Abstract
A technique for size selective discrimination of protein analytes was developed by incorporating poly(ethylene glycol) (PEG) lipopolymers into supported lipid bilayers. The membranes also contained biotinylated lipids, which recognized both streptavidin and anti-biotin IgG. By employing various PEG lipopolymer concentrations, clear discrimination against anti-biotin (m.w. = 150,000 Da) binding could be observed, which became more pronounced at higher polymer densities. On the other hand, streptavidin (m.w. = 52,800) binding to the membrane remained unaffected even at PEG concentrations that were well into the mushroom-to-brush phase transition. These observations were exploited to create an on-chip ligand-receptor binding assay that favored streptavidin binding over anti-biotin by several orders of magnitude in the presence of the lipopolymer. Control experiments revealed that the two proteins bound to similar extents from a multi-protein analyte solution in the absence of PEG.
Microfluidic platforms possess great promise in fields ranging from proteomics1 to biosensing.2 Indeed, these devices can be used for rapid analysis while consuming only trace amounts of materials.3, 4 Unfortunately, such applications often require off-chip sample purification or on-chip separations in order to carry out the desired analysis.5, 6 Such additional steps can make the monitoring process lengthy and cumbersome. Moreover, manufacturing intricate devices is more expensive and prone to contamination or failure. An ideal platform would be one that could selectively screen ligand-receptor interactions of multi-protein mixtures in the fewest possible steps.
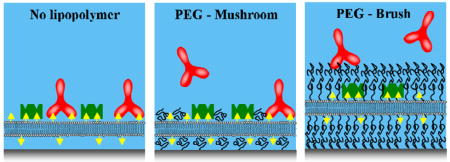
Herein, we describe a simple microfluidic method for assaying ligand-receptor binding on solid supported lipid bilayers7 (SLBs) while simultaneously applying a size-selective filter against the analytes. The lipid bilayer contains lipid-conjugated ligands, which serve to recognize incoming protein analytes with the appropriate receptor sites.8, 9 Additionally, a lipopolymer, poly(ethylene glycol) phosphatidylethanolamine (PEG-PE),10, 11 is incorporated into the fluid SLBs and serves as a nanoscale size exclusion filter. The conformation of the polymer can be controlled through the chain length of the PEG and the number density of the lipopolymer within the lipid membrane.12, 13 The particular conformation of the PEG in turn strictly regulates access to the underlying ligands in the bilayer on the basis of the size of the analyte. In other words, the PEG lipopolymers effectively screen proteins by their molecular weight, even if several species recognize the identical surface-bound ligands. A key aspect of our system is that the lipids, ligands, and PEG lipopolymers experience the same two-dimensional fluidity as found in native biomembranes.14, 15 In fact, these supramolecular architectures are to some extent reminiscent of a cell’s glycocalyx,16 which is an intricate carbohydrate network attached above the plasma membrane and might also be involved in screening incoming analytes on the basis of size.17
In a first set of experiments, solid supported lipid bilayers were prepared by fusing vesicles18, 19 inside PDMS/glass microfluidic devices.20 The lipid membranes consisted primarily of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) with 1 mol% 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(biotinyl) (sodium salt) (biotin-PE) and 0, 0.5, or 1.5 mol% of a PEG-PE lipopolymer with a degree of polymerization, np, of 114 (m.w. = 5000 Da, PEG5000-PE). It is well known that the conformation of the PEG depends on the mole fraction of PEG-PE in the membrane.12, 14, 21 At 0.5 mol% PEG5000-PE,22 the PEG moiety is at the onset of the mushroom-to-brush transition, where the interpolymer distance is just slightly less than twice the Flory radius.11 On the other hand, at 1.5 mol% PEG5000-PE, it is well into the brush transition.
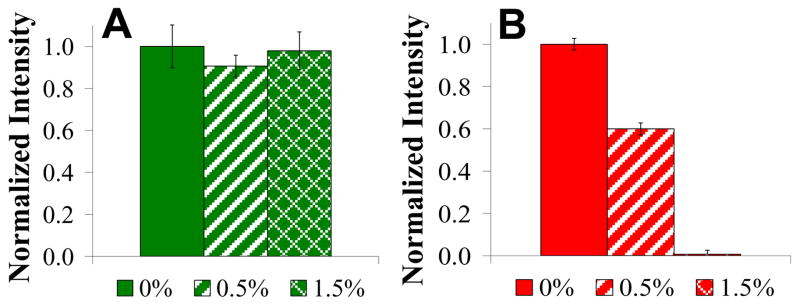
Binding assays were carried out by flowing fluorescently labeled streptavidin and anti-biotin IgG solutions (1 μM in phosphate buffered saline, pH 7.4) over biotinylated bilayers with varying PEG densities. Both proteins are known to bind tightly to the biotin moiety.23, 24 All fluorescence data were normalized to their respective controls without lipopolymer in the bilayer (POPC + 1 mol% biotin-PE). The effect of the lipopolymer concentration on the ability of the two proteins to penetrate the PEG layer and undergo ligand-receptor binding is shown in Figure 1. For the smaller protein, streptavidin (molecular weight 52.8 kD),25 there was no appreciable effect of the PEG layer even at the highest density investigated (green bars). On the other hand, the effect of the lipopolymer was dramatic for the IgG (MW 150 kD).25 At 0.5 mol% PEG5000-PE, the binding dropped to ~60% of the value obtained without PEG in the membrane. At a PEG5000-PE density of 1.5 mol%, IgG binding was almost completely inhibited (red bars).
Figure 1.
Protein filtering induced by the presence of PEG lipopolymer. (A) The level of binding of streptavidin (green) and (B) antibiotin IgG (red) without PEG are compared to membranes with PEG5000-PE at mol fractions corresponding to the onset of the mushroom-to-brush transition (0.5%) and well into the brush conformation (1.5%).
To demonstrate the ability of the PEG filter to discriminate between a mixture of proteins that recognize the same surface-bound ligand, a competitive binding assay of fluorescently labeled anti-biotin and streptavidin was carried out in a microfluidic channel containing a POPC membrane with 1 mol% biotin-PE and 1.5 mol% PEG5000-PE (Figure 2, center panel, channel 1), along with two controls (channels 2 & 3). In the second and third channels, 1 μM IgG and 1 μM streptavidin were introduced, respectively, over a biotinylated POPC bilayer without PEG. As can be seen from the green line scan (right panel), the concentration of bound streptavidin in channels 1 and 3 was identical within experimental error. By contrast, the amount of IgG in channel 1 was reduced by nearly two orders of magnitude with respect to its control, channel 2 (red line scan). Such size selective filtering is remarkable, especially considering that the proteins only differ by a factor of 2.8 in molecular weight.
Figure 2.
(Left panel) A schematic representation of the size selective filtering process for a protein mixture by using a lipopolymer membrane. (Middle panel) A working microfluidic device in which channel 1 contains a POPC + 1 mol% biotin-PE membrane with 1.5 mol% PEG5000. The other channels contain the same membrane without the lipopolymer. Fluorescence micrographs were acquired and overlaid after the bilayers were exposed to Alexa-488 streptavidin (green) and Alexa 594 IgG (red) in channel 1, Alexa 594 IgG in channel 2, and Alexa 488 streptavidin in channels 3. (Right panel) A line profile of the red and green fluorescence intensity across all three channels in the device (taken from the blue dashed line across the channels).
To insure that the screening effect was due to the PEG filter, it was necessary to show that the two proteins would bind roughly equally to the biotin-PE ligands in the absence of PEG. Therefore, a competitive binding assay was run between anti-biotin and streptavidin without any lipopolymer (Figure 3). In channel 1, a POPC bilayer with 1 mol% biotin-PE was exposed to a 1:1 mixture of fluorescently labeled IgG and unlabeled streptavidin (1 μM of each protein, 1 hour incubation, followed by rinsing out the bulk proteins). In channel 2, the same assay was run, but without the streptavidin. As can be seen, the fluorescence level from the labeled IgG was cut almost exactly by a factor of two when an equimolar concentration of streptavidin was present. The converse experiment using labeled streptavidin and unlabeled IgG gave similar results (see supplementary materials). Such data demonstrate that both proteins bind to biotin to approximately the same extent under these conditions. This occurs despite the fact that the two proteins have somewhat different equilibrium dissociation constants for biotin binding (i.e. kinetically controlled binding dominates the process).23, 24
Figure 3.
(Left) Comparison of the binding of a 1:1 mixture of unlabeled streptavidin/labeled IgG (channel 1) to a control, labeled IgG (channel 2), for 1 mol% biotin-PE in POPC bilayers inside a microfluidic device. The line scan (right) shows that the fluorescence intensity from the protein mixture is about half of that from the control.
Although the assay presented above has been optimized for filtering IgG in the presence of streptavidin, the methodology should be quite general. Polymer scaling theory26–28 predicts that the molecular weight cutoff would increase if shorter PEG lengths and lower lipopolymer densities were employed.29 On the other hand, discrimination between proteins with molecular weights below 100,000 could be achieved by using higher molecular weight PEGs and greater lipopolymer densities. Indeed, the door should now be open to “on-chip” sensor design with thin film size exclusion architectures.
Supplementary Material
Lipid preparation, fluorescent labeling, and additional microfluidic assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the NIH (R01 GM070622), the ARO (W911NF-05-1-0494) and DARPA (FA9550-06-C-0006) for support.
References
- 1.Zhu H, Bilgin M, Bangham R, Hall D, Casamayor A, Bertone P, Lan N, Jansen R, Bidlingmaier S, Houfek T, Mitchell T, Miller P, Dean RA, Gerstein M, Snyder M. Science. 2001;293:2101–2105. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]
- 2.Liu HR, Yang J, Lenigk R, Bonanno J, Grodzinski P. Anal Chem. 2004;76:1824–1831. doi: 10.1021/ac0353029. [DOI] [PubMed] [Google Scholar]
- 3.Du H, Wu M, Yang W, Yuan G, Sun Y, Lu Y, Zhao S, Du Q, Wang J, Yang S, Pan M, Lu Y, Wang S, Cheng J. Clinical Chemistry. 2005;51:368–375. doi: 10.1373/clinchem.2004.036665. [DOI] [PubMed] [Google Scholar]
- 4.Cristea LM, Gaskell SJ, Whetton AD. Blood. 2004;103:3624–3634. doi: 10.1182/blood-2003-09-3295. [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Wheeler A, Zare RN. Proc Natl Acad Sci USA. 2004;101:12809–12813. doi: 10.1073/pnas.0405299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan V, Pamula VK, Fair RB. Lab Chip. 2004;4:310–315. doi: 10.1039/b403341h. [DOI] [PubMed] [Google Scholar]
- 7.Tamm LK, McConnell HM. Biophys J. 1985;47:105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang T, Baryshnikova OK, Mao H, Holden MA, Cremer PS. J Am Chem Soc. 2003;125:4779–4784. doi: 10.1021/ja029469f. [DOI] [PubMed] [Google Scholar]
- 9.Tamm LK, Bartoldus I. Biochemistry. 1988;27:7453–7458. [Google Scholar]
- 10.De Gennes PG. Scaling Concepts in Polymer Physics. Cornell Univ. Press; Ithaca, NY: 1979. [Google Scholar]
- 11.Flory PJ. Principals of Polymer Chemistry. Cornell Univ. Press; Ithaca, NY: 1971. [Google Scholar]
- 12.Marsh D, Bartucci Sportelli L. Biochim Biophys Acta. 2003;1615:33–59. doi: 10.1016/s0005-2736(03)00197-4. [DOI] [PubMed] [Google Scholar]
- 13.Hashizaki K, Taguchi H, Itoh C, Sakai H, Abe M, Saito Y, Ogawa N. Chem Pharm Bull. 2003;51:815–820. doi: 10.1248/cpb.51.815. [DOI] [PubMed] [Google Scholar]
- 14.Albertorio F, Diaz AJ, Yang T, Chapa VA, Kataoka S, Castellana ET, Cremer PS. Langmuir. 2005;21:7476–7482. doi: 10.1021/la050871s. [DOI] [PubMed] [Google Scholar]
- 15.Soong R, Macdonald PM. Biophys J. 2005;88:255–268. doi: 10.1529/biophysj.104.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans SV, MacKenzie CR. J Mol Rec. 1999;12:155–168. doi: 10.1002/(SICI)1099-1352(199905/06)12:3<155::AID-JMR456>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Albersdorfer A, Sackmann E. Biophys J. 1997;73:245–257. doi: 10.1016/S0006-3495(97)78065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barenholtz Y, Litman BJ, Goll J, Thompson TE, Carlson FD. Biochemistry. 1977;16:2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- 19.Kalb E, Frey S, Tamm LK. Biochim Biophys Acta. 1992;1103:307–316. doi: 10.1016/0005-2736(92)90101-q. [DOI] [PubMed] [Google Scholar]
- 20.Yang TL, Jung SY, Mao HB, Cremer PS. Anal Chem. 2001;73:165–169. doi: 10.1021/ac000997o. [DOI] [PubMed] [Google Scholar]
- 21.Burridge KA, Figa MA, Wong JY. Langmuir. 2004;20:10252–10259. doi: 10.1021/la0489099. [DOI] [PubMed] [Google Scholar]
- 22.Hansen PL, Cohen JA, Podgomik R, Parsegian A. Biophys J. 2003;84:350–355. doi: 10.1016/S0006-3495(03)74855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chilkoti A, Stayton PS. J Am Chem Soc. 1995;117:10622–10628. [Google Scholar]
- 24.Bagci H, Kohen F, Kuscuoglu U, Bayer EA, Wilchek M. FEBS. 1993;322:47–50. doi: 10.1016/0014-5793(93)81108-c. [DOI] [PubMed] [Google Scholar]
- 25.Roberts CJ, Davies MC, Tendler SJB, Williams PM, Davies J, Dawkes AC, Yearwood GDL, Edwards JC. Ultramicroscopy. 1996;62:149–155. doi: 10.1016/0304-3991(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 26.Jeon SI, Lee JH, Andrade JD, de Gennes PG. J Colloid Interface Sci. 1991;142:149–158. [Google Scholar]
- 27.De Gennes PG. Adv Colloid Interface Sci. 1987;27:189–209. [Google Scholar]
- 28.De Gennes PG. Macromolecules. 1980;13:1069–1075. [Google Scholar]
- 29.Jeppesen C, Wong JY, Kuhl TL, Israelachvili GN, Mullah N, Zalipsky S, Marques CM. Science. 2001;293:465–467. doi: 10.1126/science.293.5529.465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lipid preparation, fluorescent labeling, and additional microfluidic assays. This material is available free of charge via the Internet at http://pubs.acs.org.