Abstract
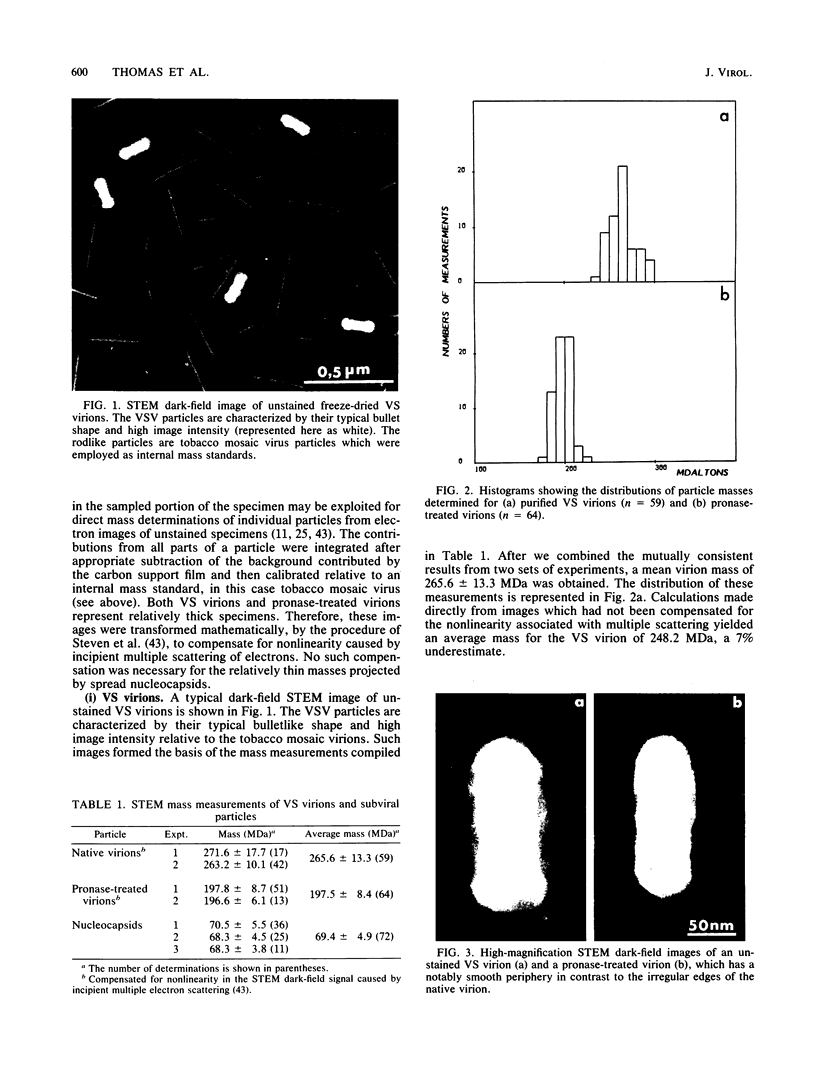
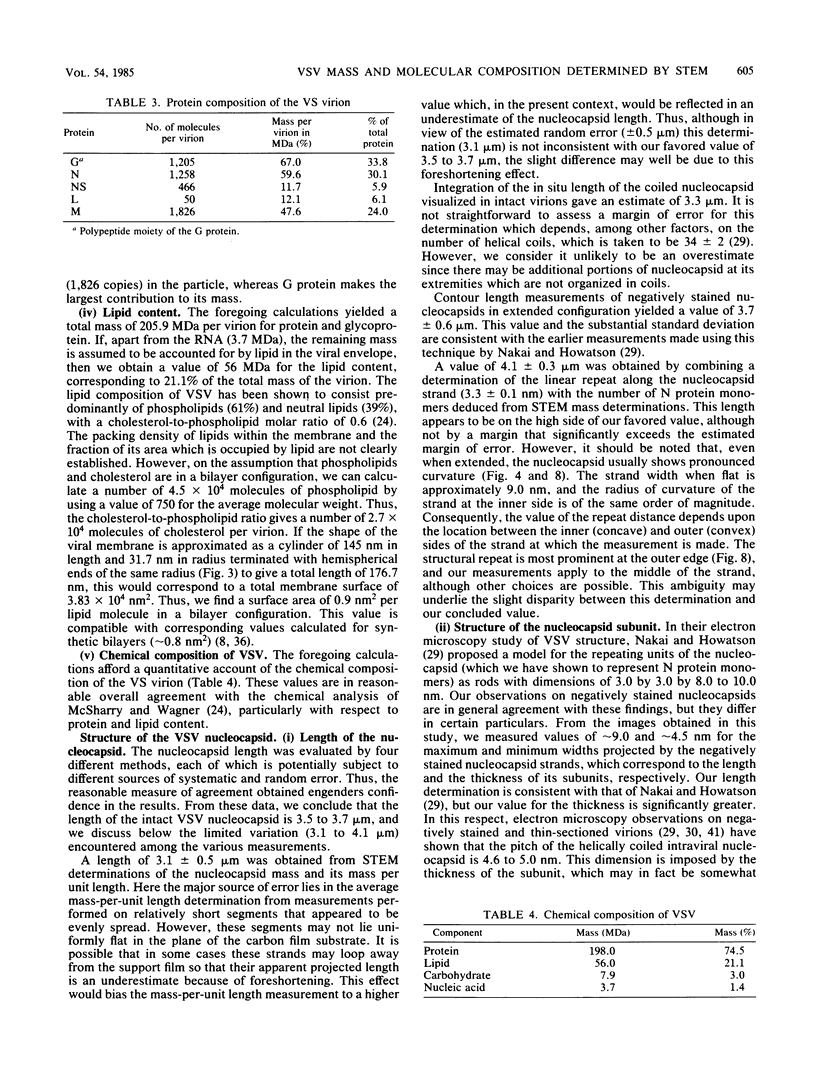
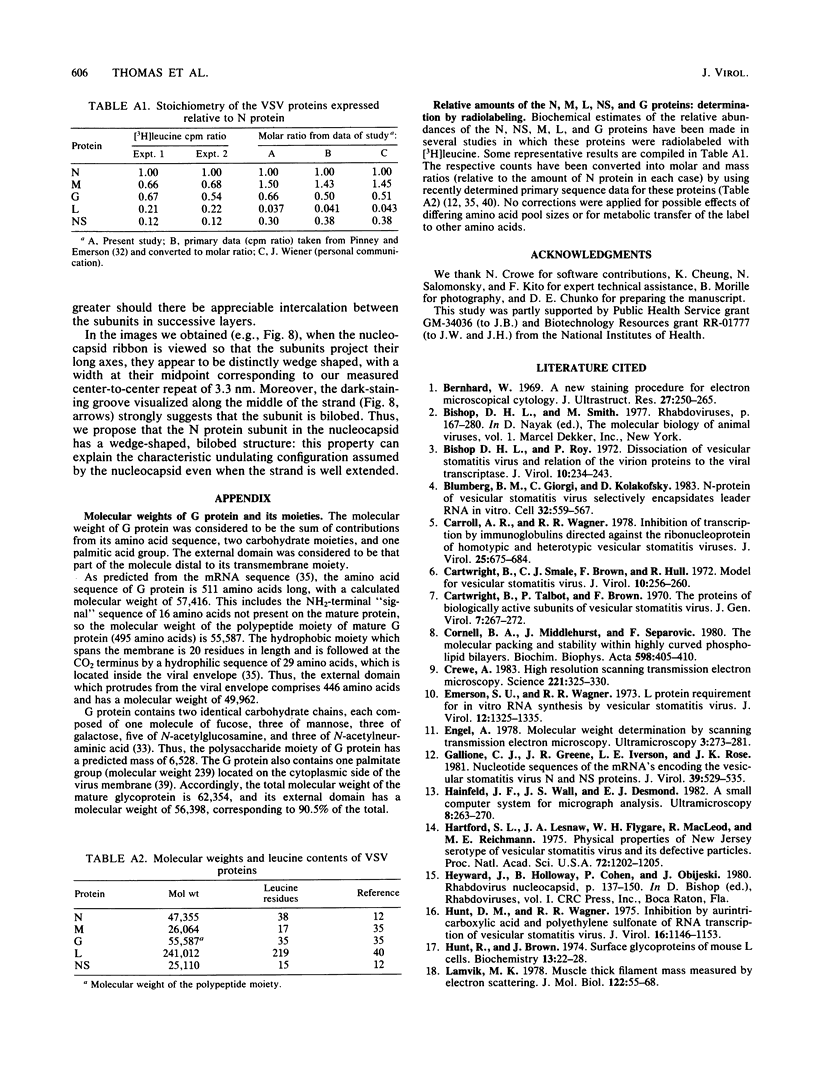
Dark-field scanning transmission electron microscopy was used to perform mass analyses of purified vesicular stomatitis virions, pronase-treated virions, and nucleocapsids, leading to a complete self-consistent account of the molecular composition of vesicular stomatitis virus. The masses obtained were 265.6 +/- 13.3 megadaltons (MDa) for the native virion, 197.5 +/- 8.4 MDa for the pronase-treated virion, and 69.4 +/- 4.9 MDa for the nucleocapsid. The reduction in mass effected by pronase treatment, which corresponds to excision of the external domains (spikes) of G protein, leads to an average of 1,205 molecules of G protein per virion. The nucleocapsid mass, after compensation for the RNA (3.7 MDa) and residual amounts of other proteins, yielded a complement of 1,258 copies of N protein. Calibration of the amounts of M, NS, and L proteins relative to N protein by biochemical quantitation yielded values of 1,826, 466, and 50 molecules, respectively, per virion. Assuming that the remaining virion mass is contributed by lipids in the viral envelope, we obtained a value of 56.1 MDa for its lipid content. In addition, four different electron microscopy procedures were applied to determine the nucleocapsid length, which we conclude to be 3.5 to 3.7 micron. The nucleocapsid comprises a strand of repeating units which have a center-to-center spacing of 3.3 nm as measured along the middle of the strand. We show that these repeating units represent monomers of N protein, each of which is associated with 9 +/- 1 bases of single-stranded RNA. From scanning transmission electron microscopy images of negatively stained nucleocapsids, we inferred that N protein has a wedge-shaped, bilobed structure with dimensions of approximately 9.0 nm (length), approximately 5.0 nm (depth), and approximately 3.3 nm (width, at the midpoint of its long axis). In the coiled configuration of the in situ nucleocapsid, the long axis of N protein is directed radially, and its depth corresponds to the pitch of the nucleocapsid helix.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969 May;27(3):250–265. doi: 10.1016/s0022-5320(69)80016-x. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Inhibition of transcription by immunoglobulins directed against the ribonucleoprotein of homotypic and heterotypic vesicular stomatitis viruses. J Virol. 1978 Feb;25(2):675–684. doi: 10.1128/jvi.25.2.675-684.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Cornell B. A., Middlehurst J., Separovic F. The molecular packing and stability within highly curved phospholipid bilayers. Biochim Biophys Acta. 1980 May 23;598(2):405–410. doi: 10.1016/0005-2736(80)90018-8. [DOI] [PubMed] [Google Scholar]
- Crewe A. V. High-resolution scanning transmission electron microscopy. Science. 1983 Jul 22;221(4608):325–330. doi: 10.1126/science.6867711. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. Molecular weight determination by scanning transmission electron microscopy. Ultramicroscopy. 1978;3(3):273–281. doi: 10.1016/s0304-3991(78)80037-0. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainfeld J. F., Wall J. S., Desmond E. J. A small computer system for micrograph analysis. Ultramicroscopy. 1982;8(3):263–270. doi: 10.1016/0304-3991(82)90242-x. [DOI] [PubMed] [Google Scholar]
- Hartford S. L., Lesnaw J. A., Flygare W. H., MacLeod R., Reichmann M. E. Physical properties of New Jersey serotype of vesicular stomatitis virus and its defective particles. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1202–1205. doi: 10.1073/pnas.72.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Wagner R. R. Inhibition by aurintricarboxylic acid and polyethylene sulfonate of RNA transcription of vesicular stomatitis virus. J Virol. 1975 Nov;16(5):1146–1153. doi: 10.1128/jvi.16.5.1146-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. C., Brown J. C. Surface glycoproteins of mouse L cells. Biochemistry. 1974 Jan 1;13(1):22–28. doi: 10.1021/bi00698a004. [DOI] [PubMed] [Google Scholar]
- Lamvik M. K. Muscle thick filament mass measured by electron scattering. J Mol Biol. 1978 Jun 15;122(1):55–68. doi: 10.1016/0022-2836(78)90108-0. [DOI] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J. Virus envelopes and plasma membranes. Annu Rev Biophys Bioeng. 1978;7:139–165. doi: 10.1146/annurev.bb.07.060178.001035. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Heterogeneity of vesicular stomatitis virus particles: implications for virion assembly. J Virol. 1980 Jan;33(1):52–58. doi: 10.1128/jvi.33.1.52-58.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Lipid composition of purified vesicular stomatitis viruses. J Virol. 1971 Jan;7(1):59–70. doi: 10.1128/jvi.7.1.59-70.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson M. W., Hainfeld J., Wall J., Haschemeyer R. H. Identification and mass analysis of human fibrinogen molecules and their domains by scanning transmission electron microscopy. J Mol Biol. 1981 Dec 15;153(3):695–718. doi: 10.1016/0022-2836(81)90414-9. [DOI] [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Nagpal M. L., Brown J. C. Protein and glycoprotein components of phagosome membranes derived from mouse L cells. Int J Biochem. 1980;11(2):127–138. doi: 10.1016/0020-711x(80)90245-1. [DOI] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J Virol. 1981 Jul;39(1):295–299. doi: 10.1128/jvi.39.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. doi: 10.1128/jvi.42.3.897-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Repik P., Bishop D. H. Determination of the molecular weight of animal RNA viral genomes by nuclease digestions. I. Vesicular stomatitis virus and its defective T particle. J Virol. 1973 Nov;12(5):969–983. doi: 10.1128/jvi.12.5.969-983.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Engelman D. M. Molecular mechanism for the interaction of phospholipid with cholesterol. Nat New Biol. 1972 May 10;237(71):42–44. doi: 10.1038/newbio237042a0. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Andree P. J., Hooft van Huysduynen R. A., Mellema J. E. Characterization of three highly purified influenza virus strains by electron microscopy. J Gen Virol. 1984 Apr;65(Pt 4):799–802. doi: 10.1099/0022-1317-65-4-799. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. doi: 10.1016/0042-6822(66)90289-3. [DOI] [PubMed] [Google Scholar]
- Smith P. R. An integrated set of computer programs for processing electron micrographs of biological structures. Ultramicroscopy. 1978;3(2):153–160. doi: 10.1016/s0304-3991(78)80021-7. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Hainfeld J. F., Wall J. S., Steer C. J. Mass distributions of coated vesicles isolated from liver and brain: analysis by scanning transmission electron microscopy. J Cell Biol. 1983 Dec;97(6):1714–1723. doi: 10.1083/jcb.97.6.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Wall J., Hainfeld J., Steinert P. M. Structure of fibroblastic intermediate filaments: analysis of scanning transmission electron microscopy. Proc Natl Acad Sci U S A. 1982 May;79(10):3101–3105. doi: 10.1073/pnas.79.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware B. R., Raj T., Flygare W. H., Lesnaw J. A., Reichmann M. E. Molecular weights of vesicular stomatitis virus and its defective particles by laser light-scattering spectroscopy. J Virol. 1973 Jan;11(1):141–145. doi: 10.1128/jvi.11.1.141-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]







