure S1). For each of these genes, we obtained deletion allele(s) that disrupt the respective coding sequences and are expected to be strong loss-of-function (lf) or null mutations (Figure S2).
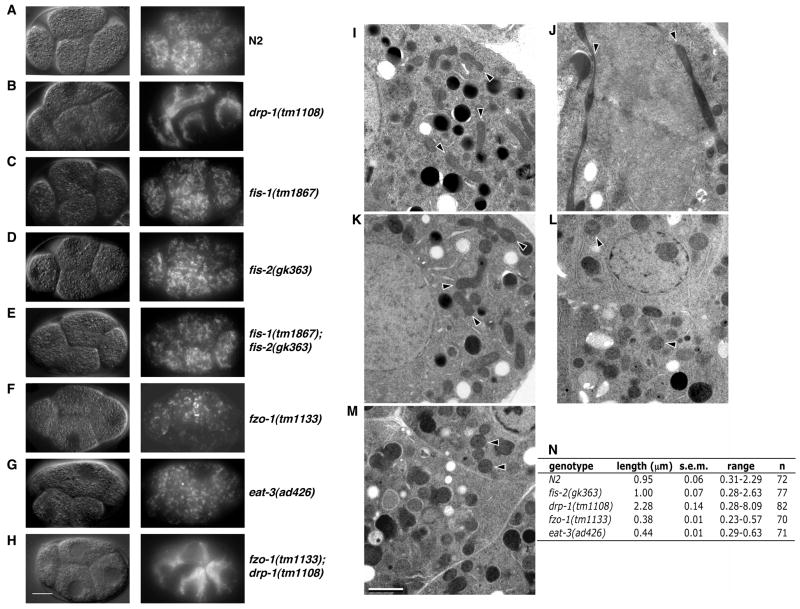
fzo-1(tm1133), eat-3(ad426) and eat-3(tm1107) animals exhibit slow growth, reduced brood sizes, and high percentages of embryonic lethality (Avery, 1993), probably due to compromised mitochondrial functions important for the vitality of the cells (Chan, 2006). The eat-3(ad426) mutation causes a Val 328 to Ile substitution within the GTPase domain (Avery, 1993; Breckenridge and Xue, unpublished results). drp-1(tm1108) animals also exhibit reduced brood sizes and a high percentage of embryonic lethality, whereas fis-1(tm1867), fis-1(tm2227), fis-2(gk363), and fis-2(tm1832) animals, as well as fis-1(tm1867); fis-2(gk363) double mutant animals, appear superficially wild type (data not shown). Staining of live embryos with tetramethyl rhodamine ester (TMRE), a mitochondria-specific dye, and thin section electron microcopy analysis, revealed striking differences in the overall connectivity of the mitochondrial network in embryos of the various mutant backgrounds (Figure 1, A to N). Compared to wild type N2 animals, TMRE-stained mitochondria in drp-1(tm1108) embryos appeared clumpy and highly fused (Figure 1, A and B), and the number of mitochondria observed in EM sections of drp-1(tm1108) embryos was low but individual mitochondria were often very long, with an increased longitudinal mean length of 2.28 μm, compared with 0.95 μm mean length in N2 animals (Figure 1, I, J, and N). These results suggest a reduction in mitochondrial fission in drp-1(tm1108) animals (Labrousse et al., 1999). Conversely, mitochondria appeared highly fragmented in fzo-1(tm1133), eat-3(ad426) and eat-3(tm1107) embryos (Figure 1, F and G, and data not shown). Electron micrographs of fzo-1(tm1133) and eat-3(ad426) embryos displayed an increased number of spherical mitochondria, with rather uniform length (mean length of 0.38 μm and 0.44 μm, respectively)(Fig 1, L and M). Therefore, fzo-1 and eat-3 appear to be required for mitochondrial fusion in C. elegans. In addition, mitochondria in eat-3(ad426) embryos had disrupted cristae structures (Figure S3), suggesting that like OPA1 in mammals EAT-3 is required for maintenance of mitochondrial cristae (Frezza et al., 2006). Mitochondria in the fzo-1(tm1133); drp-1(tm1108) double mutant were highly connected and indistinguishable from those in drp-1(tm1108) single mutant embryos (compare Figure 1B and 1H), indicating that drp-1 is required for the mitochondrial fragmentation observed in fzo-1 mutants (Bleazard et al., 1999). Similar mitochondrial morphologies were observed in body wall muscle cells, the germline, and the intestinal cells of each mutant strain (data not shown). The defects in mitochondrial morphology observed in drp-1(tm1108), fzo-1(tm1133), and eat-3(ad426) animals are similar to those described for loss-of-function mutants of their respective homologs in yeast, mouse and humans (Chan, 2006). Mitochondrial connectivity appears normal in fis-1(tm1867), fis-1(tm2227), fis-2(gk363), and fis-2(tm1832) single mutants, and in the fis-1(tm1867); fis-2(gk363) double mutant (Figure 1, C, D, E, and K and data not shown), suggesting that, unlike in yeast and mammals (Okamoto and Shaw, 2005; Chan, 2006), C. elegans fis genes are not required for mitochondrial fission. However, it is possible that fis-1 and fis-2 function redundantly with other unknown genes to regulate mitochondrial fission.
Figure 1.
Mitochondrial morphology in mitochondrial fission and fusion mutant embryos. (A–H) Live imaging of mitochondrial morphology. The indicated strains were stained with TMRE and four-cell stage embryos were visualized by Differential Interference Contrast (DIC, left) and rhodamine fluorescence (right) microscopy. Compared with the wild type embryo (A), the mitochondrial network is highly connected in the drp-1(tm1108) and fzo-1(tm1133); drp-1(tm1108) embryos (B, H), indistinguishable from that of the wild type embryo in fis-1(tm1867), fis-2(gk363), and fis-1(tm1867); fis-2(gk363) embryos (C–E), and highly fragmented in fzo-1(tm1133) and eat-3(ad426) embryos (F, G). Scale bar represents 10 μm. (I–M) Representative electron micrographs of embryos from the following strains are shown: N2 (I), drp-1(tm1108) (J), fis-2(gk363) (K), fzo-1(tm1133) (L), and eat-3(ad426) (M). Scale bar represents 1 μM. Arrows indicate the longitudinal axis of mitochondria. (N) Quantification of the mean mitochondrial length. Randomly selected mitochondria from electron micrographs were measured along their longitudinal axis. For each strain, mitochondria were selected from over 10 different embryos inside at least two thin-sectioned gravid adult animals. s.e.m., standard error of the mean. n, the number of mitochondria scored.
Cell death occurs normally in the absence of mitochondrial fission or fusion in C. elegans
We next examined the kinetics of programmed cell death in various mitochondrial fission and fusion mutants by conducting a time course analysis of cell corpse appearance during embryo development. In this assay, mutants strongly defective in cell death have few cell corpses at all stages of embryonic development (Ellis and Horvitz, 1986; Stanfield and Horvitz, 2000), mutants that are weakly defective in cell death often display a delay in cell corpse appearance or reduced cell corpse numbers (Parrish et al., 2001; Stanfield and Horvitz, 2000; Wang et al., 2002), and mutants defective in anti-apoptotic genes or genes involved in cell corpse engulfment have increased cell corpse numbers at all embryonic stages (Hedgecock et al., 1983; Ellis et al., 1991; Hengartner et al., 1992; Bloss et al., 2003). Although fzo-1(tm1133), eat-3(ad426), and eat-3(tm1107) animals had highly fragmented mitochondria (Figure 1), the numbers of cell corpses in these mutants were normal at all stages of embryonic development (Figure S4A), indicating that excessive mitochondrial fragmentation per se does not cause ectopic cell deaths in C. elegans. We confirmed this finding in a sensitized genetic background, ced-1(e1735), where engulfment of apoptotic cells is blocked and a small increase in cell deaths will result in a greater increase in the number of persistent cell corpses (Hedgecock et al., 1983; Ellis et al., 1991; Bloss et al., 2003)(Figure S4B). The cell corpse numbers or the kinetics of cell corpse appearance were also unaffected in drp-1(tm1108) animals (Figure S4C), even though mitochondria were constitutively fused (Figure 1). fis-1(tm1867), fis-2(gk363), and fis-2(tm1832) single mutants, and fis-1(tm1867); fis-2(gk363) double mutant animals also had cell corpse profiles that did not significantly differ from that of wild type animals (Figure S4C). Altogether, these results suggest that defects in mitochondria fusion or fission do not obviously affect programmed cell death in C. elegans.
We also examined whether any of these mutations can block cell death by counting the number of inappropriately surviving cells that normally die during the development of the anterior pharynx (Ellis and Horvitz, 1986; Wang et al., 2002). No extra surviving cells were found in the anterior pharynx of any of the individual mitochondrial fission or fusion mutants or in the fis-1(tm1867); fis-2(gk363) double mutant (Table 1). Thus, programmed cell death occurs normally in C. elegans in the absence of either mitochondrial fusion or fission.
Table 1.
Loss of individual mitochondrial fission or fusion genes does not cause inappropriate survival of cells that normally die in the anterior pharynx.
| Number of extra cells
|
||||
|---|---|---|---|---|
| Genotype | Mean | s.e.m. | Range | n |
| N2 | 0.1 | 0.1 | 0–1 | 40 |
| eat-3(ad426) | 0.1 | 0.1 | 0–1 | 32 |
| eat-3(tm1107) | 0.1 | 0.1 | 0–1 | 35 |
| fzo-1(tm1133) | 0.1 | 0.1 | 0–1 | 30 |
| fis-1(tm1867) | 0.1 | 0.1 | 0–1 | 30 |
| fis-1(tm2227) | 0.1 | 0.1 | 0–1 | 30 |
| fis-2(gk363) | 0.1 | 0.1 | 0–1 | 20 |
| fis-2(tm1832) | 0.2 | 0.1 | 0–1 | 30 |
| fis-1(tm1867); fis-2(gk363) | 0.1 | 0.1 | 0–1 | 20 |
| drp-1(tm1108) | 0.1 | 0.1 | 0–1 | 27 |
| drp-1(tm1108); fis-2(gk363) 1 | 0.6 | 0.2 | 0–2 | 23 |
| drp-1(tm1108); fis-2(tm1832) 2 | 0.5 | 0.1 | 0–2 | 31 |
The number of extra cells in the anterior pharynx of the indicated animals was counted as described in Experimental Procedures. s.e.m., standard error of the mean. Unpaired two tailed t-test, compared with N2 animals:
P=0.0017,
P=0.0011. Others have P values > 0.05.
drp-1 and fis-2 have minor, independent roles during programmed cell death
Our analysis of the drp-1(tm1108) mutant, which has no detectable DRP-1 protein expression (Figure S5) and thus represents a strong loss-of-function or null mutant, contrasts with the conclusion of a previous report that drp-1 plays a significant role in promoting apoptosis in C. elegans (Jagasia et al., 2005). The conclusion by Jagasia et al. was based on the observation that ectopic expression of a dominant-negative DRP-1(K40A) mutant resulted in the survival of 2–3 extra cells in the anterior pharynx of transgenic animals. The different cell death phenotypes observed between drp-1(tm1108) animals and animals overexpressing drp-1(K40A) may reflect an off-target effect of the dominant-negative mutant, since overexpresssion of DRP-1(K40A) induced a similar number of extra cells in drp-1(tm1108) animals (without endogenous DRP-1) as in wild-type animals (Table S1). This result indicates that the endogenous DRP-1 protein is not a target of DRP-1(K40A).
Since some weak cell death mutations that do not block cell death on their own can enhance the cell death defect caused by weak alleles of ced-3 or ced-4 (Parrish et al., 2001; Stanfield and Horvitz, 2000; Wang et al., 2002), we constructed strains carrying each of the mitochondrial fission or fusion mutant alleles in a weak ced-3 loss-of-function background (n2438) to determine if fzo-1, eat-3, drp-1, fis-1, or fis-2 might play a facilitatory role in programmed cell death. Analysis of fzo-1(tm1133); ced-3(n2438) animals and eat-3(ad426); ced-3(n2438) animals did not reveal a significant decrease or increase in the number of extra cells compared with ced-3(n2438) animals (Table 2), confirming that eat-3 and fzo-1 do not affect cell death in C. elegans. However, analysis of drp-1(tm1108) ced-3(n2438) animals revealed a small but significant increase in the number of extra cells over that observed in ced-3(n2438) animals; 2.5 extra cells were seen in the anterior pharynx of drp-1(tm1108) ced-3(n2438) animals compared to 1.3 extra cells seen in ced-3(n2438) animals (P < 0.005, unpaired two tailed t-test). A similar increase in extra cells was observed in ced-3(n2438); fis-2(gk363), ced-3(n2438); fis-2(tm1832), and fis-1(tm1867); ced-3(n2438); fis-2(gk363) animals, but not in fis-1(tm1867); ced-3(n2438) or fis-1(tm2227); ced-3(n2438) animals, suggesting that fis-2, but not fis-1, also affects apoptosis in the worm. The different effects of fis-1 and fis-2 on somatic apoptosis may be due to the fact that fis-1 expression is enriched in the germline, whereas fis-2 expression is enriched in the soma (Reinke et al., 2004). However, we have not detected a germ cell death defect in fis-1(tm1867) animals, even in sensitized genetic backgrounds (data not shown).
Table 2.
drp-1 and fis-2 have minor, independent roles during programmed cell death.
| Number of extra cells
|
||||
|---|---|---|---|---|
| Genotype | Mean | s.e.m. | Range | n |
| ced-3(n2438) | 1.3 | 0.2 | 0–4 | 40 |
| eat-3(ad426); ced-3(n2438) | 1.6 | 0.2 | 0–4 | 33 |
| fzo-1(tm1133); ced-3(n2438) | 1.5 | 0.3 | 0–5 | 20 |
| drp-1(tm1108) ced-3(n2438) 1 | 2.5 | 0.3 | 0–4 | 23 |
| fis-1(tm1867); ced-3(n2438) | 1.1 | 0.3 | 0–3 | 20 |
| fis-1(tm2227); ced-3(n2438) | 1.5 | 0.2 | 0–4 | 44 |
| ced-3(n2438); fis-2(gk363) 2 | 2.8 | 0.2 | 1–7 | 38 |
| ced-3(n2438); fis-2(tm1832) 3 | 2.5 | 0.2 | 1–4 | 26 |
| fis-1(tm1867); drp-1(tm1108) ced-3(n2438) | 2.4 | 0.3 | 0–4 | 20 |
| fis-1(tm1867); ced-3(n2438); fis-2(gk363) 4 | 2.6 | 0.4 | 1–6 | 20 |
| drp-1(tm1108) ced-3(n2438); fis-2(gk363) 5 | 3.9 | 0.2 | 2–6 | 20 |
| drp-1(tm1108) ced-3(n2438); fis-2(tm1832) 6 | 3.7 | 0.3 | 1–7 | 34 |
| ced-4(2273) | 1.6 | 0.3 | 0–4 | 25 |
| ced-4(n2273); fis-2(gk363) 7 | 2.9 | 0.2 | 1–4 | 25 |
| ced-4(n2273); drp-1(tm1108) 8 | 2.7 | 0.3 | 1–6 | 24 |
| ced-4(n2273); drp-1(tm1108); fis-2(gk363) 9 | 4.3 | 0.2 | 3–7 | 24 |
| ced-3(n2438); control(RNAi) | 1.5 | 0.1 | 0–4 | 50 |
| ced-3(n2438); wah-1(RNAi) 10 | 2.4 | 0.1 | 0–4 | 43 |
| ced-3(n2438); cps-6(RNAi) 11 | 2.4 | 0.2 | 0–5 | 44 |
| drp-1(tm1108) ced-3(n2438); control(RNAi) | 2.2 | 0.3 | 0–4 | 24 |
| drp-1(tm1108) ced-3(n2438); wah-1(RNAi) 12 | 3.9 | 0.2 | 1–6 | 45 |
| drp-1(tm1108) ced-3(n2438); cps-6(RNAi) 13 | 4.4 | 0.4 | 1–7 | 27 |
| ced-3(n2438); fis-2(gk363); control(RNAi) | 2.2 | 0.2 | 0–5 | 28 |
| ced-3(n2438); fis-2(gk363); wah-1(RNAi) 14 | 3.5 | 0.1 | 1–5 | 46 |
| ced-3(n2438); fis-2(gk363); cps-6(RNAi) 15 | 3.9 | 0.2 | 2–6 | 34 |
| drp-1(tm1108) ced-3(n2438); fis-2(gk363); control(RNAi) | 3.6 | 0.2 | 1–6 | 29 |
| drp-1(tm1108) ced-3(n2438); fis-2(gk363); wah-1(RNAi) 16 | 5.3 | 0.3 | 3–8 | 36 |
| drp-1(tm1108) ced-3(n2438); fis-2(gk363); cps-6(RNAi) 17 | 5.0 | 0.3 | 2–8 | 29 |
The number of extra cells in the anterior pharynx of the indicated animals was counted. All data involving the ced-3(n2438) strains were confirmed by multiple independent blind test analyses. s.e.m., standard error of the mean. Unpaired two tailed t-test, compared with ced-3(n2438) animals:
P=0.0012,
P=3.4×10−5,
P=6.3×10−5,
P=0.0018,
P=2.1×10−11,
P=2.7×10−9; compared with ced-4(n2273) animals:
P=0.006,,
P=0.043,,
P=3.0×10−9; compared with control(RNAi) treatment of the same strain:
P=8.8×10−5,
P=2.8×10−5,
P=1.3×10−5,
P=9.0×10−8,
P=6.3×10−6,
P=2.7×10−7,
P=8.7×10−6,
P=1.1×10−4. Others have P values > 0.05.
drp-1(tm1108) or fis-2(gk363) also weakly increased the number of extra cells observed in a weak ced-4(n2273) mutant background (Table 2), suggesting that drp-1 and fis-2 alleles can enhance the cell death defect in different genetic backgrounds. Thus, drp-1 and fis-2 play minor roles in cell death, which are only observable in sensitized genetic backgrounds. Importantly, a GFP::FIS-2 fusion protein localizes to mitochondria (Figure S6), suggesting that FIS-2, like DRP-1 (Labrousse et al., 1999), exerts its pro-apoptotic function at this organelle.
Given that in yeast and mammals DRP1 and FIS1 proteins can interact and promote mitochondrial fission (Okamoto and Shaw, 2005; Yu et al., 2005), it would seem likely that drp-1 and fis-2 function in the same cell death pathway in C. elegans. If so, animals doubly mutant for drp-1 and fis-2 would have the same cell death defect as either of the single mutants. Alternatively, if these two genes function in different cell death pathways, a more severe cell death defect would be observed in the drp-1; fis-2 double mutant. As shown in Figure S4C , drp-1(tm1108); fis-2(gk363) and drp-1(tm1108); fis-2(tm1832) double mutants had significantly fewer cell corpses than N2 and the single mutant animals, suggesting a reduction in cell death. Moreover, half of the drp-1(tm1108); fis-2(gk363) and drp-1(tm1108); fis-2(tm1832) animals had at least one and sometimes two extra cells in their anterior pharynx (a mean of 0.6 and 0.5 extra cell, respectively), which were only occasionally seen in N2 and the single mutant animals (Table 1), suggesting that some cells fail to undergo programmed cell death in these double mutants. Furthermore, drp-1(tm1108) and fis-2(lf) mutations additively enhanced the defect of the ced-3(n2438) mutant, resulting in an average of 3.7–3.9 extra cells in drp-1(tm1108) ced-3(n2438); fis-2(lf) animals, compared to 2.5–2.8 extra cells seen in drp-1(tm1108) ced-3(n2438) or ced-3(n2438); fis-2(lf) animals (Table 2, P < 0.005). drp-1(tm1108) and fis-2(gk363) mutations also additively enhanced the cell death defect of the ced-4(n2273) mutant (Table 2). Given that drp-1(tm1108) and fis-2(tm1832) mutations are putative null alleles (Figures S2, S5, and S7) and can additively reduce cell death, drp-1 and fis-2 likely function independent of each other during apoptosis. However, the possibility that they have partially redundant functions cannot be ruled out. Consistent with the proposal that drp-1 and fis-2 function independently during apoptosis, overexpression of FIS-2 could induce ectopic apoptosis in C. elegans independent of drp-1 (Table S2), whereas overexpression of a FIS-2(1–124) mutant lacking its transmembrane domain did not. On the other hand, overexpression of drp-1 did not induce ectopic apoptosis (Table S2), however, overexpression of drp-1 did cause excessive mitochondrial fission shortly after overexpression (data not shown) and could rescue the apoptosis and mitochondrial fission defects observed in drp-1(tm1108) ced-3(n2438) animals (Figure S8).
drp-1 and fis-2 act independently of ced-9 during programmed cell death
BAX, a mammalian proapoptotic Bcl-2 family member, was reported to promote mitochondria fission and co-localize with DRP1 at sites of mitochondrial fission during apoptosis (Frank et al., 2001; Karbowski et al., 2002), leading to the hypothesis that these two proteins function together to cause apoptotic mitochondrial fission (Youle and Karbowski, 2005). In C. elegans, Jagasia et al. reported that the Bcl-2 homolog ced-9 is required for drp-1-mediated mitochondrial fission and proposed that CED-9, like BAX, functions with DRP-1 to promote mitochondrial fragmentation and apoptosis by releasing caspase activating factors from mitochondria (Jagasia et al., 2005). A proapoptotic function for ced-9 was first suggested by the finding that in weak ced-3(lf) mutants, ced-9(lf) mutations unexpectedly increase, rather than decrease (as expected of its anti-apoptotic function), the number of extra surviving cells in the anterior pharynx (Hengartner and Horvitz, 1994). This finding was interpreted as evidence that ced-9 has both proapoptotic and antiapoptotic functions. If drp-1 or fis-2 acts in the ced-9 proapoptotic pathway, then drp-1(tm1108) and fis-2(gk363) alleles are not expected to increase the number of extra cells in the ced-9(n2812); ced-3(n2438) mutant, which contains a putative ced-9 null allele (Hengartner et al., 1992). As shown in Table 3, ced-9(n2812); ced-3(n2438) animals have an average of 7.2 extra cells in the anterior pharynx compared to 1.3 extra cells observed in ced-3(n2438) animals. ced-9(n2812); drp-1(tm1108) ced-3(n2438) animals had an average of 8.4 extra cells, displaying a small but significant increase in extra cells over ced-9(n2812); ced-3(n2438) animals (P=0.008, unpaired two-tailed t-test). In ced-9(n2812); ced-3(n2438); fis-2(gk363) animals, the number of extra cells was raised to 8.6, again significantly higher than that of the ced-9(n2812); ced-3(n2438) animals (P=0.0003, unpaired two-tailed t-test). Moreover, ced-9(n2812); drp-1(tm1108) ced-3(2438); fis-2(gk363) animals showed a further increase in extra cells to an average of 9.8 (Table 3). The fact that drp-1(tm1108) and fis-2(gk363) null alleles additively increase the number of extra cells observed in the absence of ced-9 activity strongly suggests that DRP-1 and FIS-2 function independently of CED-9 to promote programmed cell death in C. elegans.
Table 3.
drp-1 and fis-2 function independently of ced-9 during programmed cell death.
| Number of extra cells
|
||||
|---|---|---|---|---|
| Genotype | Mean | s.e.m. | Range | n |
| ced-3(n2438) | 1.3 | 0.2 | 0–4 | 20 |
| ced-9(n2812); ced-3(n2438) | 7.2 | 0.3 | 5–10 | 23 |
| ced-9(n2812); drp-1(tm1108) ced-3(n2438) 1 | 8.4 | 0.3 | 5–11 | 20 |
| ced-9(n2812); ced-3(n2438); fis-2(gk363) 2 | 8.6 | 0.2 | 6–10 | 20 |
| ced-9(n2812); ced-3(n2438) drp-1(tm1108); fis-2(gk363) 3 | 9.8 | 0.4 | 7–13 | 21 |
The number of extra cells in the anterior pharynx of the indicated animals was counted as described in Table 1. All strains carried the dpy-4(e1166) allele. All data were confirmed by multiple independent blind test analyses. s.e.m., standard error of the mean. Unpaired two-tailed t-test, compared with ced-9(n2812); ced-3(n2438) animals:
P=0.0084;
P=3.3×10−4,
P=2.3×10−6.
drp-1 and fis-2 act downstream of or in parallel to ced-3 to promote cell death
We conducted further genetic epistasis analysis to determine where drp-1 and fis-2 act in the programmed cell death pathway in relation to egl-1 and ced-3. Ectopic expression of egl-1 driven by the C. elegans heat-shock promoters (Phspegl-1) resulted in increased apoptosis in C. elegans embryos and the accumulation of over 40 cell corpses (Table 4). EGL-1-induced cell killing was completely blocked by a strong loss-of-function ced-3 mutation (n717) but was not affected by either the drp-1(tm1108) or the fis-2(gk363) mutation. EGL-1-induced killing, however, was significantly inhibited (approximately 40%) in the drp-1(tm1108); fis-2(gk363) double mutant, which is consistent with our previous observation that drp-1(tm1108) and fis-2(gk363) mutations additively reduce cell death. Similarly, expression of an activated form of CED-3 (acCED-3) under the control of the egl-1 promoter (Pegl-1acCED-3) induced ectopic cell deaths and an average of 28 cell corpses in the ced-1(e1735); ced-4(n1162) mutant (Table 4; Kokel et al., 2006). Since the ced-4(n1162) mutation abolishes almost all naturally occurring cell deaths in C. elegans (Ellis and Horvitz, 1986), cell corpses observed in the ced-1(e1735); ced-4(n1162) mutant are strictly acCED-3-induced cell deaths. As in the case of EGL-1-induced cell killing, acCED-3-induced apoptosis was not affected by either the drp-1(tm1108) mutation or the fis-2(gk363) mutation (Table 4), probably because loss of either gene alone is insufficient to block acCED-3-induced cell death. However, drp-1(tm1108); fis-2(gk363) double mutant animals displayed a 30% reduction in acCED-3-induced cell corpses (Table 4), again consistent with previous observations that these two mutations additively inhibit cell death. The observation that drp-1(tm1108) and fis-2(gk363) mutations together inhibit both EGL-1- and acCED-3-induced cell killing to a similar degree suggests that these genes function downstream of, or in parallel to, activated CED-3 to promote cell death execution. A role for drp-1 and fis-2 downstream of ced-3 is consistent with the very weak cell death defect observed in the drp-1(tm1108), fis-2(gk363), or fis-2(tm1832) mutant (Figure S4, Table 1, and Table 2), as CED-3 likely triggers multiple cell death execution pathways and disruption of only one of these downstream pathways is unlikely to block cell death (Parrish et al., 2001; Wang et al., 2002).
Table 4.
drp-1 and fis-2 function downstream of, or in parallel to, egl-1 and ced-3, and independently of cps-6 and wah-1.
| Number of extra cells
|
||||
|---|---|---|---|---|
| Genotype | Mean | s.e.m. | Range | n |
| N2 | 10.4 | 0.6 | 7–13 | 15 |
| N2 (heat-shock control) | 10.7 | 0.7 | 7–15 | 15 |
| Phspegl-1 | 40.7 | 2.8 | 25–61 | 15 |
| Phspegl-1; ced-3(n717) | 0.1 | 0 | 0–1 | 15 |
| Phspegl-1; drp-1(tm1108) | 39.7 | 2.3 | 30–64 | 15 |
| Phspegl-1; fis-2(gk363) | 38.7 | 1.6 | 35–51 | 15 |
| Phspegl-1; fis-1(tm1867); fis-2(gk363) | 37.6 | 2.0 | 31–56 | 15 |
| Phspegl-1; drp-1(tm1108); fis-2(gk363) 1 | 24.8 | 2.0 | 13–40 | 15 |
| Pegl-1acCED-3 | 27.7 | 1.1 | 12–41 | 36 |
| Pegl-1acCED-3; drp-1(tm1108) | 27.1 | 0.7 | 18–36 | 33 |
| Pegl-1acCED-3; fis-2(gk363) | 28.2 | 1.2 | 11–37 | 31 |
| Pegl-1acCED-3; drp-1(tm1108); fis-2(gk363) 2 | 19.6 | 1.1 | 7–28 | 34 |
| Pegl-1acCED-3; control(RNAi) | 29.0 | 1.1 | 20–36 | 21 |
| Pegl-1acCED-3; cps-6(RNAi) | 28.5 | 0.8 | 19–35 | 24 |
| Pegl-1acCED-3; wah-1(RNAi) | 27.7 | 1.1 | 17–35 | 23 |
| Pegl-1acCED-3; fis-2(gk363); control(RNAi) | 26.3 | 0.6 | 16–35 | 58 |
| Pegl-1acCED-3; fis-2(gk363); cps-6(RNAi) 3 | 22.3 | 0.9 | 14–31 | 24 |
| Pegl-1acCED-3; fis-2(gk363); wah-1(RNAi) 4 | 23.8 | 0.6 | 12–37 | 77 |
| Pegl-1acCED-3; drp-1(tm1108); fis-2(gk363); control(RNAi) | 21.8 | 0.8 | 13–35 | 47 |
| Pegl-1acCED-3; drp-1(tm1108); fis-2(gk363); cps-6(RNAi) 5 | 17.0 | 0.7 | 11–26 | 33 |
| Pegl-1acCED-3; drp-1(tm1108); fis-2(gk363); wah-1(RNAi) 6 | 16.2 | 0.7 | 9–26 | 30 |
Phspegl-1 (smIs82) is an integrated transgene containing the Phspegl-1 constructs. EGL-1 expression was activated by heat-shock treatment and the number of cell corpses in the head region of 1.5-fold stage embryos was scored. Pegl-1acCED-3 (smIs111) is an integrated transgene containing the Pegl-1acCED-3 construct (Kokel et al., 2006). acCED-3 is therefore expressed in cells normally destined to die. The number of cell corpses was counted in the head region of the 4-fold stage embryos. RNAi experiments were carried out as described in Experimental Procedures. All strains were in the ced-1(e1735); ced-4(n1162) background and confirmed by multiple independent blind test analyses. s.e.m., standard error of the mean. Unpaired two tailed t-test, compared with Phspegl-1 animals:
P = 8.7×10−5; compared with Pegl-1acCED-3 animals:
P=1.2×10−6; compared with control(RNAi) treatment of the same strain:
P=0.00035,
P=0.0063,
P=1.7×10−5,
P=1.5×10−6. Others have P values > 0.05.
drp-1 and fis-2 function independently of the cps-6/wah-1 DNA degradation pathway
A conserved aspect of apoptosis between C. elegans and humans is the role of apoptosis-inducing factor (AIF) and endonuclease G (wah-1 and cps-6 in C. elegans, respectively) in mediating apoptotic DNA degradation. AIF and endonuclease G reside in mitochondria of healthy cells but are released from mitochondria and translocate to the nucleus during apoptosis (Li et al., 2001; Susin et al., 1999). In C. elegans, CPS-6 and WAH-1 function together to promote apoptotic DNA degradation, most likely acting downstream of activated CED-3, since cps-6 was identified as a suppressor of acCED-3-induced cell deaths and WAH-1 is released from mitochondria in a ced-3-dependent manner (Parrish et al., 2001; Wang et al., 2002). We thus investigated whether drp-1 or fis-2 might function in the CPS-6/WAH-1 mitochondrial cell death pathway. Like the drp-1(tm1108) and fis-2(gk363) mutations, wah-1(RNAi) and cps-6(RNAi) weakly increased the number of extra cells observed in the anterior pharynx of ced-3(n2438) animals (Table 2; Parrish et al., 2001; Wang et al., 2002). Importantly, wah-1(RNAi) and cps-6(RNAi) also increased the number of extra cells observed in drp-1(tm1108) ced-3(n2438), ced-3(n2438); fis-2(gk363), and drp-1(tm1108) ced-3(n2438); fis-2(gk363) animals, compared with corresponding control(RNAi) treatment (Table 2). Moreover, cps-6(RNAi) or wah-1(RNAi) treatment of the fis-2(gk363) single mutant or drp-1(tm1108); fis-2(gk363) double mutant also resulted in stronger inhibition of acCED-3-induced cell killing than when control(RNAi) was used (Table 4). Taken together, these data suggest that drp-1 and fis-2 do not function in the cps-6/wah-1 DNA degradation pathway and that these genes may act in three independent mitochondrial pathways downstream of the CED-3 caspase to facilitate execution of cell death.
CED-3 cleaves DRP-1 in vitro and may activate DRP-1’s proapoptotic function in vivo
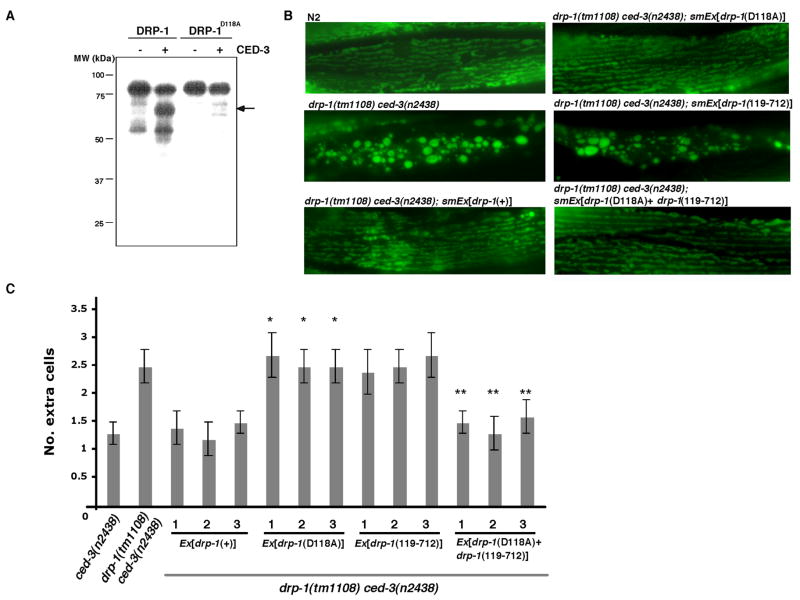
Given that DRP-1 and FIS-2 act downstream of the CED-3 caspase, we tested if they are CED-3 substrates. DRP-1, but not FIS-2, was cleaved by CED-3 in vitro: [35S]-Methionine labeled DRP-1 was cleaved by the recombinant CED-3 protease to create a major cleavage product of approximately 67 kDa (Fig. 2A and data not shown). Microsequencing of the amino terminus of this CED-3 cleavage product derived from cleavage of a recombinant DRP-1 protein revealed that proteolysis occurred between Asp118 and Arg119 (data not shown), with an adjacent tetrapeptide recognition motif (DETD) that is similar to the established CED-3 recognition motif (DEXD)(Thornberry et al., 1997). A mutant DRP-1 protein carrying an Asp118 to Ala substitution (DRP-1D118A) failed to be cleaved by CED-3 (Fig. 2A), confirming that D118 is the major CED-3 cleavage site in DRP-1.
Figure 2.
Cleavage of DRP-1 by CED-3 is important for the proapoptotic function of DRP-1 but not its function in regulating mitochondria fission. (A) DRP-1 can be cleaved by CED-3 in vitro. [35S] Methionine-labeled DRP-1 or DRP-1D118A was incubated with purified CED-3 protease. The 67 kDa major cleavage product is indicated by an arrow. (B) Cleavage of DRP-1 by CED-3 is not required for its function in regulating mitochondria fission. Representative images of mitoGFP in body wall muscle cells from wild type (N2), drp-1(tm1108) ced-3(n2438), and drp-1(tm1108) ced-3(n2438) animals carrying transgenes with a 3994 bp drp-1 genomic fragment [drp-1(+)], the drp-1 genomic fragment harboring the DRP-1D118A mutation [drp-1(D118A)], or a drp-1 construct expressing DRP-1119–712 [drp-1(119–712)] under the control of the drp-1 promoter, or transgenes containing both drp-1(119–712) and drp-1(D118A) (Supplemental Experimental Procedures). (C) Cleavage of DRP-1 by CED-3 at Asp118 is important for the proapoptotic function of DRP-1 in vivo. drp-1(tm1108) ced-3(n2438) animals carrying the indicated transgene were analyzed for the number of extra cells in the anterior pharynx. Three independent transgenic lines were examined for each construct. Error bars indicate s.e.m. (n = 20). Unpaired two tailed t-test, compared with ced-3(n2438) animals: * P < 0.005, compared to drp-1(tm1108) ced-3(n2438) animals: **P < 0.005
We then determined whether cleavage of DRP-1 by CED-3 is important for DRP-1’s proapoptotic function by introducing a construct containing a 3994 bp drp-1 genomic fragment [drp-1(+)] or the drp-1 fragment carrying the CED-3-resistant DRP-1D118A mutation [drp-1(D118A)] into drp-1(tm1108) ced-3(n2438) animals along with Pmyo-3mitoGFP. Pmyo-3mitoGFP drives the expression of mitochondrial matrix localized GFP in body wall muscle cells (Labrousse et al., 1999) and can be conveniently used to assess the rescue of the mitochondrial fission defect in drp-1(tm1108) ced-3(n2438) animals. As shown in Figure 2B, mitochondria in body wall muscle cells were labeled brightly by mitoGFP and appeared as connected clumps and blebs in drp-1(tm1108) ced-3(n2438) animals compared to the regular pattern of mitochondrial tubules observed in N2 animals. Both drp-1(+) and drp-1(D118A) transgenes completely rescued the mitochondria fission defect in drp-1(tm1108) ced-3(n2438) animals (Fig 2B), indicating that DRP-1D118A has normal mitochondrial fission activity. However, in contrast to the drp-1(+) transgene, which could rescue the enhanced cell death defect in drp-1(tm1108) ced-3(n2438) animals (a mean of 2.5 extra cells) back to the level of ced-3(n2438) animals (a mean of 1.3 extra cells), the drp-1(D118A) transgene failed to do so (Figure 2C), even though DRP-1 and DRP-1D118A were expressed at similar levels (Figure S9). The drp-1(D118A) transgene also failed to rescue the enhanced cell death defect in ced-4(n2273); drp-1(tm1108) animals back to the level seen in ced-4(n2273) animals (Figure S10). Expression of the predicted caspase cleavage fragments of DRP-1 (DRP-11–118 and DRP-1119–712) either alone or together failed to rescue the mitochondrial fission and cell death defects in drp-1(tm1108) ced-3(n2438) animals (Figure 2C, Figure S11, and data not shown). However, the enhanced cell death defect in drp-1(tm1108) ced-3(n2438) animals was completely rescued by co-expression of DRP-1119–712 with DRP-1D118A, even though expression of each protein alone had no rescuing activity (Figure 2C). The fact that drp-1(D118A) completely rescues the mitochondrial fission defect in drp-1(tm1108) ced-3(n2438) animals, but not the apoptosis defect caused by drp-1(tm1108), and that co-expression of DRP-1119–712 and DRP-1D118A could bypass the requirement for DRP-1 cleavage in drp-1(tm1108) ced-3(n2438) animals, suggests that the pro-apoptotic function of DRP-1 is dependent on its cleavage by the CED-3 caspase and that one of the CED-3 cleavage products, DRP-1119–712, functions with full-length DRP-1 to exert its proapoptotic effect. These results also provide direct evidence that DRP-1 acts downstream of the CED-3 caspase to promote apoptosis.
Changes in mitochondria in apoptotic cells
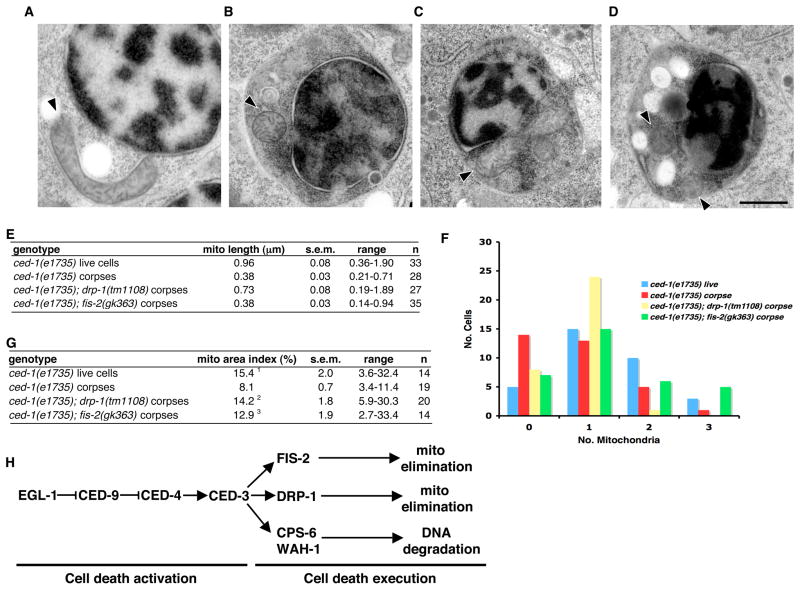
To determine the roles of drp-1 and fis-2 in apoptosis, we performed EM analysis of mitochondria in apoptotic cells of drp-1(tm1108) and fis-2(gk363) embryos that also carried a ced-1(e1735) mutation. ced-1(e1735) disables cell corpse engulfment and allows sampling of more cell corpses (Hedgecock et al., 1983). Serial EM sections through ced-1(e1735), ced-1(e1735); drp-1(tm1108), or ced-1(e1735); fis-2(gk363) 4-fold stage embryos were obtained and the mean mitochondrial length (Figure 3E), the number of mitochondria observed in cell corpses (Figure 3F), and the percentage of mitochondrial content in the cell corpses (mitochondria area index) (Figure 3G) were quantified. Living cells in ced-1(e1735) embryos typically had one to three large mitochondria, with a mean mitochondrial length of 0.96 μm, and a 15.4% mitochondria area index (Figure 3, A, E–G). In contrast, 58% of ced-1(e1735) cell corpses observed in EM sections contained 1–2 small and spherical mitochondria (mean length 0.38 μm, Figure 3, B and E) and 42% of them had no identifiable mitochondria at all (Figure 3F). In addition, the mitochondria area index was reduced to 8.1% in ced-1(e1735) cell corpses (Figure 3G). These results suggest that mitochondria are reduced or lost during apoptosis, which would eliminate cellular energy production and thus contribute to the demise of the cell (Arnoult et al., 2005; Skulachev et al., 2004). Interestingly, the mean length of remaining mitochondria in ced-1(e1735) cell corpses was close to the size of mitochondria in fzo-1(tm1133) or eat-3(ad426) living cells (0.38 μm and 0.44μm, Fig. 1N), again indicating that fragmentation of mitochondria per se does not cause apoptosis but rather is a consequence of the apoptotic cell disassembly process. In cell corpses of ced-1(e1735); drp-1(tm1108) embryos, we observed both large, elongated and small, spherical mitochondria (Figure 3C and Figure S12), with a mean length of 0.73 μm (Figure 3E), which is significantly shorter than that in drp-1(tm1108) living cells (2.28 μm, Figure 1N). This observation suggests that mitochondria breakdown or fragmentation can still occur in the drp-1(tm1108) apoptotic cells in the absence of the DRP-1 activity but may not proceed to completion due to the much larger size of mitochondria before the onset of apoptosis. However, there was no obvious reduction of the mitochondrial area index (14.2%) in ced-1(e1735); drp-1(tm1108) cell corpses. drp-1 may therefore affect elimination of mitochondria during apoptosis. Mitochondria were small and spherical in ced-1(e1735); fis-2(gk363) cell corpses, with a mean length similar to that observed in ced-1(e1735) cell corpses (Figure 3, D and E). However the number of mitochondria observed in ced-1(e1735); fis-2(gk363) cell corpses was generally higher than in ced-1(e1735) corpses (Figure 3F), with fewer instances of no mitochondria observed and more instances of 2–3 mitochondria observed in single cross sections of a corpse. Consistently, there was little reduction in mitochondria area index in ced-1(e1735); fis-2(gk363) cell corpses (Figure 3G), suggesting that fis-2 is also involved in the removal or elimination of mitochondria from dying cells. Mitochondria in cell corpses did not appear swollen, nor did we observe rupture of the outer mitochondrial membrane or obviously remodeled cristae (Figure 3, A to D). Therefore, some of the apoptotic mitochondrial morphology transitions reported in mammals (Scorrano et al., 2002) may not occur in C. elegans.
Figure 3.
Electron microscopy analysis of mitochondria in cell corpses of drp-1 and fis-2 mutants. Representative EM micrographs from serial sections of ced-1(e1735) living cells (A), cell corpses in ced-1(e1735) (B), ced-1(e1735); drp-1(tm1108) (C), and ced-1(e1735); fis-2(gk363) (D) 4-fold stage embryos are shown. Arrowheads denote the longitudinal axis of mitochondria. Compared with small, spherical mitochondria seen in ced-1(e1735) and ced-1(e1735); fis-2(gk363) corpses, some larger, elongated mitochondria are seen in the ced-1(e1735); drp-1(tm1108) cell corpses. Scale bar represents 0.5 μm. (E) Quantification of the mitochondrial length in cell corpses. The mean mitochondria length, range of mitochondria length, and standard error of the mean (s.e.m.) are shown. n, the number of mitochondria scored. (F) Quantification of the number of mitochondria in cell corpses. Randomly chosen EM sections of ced-1(e1735) living cells, or cell corpses in the indicated mutant embryos were analyzed and the number of mitochondria in each section was counted (n = 33). Data are shown as the number of cells or cell corpses (Y axis) containing a particular number of mitochondria (X axis). (G) Comparison of the mitochondrial area index. The mitochondrial area index represents the percentage of total mitochondria content within the whole cell. The mean mitochondrial area index, the range of the index, the standard error of the mean (s.e.m.), and the number of corpses scored (n) are shown. Unpaired two tailed t-test, compared with ced-1(e1735) cell corpses: 1P = 4.4×10−4; 2P = 7.6×10−4, 3P = 1.1×10−2. (H) A model for programmed cell death in C. elegans. In cells programmed to die, EGL-1 antagonizes the CED-4-inhibitory activity of CED-9, allowing CED-4 to activate the CED-3 zymogen. Activated CED-3 triggers three independent cell death pathways derived from the mitochondrion, mediated by DRP-1, FIS-2, and CPS-6/WAH-1. Cleavage of DRP-1 by CED-3 is important for DRP-1’s pro-apoptotic function, which may contribute to the removal of mitochondria. FIS-2 may also independently promote the elimination of mitochondria from dying cells. The release of CPS-6 and WAH-1 from mitochondria and translocation to the nucleus triggers apoptotic DNA degradation (Parrish et al., 2001; Wang et al., 2002).
DISCUSSION
Mitochondrial fission and fusion processes have recently been proposed as important mechanisms in regulating the release of mitochondrial apoptogenic factors such as cytochrome c and subsequent activation of caspases in C. elegans, D. melanogaster, and mammals (Abdelwahid et al., 2007; Frank et al., 2001; Frezza et al., 2006; Jagasia et al., 2005; James et al., 2003; Karbowski et al., 2004; Karbowski et al., 2002; Lee et al., 2004; Sugioka et al., 2004). However, a comprehensive genetic analysis of the roles of mitochondrial dynamin genes in apoptosis has not been reported. Using live imaging and high-resolution electron microscopy, we show that mitochondria are constitutively fragmented in C. elegans animals deficient in the profusion genes fzo-1 and eat-3 and that normal cristae structure is lost in the eat-3 mutant. However, no excess cell death is observed in these mutants, despite the fact that mitochondria in living cells of these animals are of a similar size to those observed in apoptotic cells (Fig. 1N and Fig. 3E). On the other hand, in the drp-1(tm1108) strong loss-of-function mutant, mitochondria are fused together into a long, connected network, yet programmed cell death appears to occur normally, although a mild cell death defect could be detected in sensitized genetic backgrounds. These observations argue against a casual relationship between the connectivity of the mitochondrial network and the extent of programmed cell death and the hypothesis that the mitochondrial fission process activates apoptosis in C. elegans.
Our study reaches a different conclusion from a previous report (Jagasia et al., 2005), which suggested that DRP-1-mediated mitochondrial fission functions upstream of caspase activation in C. elegans. The different conclusions could be attributed to the different methods used to inactivate the drp-1 gene. Our finding was based on analysis of the drp-1 null mutant, whereas the other study was based on analysis of animals overexpressing a dominant-negative DRP-1(K40A) mutant. However, we found that overexpression of DRP-1(K40A) still inhibits apoptosis in drp-1(tm1108) animals that have no endogenous drp-1 protein and have no cell death defect on its own (Table S1), suggesting that DRP-1(K40A) may have an off-target effect on apoptosis. Furthermore, we found that overexpression of drp-1 failed to induce ectopic apoptosis (Table S2), which is consistent with the findings that cleavage of DRP-1 by CED-3 could be important for activating DRP-1’s pro-apoptotic function and that ectopic apoptosis is not observed in either fzo-1(tm1133) or eat-3(ad426) animals (Figure S4), where mitochondria are highly fragmented. These findings underscore the need to study mitochondrial dynamins in a physiological context.
In mammals, the profusion protein OPA1 forms oligomers that maintain tight cristae junctions, preventing release of cytochrome c from cristae folds until cells are committed to die (Cipolat et al., 2006; Frezza et al., 2006). This OPA1 checkpoint does not appear to exist in the worm, possibly because CED-4 can directly activate the CED-3 zymogen independent of cytochrome c or other mitochondrial factors (Yan et al., 2005). DRP1-mediated mitochondrial fission has been proposed to be an important cellular event that occurs upstream of cytochrome c release and caspase activation in mammals (Frank et al., 2001) and an event that may activate the CED-3 caspase in C. elegans via a mechanism dependent on a novel ced-9 cell killing activity (Jagasia et al., 2005). However, this proposed ced-9-dependent, caspase-activating role by DRP-1-mediated mitochondria fission is contradicted by the following findings: 1) drp-1 promotes apoptosis independent of ced-9 in C. elegans (strong loss-of-function drp-1 and ced-9 mutations additively cause defects in cell death; Table 3); 2) drp-1 genetically acts downstream of the ced-3 caspase (Table 4) and DRP-1 can be cleaved by the CED-3 caspase; and 3) the CED-3-resistant DRP-1D118A mutant has normal mitochondrial fission function but is defective in its proapoptotic function (Figure 2), providing strong evidence that DRP-1 and mitochondria fission do not regulate CED-3 activation. Instead, we propose that CED-9 functions at an early step to regulate caspase activation by preventing the formation of CED-4 oligomers needed for CED-3 activation (Chinnaiyan et al., 1997; Conradt and Horvitz, 1998; Parrish et al., 2000; Yan et al., 2005), whereas drp-1 and other genes such as fis-2 and cps-6/wah-1 act late in the apoptosis program, after the CED-3 caspase is already activated, to independently mediate various important cell death execution events, including removal of mitochondria and fragmentation of chromosomes (Figure 3H), all of which concertedly facilitates the demise of a cell.
The downstream cell dismantling pathways mediated by drp-1, fis-2, and cps-6/wah-1 may be conserved between C. elegans and mammals. Indeed, CPS-6/endoG and WAH-1/AIF are conserved regulators of the apoptotic DNA degradation event (Susin et al., 1999; Parrish et al., 2001; Li et al., 2001; Wang et al., 2002) and mitochondrial release of AIF and WAH-1 appears to occur after caspase activation in both C. elegans and mammalian apoptosis (Wang et al., 2002; Arnoult et al., 2003; Lakhani et al., 2006). In C. elegans, CED-3 could cleave a fraction of DRP-1, leading to the formation of DRP-1 complexes containing both full-length DRP-1 and DRP-1119–712, which may redirect DRP-1 activity towards apoptotic mitochondrial elimination. Thus, the primordial apoptotic role for drp-1 may be to eliminate mitochondria after caspase activation, although Drp1 may have evolved an additional apoptotic role in mediating the release of cytochrome c from mitochondria in mammals (Cassidy-Stone et al., 2008; Frank et al., 2001). Altogether, our findings underscore an important role of mitochondria in the cell disassembly processes rather than the activation of apoptosis in C. elegans.
EXPERIMENTAL PROCEDURES
Counting of cell corpses and extra cells
The number of cell corpses in the head region of living embryos or L1 larvae and the number of extra surviving cells in the anterior pharynx of L4 larvae were determined as described previously (Hengartner et al., 1992; Stanfield and Horvitz, 2000). Statistical analysis was preformed using Microsoft Excel 2004 software.
DRP-1 antibody
cDNA fragments encoding the full-length or the first 270 residues of DRP-1 were subcloned into the pGEX-4T-3 vector (Amersham Biosciences) and expressed in E. coli BL21 strain. Recombinant GST-DRP-1 proteins were purified from the soluble fraction of the bacterial lysates using Glutathione Sepharose™ 4B beads (GE Healthcare). Rats were immunized with a mixture of purified GST-DRP-1 and GST-DRP-1(1–270) proteins and terminal bleeds were affinity purified against GST-DRP-1(1–270) as previously described (Wang et al., 2007).
CED-3 cleavage assay
1 μl of [35S] Methionine-labeled DRP-1 and DRP-1D118A (TNT Reticulocyte Lysate, Promega) was incubated with 5 ng of recombinant CED-3 protein in 10 μl of CED-3 buffer [50 mM Tris HCl (pH 8.0), 0.5 mM EDTA, 0.5 mM sucrose, and 5% glycerol] at 37°C for 2 hours. The reactions were terminated by the addition of SDS gel loading buffer, resolved on a 10% SDS-PAGE gel, and subjected to autoradiography.
Live imaging of C. elegans mitochondria and electron microscopy
Labeling of mitochondria with tetramethylrhodamine ester (TMRE; Molecular Probes) was carried out as previously described (Jagasia et al., 2005). Imaging of MitoGFP in body wall muscle cells was done as described previously (Labrousse et al., 1999). Detailed protocols for visualization of mitochondria by electron microscopy and quantification of mitochondrial length, mitochondria number in cell corpses, and mitochondrial area index are described in Supplemental Data.
Supplementary Material
Supplemental data includes additional experimental procedures, 12 additional supplemental figures, and two supplemental tables.
Acknowledgments
We thank Eric Griffiths for technical assistance, T. Blumenthal and members of the Xue lab for comments and discussions, Caenorhabditis Genetics Center for strains, and Y. Kohara for drp-1 and fis-2 cDNAs. This research was supported by fellowships from the Jane Coffins Child Memorial Fund for Medical Research (D.G.B) and the Canadian Institutes of Health Research (D.G.B), the Burroughs Wellcome Fund Career Award (D.X.), and the NIH R01 GM059083 and GM079097 grants (D.X.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K. Mitochondrial disruption in Drosophila apoptosis. Dev Cell. 2007;12:793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Alirol E, James D, Huber D, Marchetto A, Vergani L, Martinou JC, Scorrano L. The mitochondrial fission protein hFis1 requires the endoplasmic reticulum gateway to induce apoptosis. Mol Biol Cell. 2006;17:4593–4605. doi: 10.1091/mbc.E06-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Arnoult D, Karbowski M, Youle RJ. Caspase inhibition prevents the mitochondrial release of apoptosis-inducing factor. Cell Death Differ. 2003;10:845–849. doi: 10.1038/sj.cdd.4401240. [DOI] [PubMed] [Google Scholar]
- Arnoult D, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J, Sheng M, Blackstone C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15:2112–2118. doi: 10.1016/j.cub.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw JM. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss TA, Witze ES, Rothman JH. Suppression of CED-3-independent apoptosis by mitochondrial betaNAC in Caenorhabditis elegans. Nature. 2003;424:1066–1071. doi: 10.1038/nature01920. [DOI] [PubMed] [Google Scholar]
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM, Scorrano L. The many shapes of mitochondrial death. Oncogene. 2006;25:4717–4724. doi: 10.1038/sj.onc.1209605. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, O’Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Conradt B, Horvitz HR. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Jacobson DM, Horvitz HR. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics. 1991;129:79–94. doi: 10.1093/genetics/129.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14:1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, Hill RB, Basanez G, Hardwick JM. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004;18:2785–2797. doi: 10.1101/gad.1247904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Goyal G, Fell B, Sarin A, Youle RJ, Sriram V. Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster. Dev Cell. 2007;12:807–816. doi: 10.1016/j.devcel.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock EM, Sulston JE, Thomson JN. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science. 1983;220:1277–1279. doi: 10.1126/science.6857247. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356:494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR. Activation of C. elegans cell death protein CED-9 by an amino-acid substitution in a domain conserved in Bcl-2. Nature. 1994;369:318–320. doi: 10.1038/369318a0. [DOI] [PubMed] [Google Scholar]
- Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701s–1706s. [PubMed] [Google Scholar]
- Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol. 2004;164:493–499. doi: 10.1083/jcb.200309082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel D, Li Y, Qin J, Xue D. The nongenotoxic carcinogens naphthalene and para-dichlorobenzene suppress apoptosis in Caenorhabditis elegans. Nat Chem Biol. 2006;2:338–345. doi: 10.1038/nchembio791. [DOI] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone PA, Martinou JC. Mitochondrial fission and apoptosis: an ongoing trial. Biochim Biophys Acta. 2006;1763:522–530. doi: 10.1016/j.bbamcr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Parrish J, Li L, Klotz K, Ledwich D, Wang X, Xue D. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature. 2001;412:90–94. doi: 10.1038/35083608. [DOI] [PubMed] [Google Scholar]
- Parrish J, Metters H, Chen L, Xue D. Demonstration of the in vivo interaction of key cell death regulators by structure-based design of second-site suppressors. Proc Natl Acad Sci USA. 2000;97:11916–11921. doi: 10.1073/pnas.210391597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Horvitz HR. The engulfment process of programmed cell death in caenorhabditis elegans. Annu Rev Cell Dev Biol. 2004;20:193–221. doi: 10.1146/annurev.cellbio.20.022003.114619. [DOI] [PubMed] [Google Scholar]
- Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Skulachev VP, Bakeeva LE, Chernyak BV, Domnina LV, Minin AA, Pletjushkina OY, Saprunova VB, Skulachev IV, Tsyplenkova VG, Vasiliev JM, et al. Thread-grain transition of mitochondrial reticulum as a step of mitoptosis and apoptosis. Mol Cell Biochem . 2004;256–257:341–358. doi: 10.1023/b:mcbi.0000009880.94044.49. [DOI] [PubMed] [Google Scholar]
- Stanfield GM, Horvitz HR. The ced-8 gene controls the timing of programmed cell deaths in C. elegans. Mol Cell. 2000;5:423–433. doi: 10.1016/s1097-2765(00)80437-2. [DOI] [PubMed] [Google Scholar]
- Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–52734. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang J, Gengyo-Ando K, Gu L, Sun CL, Yang C, Shi Y, Kobayashi T, Shi Y, Mitani S, et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol. 2007;9:541–549. doi: 10.1038/ncb1574. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang C, Chai J, Shi Y, Xue D. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science. 2002;298:1587–1592. doi: 10.1126/science.1076194. [DOI] [PubMed] [Google Scholar]
- Yan N, Chai J, Lee ES, Gu L, Liu Q, He J, Wu JW, Kokel D, Li H, Hao Q, et al. Structure of the CED-4-CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nature. 2005;437:831–837. doi: 10.1038/nature04002. [DOI] [PubMed] [Google Scholar]
- Yan N, Xu Y, Shi Y. 2:1 Stoichiometry of the CED-4-CED-9 complex and the tetrameric CED-4: insights into the regulation of CED-3 activation. Cell Cycle. 2006;5:31–34. doi: 10.4161/cc.5.1.2263. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Yu T, Fox RJ, Burwell LS, Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J Cell Sci. 2005;118:4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data includes additional experimental procedures, 12 additional supplemental figures, and two supplemental tables.