Abstract
Purification of recombinant proteins is often a challenging process involving several chromatographic steps that must be optimized for each target protein. Here, we developed a self-excising module allowing single-step affinity chromatography purification of untagged recombinant proteins. It consists of a 250-residue-long self-processing module of the Neisseria meningitidis FrpC protein with a C-terminal affinity tag. The N terminus of the module is fused to the C terminus of a target protein of interest. Upon binding of the fusion protein to an affinity matrix from cell lysate and washing out contaminating proteins, site-specific cleavage of the Asp–Pro bond linking the target protein to the self-excising module is induced by calcium ions. This results in the release of the target protein with only a single aspartic acid residue added at the C terminus, while the self-excising affinity module remains trapped on the affinity matrix. The system was successfully tested with several target proteins, including glutathione-S-transferase, maltose-binding protein, β-galactosidase, chloramphenicol acetyltransferase, and adenylate cyclase, and two different affinity tags, chitin-binding domain or poly-His. Moreover, it was demonstrated that it can be applied as an alternative to two currently existing systems, based on the self-splicing intein of Saccharomyces cerevisiae and sortase A of Staphylococcus aureus.
Keywords: Asp–Pro bond, FrpC, purification, self-cleavage, self-excising, self-processing
Production of recombinant proteins in an active and highly purified form is an important task in biomedical research, biotechnology, and the pharmaceutical industry. Various systems and methods for protein overproduction, isolation, and purification have been developed (Hunt 2005; Hartley 2006; Graslund et al. 2008), but purification of recombinant proteins to homogeneity is still a challenging process. In general, several chromatographic steps have to be individually optimized for each target protein of interest. Recently, various affinity-tag systems for single-step purification of recombinant proteins have been developed (Ford et al. 1991; Nilsson et al. 1997; Arnau et al. 2006). Typically, an affinity-tag sequence is genetically fused to the N or C terminus of the target protein, which enables purification of the tagged protein from cell extracts or culture media by high-affinity binding to a purification matrix. Both small peptide affinity tags, such as poly-His, poly-Arg, c-myc, Flag, S, or Strep, as well as large peptide or protein tags such as cellulose- or chitin-binding domain, maltose-binding protein, or glutathione-S-transferase, have been widely and successfully used for protein purification (Ford et al. 1991; Nilsson et al. 1997; Arnau et al. 2006). The drawback of all of these systems, however, is that the affinity tag usually has to be removed prior to biochemical or biological activity characterization, crystallization, or use in antibody production. Therefore, a specific cleavage site is engineered between the affinity tag and the protein of interest and the tag is cleaved off by treatment with a site-specific protease (Nilsson et al. 1997; Jenny et al. 2003; Arnau et al. 2006). This approach, however, suffers several limitations: (1) separation of the protein of interest from the affinity tag, the unprocessed fusion, and the added protease typically requires an extra purification step; (2) the used proteases (e.g., factor Xa, enterokinase, or thrombin) are not fully specific and may cleave additional bonds within the target protein; (3) the cleavage site in the fusion protein may be inaccessible to the protease; (4) the cleavage of the fusion usually requires long reaction times; and (5) the process is rather costly.
To overcome these limitations, affinity-tag systems have been combined with site-specific processing modules in current one-step purification systems based on self-cleaving affinity tags (Chong et al. 1997; Mao 2004). These consist of a self-processing module that is genetically fused at one terminus to an affinity tag, enabling simple one-step purification, and at the second terminus to a protein of interest. After binding of the fusion protein to an affinity matrix and washing out of contaminating proteins, the cleavage activity of the self-processing module is induced by a low-molecular weight compound, resulting in release of the protein of interest, while the self-processing module–affinity tag remains trapped on the matrix. A suitable self-processing module has to fulfill two basic conditions: (1) It must undergo cleavage only at the specific peptide bond between the protein of interest and the self-processing module–affinity tag, and (2) it must cleave off only after induction in vitro and not inside of the producing organism. Therefore, self-processing modules matching these requirements are rare; only two types of such self-cleaving affinity tags have been reported. One is based on a self-splicing protein element of Saccharomyces cerevisiae, called intein (Chong et al. 1997), and the other utilizes the catalytic core of sortase A from Staphylococcus aureus (Mao 2004).
Recently, we demonstrated that upon activation by calcium ions, the FrpC protein of Neisseria meningitidis undergoes an autocatalytic and highly site-specific processing at the Asp414 and Pro415 bond (Osicka et al. 2004) that is mediated by an adjacent segment of 250 residues (Osicka et al. 2004). Here, we used this module of FrpC to design a novel type of a self-cleaving affinity tag for effective single-step purification of native recombinant proteins.
Results
The FrpC self-processing module specifically and efficiently cleaves off from recombinant fusion proteins
We recently reported that the FrpC protein of Neisseria meningitidis (Fig. 1A) undergoes a unique calcium-dependent autocatalytic processing at the peptide bond between residues Asp414 and Pro415, and that this processing is catalyzed by a self-processing module (SPM) located next to the cleavage site (Osicka et al. 2004). We therefore reasoned that the SPM might be useful in constructing self-cleaving affinity tags (Fig. 1B). An obstacle to its use, however, appeared to consist in some reactivity of carboxyl groups of the C-terminal Asp414 residues released by SPM-mediated cleavage, as these were observed to form covalent isopeptide bonds with ε-amino groups of internal lysine residues of other proteins (Osicka et al. 2004).
Figure 1.
Schemes of the FrpC protein and a recombinant protein purification based on the self-processing module of FrpC. (A) The 198-kDa FrpC protein of N. meningitidis consists of an N-terminal part harboring the self-processing module (SPM), a C-terminal portion with the putative calcium-binding nonapeptide repeats (RTX), and the C-proximal secretion signal. (Arrowhead) Asp414–Pro415 bond autocatalytically and specifically cleaved upon activation by calcium ions. (B) The fusion protein used for one-step SPM-mediated protein purification consists of SPM that is genetically fused at the N terminus to a target protein and at the C terminus to an affinity tag. After binding of the fusion protein to an affinity matrix from a crude cell lysate and washing out contaminating proteins, the cleavage activity of SPM is induced by addition of calcium ions. It results in release and elution of the target protein harboring only one extra aspartate residue at the C terminus, while the SPM-affinity tag fusion remains trapped on the matrix.
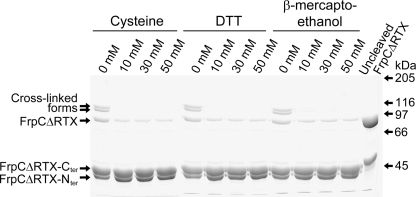
On the basis of the previously proposed reaction mechanism (Osicka et al. 2004), we reasoned that the calcium-induced cleavage activity of SPM might be efficiently separated from the cross-linking activity of liberated N-terminal fragments in the presence of reactive thiol compounds, such as DTT, β-mercaptoethanol, or cysteine. As shown in Figure 2, already at a 10 mM concentration the thiol compounds indeed completely inhibited formation of the cross-linked protein species upon calcium-induced processing of FrpCΔRTX, a previously described deletion variant of FrpC (Osicka et al. 2004).
Figure 2.
Calcium-induced cleavage activity of FrpCΔRTX can be efficiently separated from its cross-linking activity by thiol compounds. Purified FrpCΔRTX protein was incubated for 1 h at 37°C in the absence of thiol compounds (0 mM) or in the presence of 10 mM, 30 mM, or 50 mM cysteine, DTT, or β-mercaptoethanol, respectively. Calcium ions (2 mM) were added to each reaction to induce processing. The protein fragments were separated by SDS-PAGE (7.5%) and stained with Coomassie blue. (Last lane) Purified FrpCΔRTX incubated for 1 h at 37°C in the absence of calcium ions. FrpCΔRTX-Cter or FrpCΔRTX-Nter, the C- or N-terminal fragments of FrpCΔRTX, respectively, are formed by the calcium-dependent cleavage between residues Asp414 and Pro415.
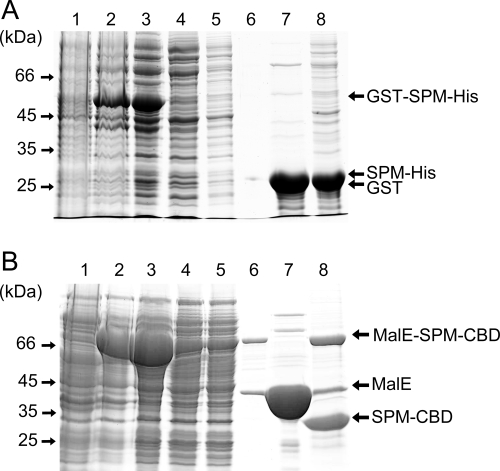
To further investigate whether the SPM could be used for construction of a functional self-cleaving affinity tag (Fig. 1B), two fusion proteins with model targets and affinity tags were generated, using a truncated SPM consisting of residues 414–657 of FrpC. The first fusion construct, GST-SPM-His (52.9 kDa), contained a Schistosoma japonicum glutathione-S-transferase (GST, 26.3 kDa) as an N-terminal moiety fused to SPM (26 kDa) and a 6× poly-His tag (His, 0.6 kDa) at the C terminus. The second, MalE-SPM-CBD (74 kDa), consisted of Escherichia coli maltose-binding protein (MalE, 41.2 kDa) fused to SPM and a C-terminal chitin-binding domain from Bacillus circulans (CBD, 6.8 kDa). These fusions were constructed so as to result in a single extra residue (Asp) left over at the C terminus of the target protein upon calcium-induced processing (Fig. 1B). The GST-SPM-His and MalE-SPM-CBD proteins were produced in E. coli BL21(λDE3) as soluble cytosolic proteins and were purified by single-step chromatography on Ni-NTA agarose or amylose columns, respectively.
To analyze the SPM-mediated cleavage of the GST-SPM-His and MalE-SPM-CBD proteins in solution, first the kinetics of the cleavage reactions were compared with those of the original FrpCΔRTX protein (Osicka et al. 2004). For this purpose, the processing products of the fusion proteins and of FrpCΔRTX were sampled at various time points, separated by SDS-PAGE, and quantified by densitometric analysis of Coomassie-stained gels. As shown in Figure 3A, incubation of GST-SPM-His and MalE-SPM-CBD with 2 mM free calcium ions at 37°C resulted in rapid processing of both proteins, with a reaction half-time of ∼5 min and a maximal conversion yield of ∼80% reached within 120 min. This was fully comparable to the kinetics of calcium-induced cleavage of FrpCΔRTX. In the absence of calcium ions, both fusion proteins were stable and remained unprocessed at 37°C over the 24-h testing period (data not shown).
Figure 3.
Time course of SPM-mediated cleavage of purified fusion proteins at different calcium concentrations and temperatures. (A) Purified GST-SPM-His, MalE-SPM-CBD, and FrpCΔRTX, respectively, were incubated in the presence of 2 mM free calcium ions at 37°C and pH 7.4. Aliquots of the processed proteins were withdrawn at the indicated times and separated by SDS-PAGE. The processing was quantified by densitometric analysis of the Coomassie-stained gels. (Inset) An example showing the cleavage of the GST-SPM-His fusion protein over time. GST-SPM-His (B,C) and MalE-SPM-CBD (D,E) were incubated for the indicated times with 2, 5, 10, and 50 mM calcium ions at 23°C (B,D) or 4°C (C,E) and pH 7.4. Aliquots of the processed fusion proteins were separated by SDS-PAGE and stained with Coomassie blue. Extent of cleavage of GST-SPM-His and MalE-SPM-CBD was quantified by densitometric analysis of SDS-PAGE gels. The values represent an average of four independent determinations ± standard deviations.
To determine the optimal concentrations of calcium ions for SPM-mediated cleavage, the purified GST-SPM-His and MalE-SPM-CBD fusions were treated with Ca2+ at four different concentrations (2, 5, 10, or 50 mM) and at 4°C and room temperature. As summarized in Figure 3B–E, already a 2 mM concentration of free calcium ions effectively induced cleavage of both fusion proteins; further elevation of Ca2+ up to 10 mM had only a minor enhancing effect on the processing rate (the increment was <20%). The rates of protein cleavage induced at 50 mM Ca2+ were similar or even lower than those at 10 mM Ca2+ (Fig. 3B–E). Therefore, a 10 mM concentration of Ca2+ was used for all further experiments. At room temperature, cleavage of both fusion proteins was highly efficient; ∼80% of both GST-SPM-His and MalE-SPM-CBD was cleaved during 4 h (Fig. 3B,D). At 4°C, the rate of SPM-mediated processing slowed down, and the maximal cleavage extent (80%) was achieved after 8 h for GST-SPM-His (Fig. 3C), whereas MalE-SPM-CBD was cleaved during the same time period only to ∼60% (Fig. 3E). Prolonged incubation with 10 mM Ca2+ increased the conversion yield to 75% in 20 h (Fig. 3E). All these data demonstrate that SPM derived from the FrpC protein of N. meningitidis can be combined with different target proteins and affinity tags without losing its efficient and specific calcium-induced self-processing activity.
SPM-mediated cleavage of proteins immobilized on an affinity matrix
For use in protein purification, it was important to characterize the specificity and efficiency of SPM-mediated cleavage of the model fusion proteins when bound to an affinity matrix. Cytosolic extracts containing GST-SPM-His (Fig. 4A, lane 3) or MalE-SPM-CBD (Fig. 4B, lane 3) were loaded at 23°C onto an Ni-NTA agarose or chitin column, respectively, under conditions where most of the fusion protein was bound (Fig. 4A,B, lane 4), while contaminating proteins were removed by extensive washing of the affinity matrix with column buffer (Fig. 4A,B, lane 5). Next, the column buffer supplemented with 10 mM Ca2+ and 10 mM DTT was rapidly passed through the columns to induce SPM-mediated cleavage of the fusion proteins. Only minimal amounts of the cleaved-off GST and MalE were eluted from the columns at this stage (Fig. 4A,B, lane 6). After 6 h of incubation of the column buffer with calcium ions, the released GST and MalE proteins were eluted with yields of ∼30 mg of GST and 50 mg of MalE per liter of culture, respectively (Fig. 4A,B, lane 7). Only negligible amounts of remaining uncleaved GST-SPM-His fusion could be subsequently eluted from the Ni-NTA matrix with 250 mM imidazole, suggesting that in 6 h at 23°C the cleavage of the fusion protein was nearly complete (Fig. 4A, lane 8). In turn, higher amounts of uncleaved protein were still eluted from the chitin column with 2% SDS (Fig. 4B, lane 8), thus confirming that processing of the MalE-SPM-CBD fusion was less efficient.
Figure 4.
Affinity purification of GST from GST-SPM-His on a Ni-NTA agarose column (A) and MalE from MalE-SPM-CBD on a chitin column (B). Lanes in each gel: (1) crude extract from uninduced E. coli BL21(λDE3) cells; (2) crude extract from cells induced for production of the fusion protein; (3) clarified crude extract from induced cells loaded onto a column; (4) column flow-through; (5) column wash; (6) fraction of the column buffer complemented with 10 mM Ca2+ and 10 mM DTT that was rapidly passed (not more than 15 min) through the column to induce SPM-mediated cleavage of the fusion proteins; (7) eluted protein after SPM-mediated excision for 6 h; (8) proteins that remained bound to affinity column. The protein samples were analyzed on a 10% polyacrylamide gel and stained with Coomassie blue.
As handling at room temperature may need to be avoided for many proteins, affinity purification of both GST-SPM-His and MalE-SPM-CBD also was performed at 4°C. With cleavage times extended from 6 to 16 h, identical yields of purified GST and MalE proteins were indeed obtained (data not shown). These results thus show that when immobilized on an affinity matrix, the SPM also can specifically and efficiently cleave off a target protein at 4°C.
Recombinant enzymes purified as self-cleaving SPM fusions retain specific catalytic activities
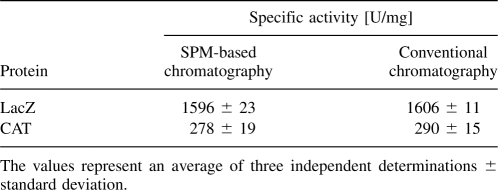
To test whether recombinant proteins obtained by SPM-based affinity purification do not have their activities altered, well-characterized enzymes such as the tetrameric β-galactosidase (LacZ) and the chloramphenicol acetyltransferase (CAT), were used as reporter proteins in fusion with SPM-CBD. First, the open reading frames encoding LacZ (119.8 kDa) and CAT (26.7 kDa) were subcloned in-frame with the 5′-end of the gene for SPM-CBD, to obtain the constructs for expression of the LacZ-SPM-CBD (152.6 kDa) and CAT-SPM-CBD (59.5 kDa) fusion proteins, respectively. Both LacZ and CAT were purified from clarified E. coli BL21(λDE3) extracts on a chitin column at 23°C. In parallel, to have appropriate controls for determination of specific enzyme activities, the LacZ and CAT enzymes were produced without the SPM-CBD extension, being tagged with a 6× poly-His affinity purification tag, and were purified by a single-step metal affinity chromatography using Ni-NTA resins. As shown in Table 1, comparison of the specific catalytic activities revealed that the LacZ and CAT enzymes purified from SPM fusions and as free 6× poly-His-tagged enzymes indeed exhibited essentially identical specific catalytic activities. These results thus show that at least some recombinant enzymes can be purified from the self-cleaving SPM fusions without losing activities.
Table 1.
Specific catalytic activities of the LacZ and CAT enzymes purified by SPM-based purification system and by metal affinity chromatography
SPM- and intein-based purification systems exhibit comparable yields
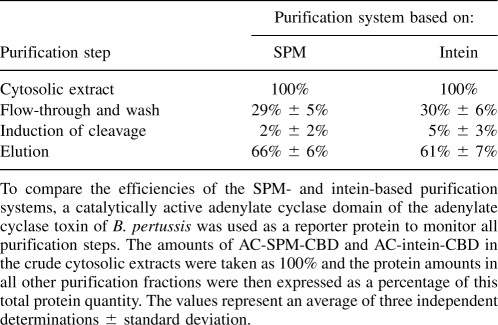
To compare the efficiency of the SPM-based protein purification system developed here to that based on the self-splicing intein, AC-SPM-CBD (74.1 kDa) or AC-intein-CBD (97.6 kDa) fusion proteins were produced, using a catalytically active adenylate cyclase domain (AC) derived from the adenylate cyclase toxin of Bordetella pertussis as a reporter protein. This enzyme was chosen because it enables a simple and accurate monitoring of AC activity recovery throughout the entire protein purification process. To allow a comparison of AC activity yields resulting from the SPM- and intein-based purification processes, AC-SPM-CBD and AC-intein-CBD proteins were produced in E. coli BL21(λDE3), and prior to purification the amounts of the fusion proteins in cell extracts were equalized on the basis of the AC activity (Table 2). The equalized extracts were then loaded onto the columns filled with equivalent volumes of chitin beads, and all fractions over the entire purification procedure were collected and monitored for AC activity. As summarized in Table 2, ∼30% of both fusion proteins failed to bind to the matrix or were removed from the chitin beads by extensive washing with column buffer. Upon rapid passage of the column buffer containing 10 mM Ca2+ and 50 mM DTT (to induce SPM- and intein-mediated cleavage, respectively), only traces of the released AC protein (2%–5%) were eluted (Table 2), while the majority of the free AC domain was eluted from the chitin matrix only after incubation with cleavage-inducing buffer for 6 h. The yield of eluted AC was >60% for both types of fusion proteins, and only low amounts of the AC-SPM-CBD and AC-intein-CBD fusions remained uncleaved and bound to the column (Table 2). The yield of the purified AC domain was in both cases ∼20 mg of the protein per liter of culture, and quite similar purity of the AC domain (>90%) in the elution fractions was achieved using the two different purification systems (data not shown). These results show that the efficiency of the SPM-based protein purification system developed here was quite comparable to the system based on the modified self-cleavable intein.
Table 2.
Comparison of the SPM- and intein-based purification systems
Target proteins are not modified by DTT used in SPM-mediated cleavage reactions
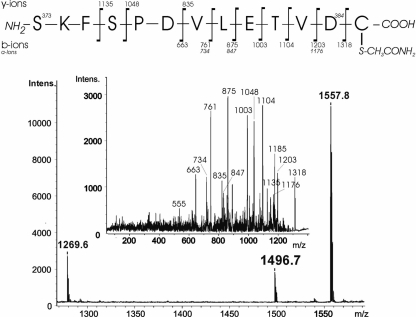
It was important to examine whether a protein released by SPM-mediated cleavage in the presence of a thiol compound (used to inhibit target protein cross-linking) harbors the thiol compound covalently linked to the C-terminal aspartate residue. Therefore, the AC-SPM-CBD fusion was processed in the presence of 10 mM DTT or 10 mM cysteine and the purified AC enzyme was examined for covalent modification at the Asp residue by MALDI-TOF mass spectrometry. Tryptic digests of the AC protein processed in the presence of DTT, however, contained repeatedly only a protonated species at m/z 1336.5, corresponding to the C-terminal SKFSPDVLETVD peptide of AC, the identity of which was confirmed by the post-source decay (PSD) fragmentation analysis (data not shown). We were unable to find in the numerous acquired spectra any AC fragment that would correspond to a C-terminal peptide with covalently linked DTT. This suggests that DTT used to inhibit the cross-linking reaction during SPM-mediated processing of the AC-SPM-CBD fusion proteins was efficiently hydrolyzed from the AC protein. On the other hand, AC released from AC-SPM-CBD upon processing in the presence of 10 mM cysteine contained a tryptic fragment detected as a new protonated species at m/z 1496.7 (Fig. 5). This would correspond to the C-terminal SKFSPDVLETVD peptide with a covalently linked carbamidomethylated cysteine. The identity and structure of the tryptic fragment was confirmed by analysis of daughter ion spectra obtained by PSD fragmentation (Fig. 5), as well as by high-resolution MALDI-FT-ICR analysis (Supplemental Table 1). Both analyses confirmed that the cysteine residue was covalently linked to the C terminus of AC through a peptide bond, having the thiol group accessible to carbamidomethylation with iodoacetamide during sample preparation.
Figure 5.
Cysteine used for inhibition of cross-linking activity upon SPM-mediated cleavage of AC-SPM-CBD is covalently bound to the C terminus of the released AC protein. The tryptic fragment not matching any of the masses in the theoretical AC digest was detected in one of the peptide fractions at m/z 1496.7, and its structure was determined from the PSD daughter ion spectra. Analyzed peptide corresponds to the C-terminal peptide of AC, SKFSPDVLETVD, with covalently linked carbamidomethylated cysteine through a peptide bond.
Discussion
We show here that the self-processing module of the FrpC protein of Neisseria meningitidis can be used as a functional self-cleaving affinity tag for single-step affinity purification of free recombinant proteins.
The GST-SPM-His and MalE-SPM-CBD fusions were used as model proteins to study the SPM-mediated cleavage reaction. It was demonstrated that SPM can be fused to different affinity purification tags (6× poly-His tag or CBD) and different recombinant proteins (GST or MalE) without losing the specific and highly efficient calcium-induced self-processing activity. Incubation of both fusion proteins with 2 mM free calcium ions at 37°C resulted in rapid processing, the kinetics of which were fully comparable to that of the original FrpCΔRTX protein. However, when the kinetics of the cleavage reactions were compared at lower temperatures, significant reduction of the cleavage rate was observed for MalE-SPM-CBD. This difference can be explained by a different conformation of MalE, in comparison to that of GST or of the cleaved-out N-terminal portion of FrpCΔRTX, which might sterically hinder the cleavage reaction catalyzed by SPM. Nevertheless, the cleavage reaction of MalE-SPM-CBD occurred with sufficient efficiency, showing that the SPM-based self-cleaving affinity tag can be used for purification of proteins also at low temperatures, as required for the purification of many recombinant proteins.
Fusion proteins used throughout this work were highly stable both in the prepared clarified crude extracts as well as during purification. Therefore, no protease inhibitors had to be used to avoid their unspecific degradation by E. coli proteolytic enzymes. However, since the self-processing activity of SPM is not blocked by any typical inhibitor of conventional proteases, other than calcium chelating agents (Osicka et al. 2004), protease inhibitors can be used during SPM-based purification of recombinant proteins sensitive to proteolytic degradation.
It has been described that some proteases are not able to cleave a peptide bond when certain amino acid residues are placed N-terminally adjacent to the cleavage site (Shuker et al. 2003; Chu et al. 2006). Similarly, the intein-based self-cleaving affinity tag is not able to efficiently cleave the peptide bond between a protein of interest and intein, when an asparagine, cysteine, or proline residue (Xxx) is immediately linked to the cysteine residue of the cleaved Xxx/Cys bond (see, IMPACT-CN instruction manual at www.neb.com). Here, different recombinant proteins of interest were fused in-frame with SPM, having their C-terminal residue (alanine, glutamate, histidine, serine, tryptophan, tyrosine, or valine) directly fused to the cleaved Asp–Pro bond of SPM. All constructed fusions were found to be efficiently cleaved by SPM, suggesting that those residues are allowed in the given position and that their presence has no negative effect on the SPM-mediated cleavage of the fusion proteins. It also confirms that no extra residues from the original FrpC sequence adjacent N-terminally to the Asp–Pro cleavage site are necessary for self-processing activity of SPM. Moreover, SPM demonstrated strong specificity only for the Asp–Pro cleavage site localized at the N terminus of SPM, and it was not able to nonspecifically cleave Asp–Pro bonds occurring in proteins of interest that were fused to SPM (an Asp–Pro bond occurred once in GST, once in MalE, and four times in LacZ). Moreover, due to the high cleavage specificity of SPM, the released proteins of interest should all have a homogeneous C terminus. This was indeed demonstrated for the AC protein that was, after SPM-mediated cleavage of the AC-SPM-CBD fusion, extensively analyzed by mass spectrometry.
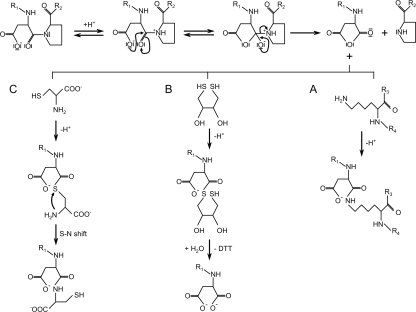
We have recently proposed a possible reaction scheme of processing and cross-linking of FrpC (Osicka et al. 2004), in which binding of calcium ions to SPM of FrpC promotes a conformational change in the molecule that allows interaction of an as yet uncharacterized residue(s) of FrpC with Pro415 and promotes its protonation (Fig. 6). This subsequently promotes a nucleophilic attack of the β-carboxyl group of Asp414 on the carbonyl carbon and rupture of the amide bond to Pro415. A reactive anhydride at Asp414 could then form and either hydrolyze or get attacked by adjacent ε-amino groups of another protein molecule to form a new isopeptide Asp–Lys amide bond (Fig. 6).
Figure 6.
Proposed mechanism of the SPM-mediated cleavage of the Asp–Pro bond of a fusion protein and of the inhibition of the subsequent cross-linking reaction by thiol compounds. Upon calcium binding, the β-carboxyl group of Asp would initiate a nucleophilic attack on the carbonyl carbon of the amide bond with Pro, resulting in an unstable intermediate and disruption of the peptide bond with subsequent formation of a reactive Asp anhydride at the C terminus of the released protein of interest. This can be attacked at the carboxyl carbon of the anhydride ring by the free ε-amino group of a lysine residue of the SPM-based self-cleaving affinity tag, leading to formation of an amide (isopeptide) bond with either α- or β-carboxyl of the Asp residue (only reaction with α-carboxyl is shown) (A). This cross-linking reaction can be efficiently blocked by DTT, the free thiol group of which attacks the carboxyl carbon of the anhydride ring with higher efficiency than ε-amino group of lysine residue (B). A similar reaction occurs when cysteine is used instead of DTT (C). However, while DTT is subsequently hydrolyzed from the aspartate (B), cysteine undergoes a spontaneous S–N acyl shift that results in formation of a peptide bond between aspartate and cysteine (C).
Here, the cross-linking activity was not observed when the purified GST-SPM-His and MalE-SPM-CBD fusion proteins were autocatalytically cleaved in the presence of calcium ions in solution (data not shown). However, when the same proteins were bound and processed on an affinity matrix, much higher yields of the eluted GST or MalE protein were obtained when the immobilized fusion proteins were cleaved in the presence of 10 mM DTT (data not shown). This suggests that the cross-linking activity upon SPM-mediated cleavage was highly increased when the fusion proteins were concentrated on the surface of affinity beads. Hence, to avoid trapping of the proteins of interest on the column by cross-linking to the immobilized self-cleaving affinity tags, the cleavage reaction of immobilized proteins should always be performed in the presence of thiol compounds.
Thiol groups of DTT or cysteine appear indeed to be capable of promoting a nucleophilic attack of the reactive aspartic anhydride with higher efficiency than ε-amino groups of lysine residues and form a thioester bond with the C terminus of the released protein (Fig. 6). While DTT is subsequently hydrolyzed from the C-terminal aspartate of the released protein, cysteine undergoes a spontaneous S–N acyl shift that results in formation of a peptide bond between the cysteine and the C-terminal aspartate of the released protein (Fig. 6). Therefore, while the calcium-induced SPM-mediated cleavage of a fusion protein in the presence of DTT leads to the recombinant protein of interest having only one extra aspartate residue at its C terminus, the cleavage in the presence of cysteine yields the protein of interest with two extra amino acid residues, aspartate and cysteine, covalently attached to its C terminus.
Thiol compounds are also used during the purification process that is based on the intein self-cleaving affinity tag; they are used for induction of the intein-mediated cleavage of fusion proteins. Similarly to the SPM-based purification system, DTT is hydrolyzed from a released protein after intein-catalyzed processing of a fusion protein, while cysteine remains covalently bound to its C terminus (Chong et al. 1997). This observation was also confirmed here by high-resolution mass spectrometric analysis of the trypsinized AC protein released upon the intein-mediated cleavage of the AC-intein-CBD fusion (Supplemental Table 1). However, in contrast to the intein-based purification system, in which the cleavage of fusion proteins can be also induced by hydroxylamine, the SPM-based system is not able to utilize primary amines (such as methylamine or ethylamine) for blocking of the cross-linking reaction that ensues SPM-mediated cleavage of the fusion proteins (data not shown). Hence, primary amines were able to induce the intein-mediated cleavage, but did not compete with the adjacent ε-amino groups of internal lysine residues of the fusion proteins to inhibit the cross-linking reaction.
In conclusion, we present here a new functional self-cleaving affinity tag based on the self-processing module of FrpC and show that it can be simply, specifically, inexpensively, and effectively used for rapid single-step purification of free recombinant proteins.
Materials and Methods
Bacterial strains and growth conditions
The E. coli K12 strain XL1-Blue (Stratagene), used throughout this work for DNA manipulation, was grown in Luria-Bertani (LB) medium supplemented with 150 μg/mL ampicillin or 60 μg/mL kanamycin. The BL21(λDE3) E. coli strain (Novagen) was used for expression of the recombinant proteins and was grown at 30°C in LB medium containing 150 μg/mL ampicillin or 60 μg/mL kanamycin.
Plasmids
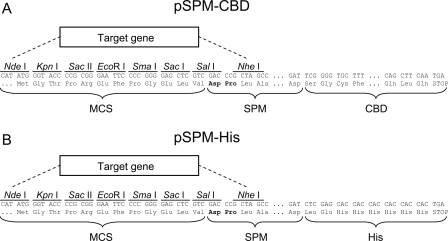
pSPM-CBD and pSPM-His
Expression vectors harboring a DNA fragment coding for a self-processing module (SPM) of FrpC (amino acid residues 414–657) fused in-frame to a sequence coding for the chitin-binding domain (CBD), or 6× poly-His tag (His) at the 3′-terminus and to a multiple cloning sequence (MCS) at the 5′-terminus (Fig. 7). The MCS was designed for insertion of a target gene in-frame with the sequence for SPM-CBD or SPM-His, thus allowing the expression of a target protein fused to SPM-CBD or SPM-His, respectively. The fusion gene is placed in both vectors under the control of the transcription and translation initiation signals of gene 10 from bacteriophage T7. Construction of pSPM-CBD and pSPM-His and their derivatives for expression of fusion proteins used throughout this work is described in detail in the Supplemental material.
Figure 7.
Cloning regions of pSPM-CBD and pSPM-His vectors. The pSPM-CBD (A) and pSPM-His (B) cloning regions harbor the same multiple cloning site (MCS), containing recognition sequences for eight different restriction endonucleases and allowing simple insertion of a target gene, which is fused in-frame to a DNA fragment coding for self-processing module (SPM) of FrpC. MCS-SPM is simultaneously fused in-frame to a chitin-binding domain of Bacillus circulans (CBD) in pSPM-CBD or to a 6× poly-His tag (His) in pSPM-His, respectively. The Asp–Pro bond autocatalytically and specifically cleaved in a fusion protein upon activation by calcium ions is indicated (bold).
pTYB2frpCΔrtx
This plasmid was used for expression of the truncated FrpCΔRTX protein, lacking the 967 C-terminal residues of the RTX domain. Plasmid construction and FrpCΔRTX purification were described previously (Osicka et al. 2004).
Protein production and purification
All recombinant proteins were produced in E. coli strain BL21(λDE3) transformed with the appropriate plasmid. Exponential 500-mL cultures grown with shaking at 30°C in LB medium supplemented with 150 μg/mL ampicillin or 60 μg/mL kanamycin were induced at OD600 = 0.6–0.8 with 1 mM IPTG and grown for an additional 4 h. Then the cells were harvested by centrifugation, washed twice with 50 mM Tris-HCl (pH 7.4), 150 mM NaCl (TN buffer), 5 mM EDTA to remove Ca2+ ions associated with the cell surface, resuspended in TN buffer containing 1 mM EDTA (EDTA buffer) or TN buffer alone (cells expressing proteins to be purified on a Ni-NTA agarose), and disrupted by sonication at 4°C. The homogenates were cleared at 20,000g for 20 min and the obtained clarified extracts were used for purifications.
For SPM-based protein purification on chitin resins (New England Biolabs), the clarified extract containing MalE-SPM-CBD, LacZ-SPM-CBD, CAT-SPM-CBD, or AC-SPM-CBD was loaded on a chitin bead column equilibrated with EDTA buffer. After washing of the column with 10 bed volumes of the same buffer, EDTA buffer containing 10 mM free calcium ions and 10 mM DTT (or β-mercaptoethanol or cysteine) was loaded on the column and the flow was stopped for 6 h of incubation at 23°C or for overnight incubation at 4°C, to promote self-excision of the SPM-CBD domain from the fusion protein. The excised free target protein (MalE, LacZ, CAT, or AC) was then eluted by restoring buffer flow through the column. In the final step, Ca2+ and DTT (or β-mercaptoethanol or cysteine) were removed from the purified protein samples by gel filtration on Sephadex G-25 equilibrated with EDTA buffer. When the AC-intein-CBD fusion protein was purified, TN buffer containing 10 mM Ca2+ and 50 mM DTT was used to induce self-excision of the intein-CBD domain from the fusion protein, while all further steps were identical to those described above for fusions containing SPM-CBD.
Recombinant GST-SPM-His, LacZ, and CAT harboring a 6× poly-His tag were purified by affinity chromatography on a Ni-NTA agarose (Qiagen) equilibrated with TN buffer. After washing of the column with 10 bed volumes of the same buffer, the proteins were eluted with TN buffer complemented with 250 mM imidazole that was removed by gel filtration on Sephadex G-25 equilibrated with EDTA buffer. When the GST-SPM-His fusion protein was purified on a Ni-NTA agarose using SPM-mediated cleavage, the clarified extract was loaded on the Ni-NTA column equilibrated with TN buffer, contaminating proteins were removed with 10 bed volumes of the same buffer, and self-excision of the SPM-His domain from the fusion protein was induced by addition of TN buffer supplemented with 10 mM Ca2+ and 10 mM DTT. The released GST protein was eluted from the column after 6 h of incubation at 23°C or after overnight incubation at 4°C with TN buffer. In the final step, Ca2+ and DTT were removed from the purified protein samples by gel filtration on a Sephadex G-25 equilibrated with EDTA-buffer.
The uncleaved MalE-SPM-CBD fusion for in vitro processing studies was purified on an amylose affinity resin (New England Biolabs). Equilibration of the column and all washes were performed in EDTA buffer, and MalE-SPM-CBD was eluted with the same buffer complemented with 10 mM maltose. Finally, maltose was removed from the purified fusion protein by gel filtration on Sephadex G-25 equilibrated with EDTA buffer.
Standard techniques
SDS polyacrylamide gel electrophoresis (SDS-PAGE), determination of protein concentration, and in vitro DNA manipulations were performed according to standard protocols (Sambrook et al. 1989).
In vitro calcium-dependent processing and densitometric analysis
Processing of the purified GST-SPM-His, MalE-SPM-CBD, and FrpCΔRTX proteins was initiated by addition of EDTA buffer supplemented with indicated concentrations of CaCl2. The concentrations of free calcium ions were adjusted in the reaction mixtures to 2, 5, 10, or 50 mM by a Ca2+-EDTA buffering system, based on calculations using the WEBMAXC 2.10 software (http://www.stanford.edu/∼cpatton/webmaxcS.htm). The reaction mixtures were incubated for indicated times at 4°C or 23°C, and the reactions were stopped by mixing the samples with SDS-PAGE loading buffer containing 10 mM EDTA. In unprocessed controls, calcium addition was omitted and the purified fusion proteins were incubated in EDTA buffer under otherwise identical conditions. Samples were separated by SDS-PAGE, and Coomassie-stained gels were digitalized and analyzed as described elsewhere (Osicka et al. 2004). The experiments were repeated four times; the given values represent the average ± standard deviation.
Assay of β-galactosidase, chloramphenicol acetyltransferase, and adenylate cyclase activities
β-galactosidase (LacZ), chloramphenicol acetyltransferase (CAT), and adenylate cyclase (AC) activities were measured as previously described (Miller 1972; Shaw 1975; Ladant 1988).
Mass spectrometric analysis
Mass spectra of tryptic peptides of the AC protein were acquired using the MALDI-TOF mass spectrometer BIFLEX (Bruker-Franzen) and high-resolution MALDI-FT-ICR mass spectrometer APEX-Q FTMS (Bruker Daltonics). Details are given in the Supplemental material.
Electronic supplemental material
Supplemental material includes a detailed description of the construction of expression vectors; details regarding sample preparation and analysis of the C-terminal peptide of the AC protein by mass spectrometry; and a table summarizing results of analysis of the C-terminal peptide of AC by MALDI-FT-ICR mass spectrometry.
Acknowledgments
This work was supported by a grant from the National Science Foundation of the Czech Republic (No. GACR 310/06/720), grant No. KAN200520702 from the Academy of Sciences of the Czech Republic, and the Institutional Research Concept AV0Z50200510. We thank S. Charvatova and H. Kubinova for excellent technical help.
Footnotes
Supplemental material: see www.proteinscience.org
Reprint requests to: Radim Osicka, Institute of Microbiology AS CR, v.v.i., Videnska 1083, CZ-142 20 Prague 4, Czech Republic; e-mail: osicka@biomed.cas.cz; fax: (420) 241-062-152.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.035733.108.
References
- Arnau, J., Lauritzen, C., Petersen, G.E., Pedersen, J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif. 2006;48:1–13. doi: 10.1016/j.pep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Chong, S., Mersha, F.B., Comb, D.G., Scott, M.E., Landry, D., Vence, L.M., Perler, F.B., Benner, J., Kucera, R.B., Hirvonen, C.A., et al. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene. 1997;192:271–281. doi: 10.1016/s0378-1119(97)00105-4. [DOI] [PubMed] [Google Scholar]
- Chu, L.H., Choy, W.Y., Tsai, S.N., Rao, Z., Ngai, S.M. Rapid peptide-based screening on the substrate specificity of severe acute respiratory syndrome (SARS) coronavirus 3C-like protease by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Protein Sci. 2006;15:699–709. doi: 10.1110/ps.052007306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, C.F., Suominen, I., Glatz, C.E. Fusion tails for the recovery and purification of recombinant proteins. Protein Expr. Purif. 1991;2:95–107. doi: 10.1016/1046-5928(91)90057-p. [DOI] [PubMed] [Google Scholar]
- Graslund, S., Nordlund, P., Weigelt, J., Bray, J., Gileadi, O., Knapp, S., Oppermann, U., Arrowsmith, C., Hui, R., Ming, J., et al. Protein production and purification. Nat. Methods. 2008;5:135–146. doi: 10.1038/nmeth.f.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, J.L. Cloning technologies for protein expression and purification. Curr. Opin. Biotechnol. 2006;17:359–366. doi: 10.1016/j.copbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Hunt, I. From gene to protein: A review of new and enabling technologies for multi-parallel protein expression. Protein Expr. Purif. 2005;40:1–22. doi: 10.1016/j.pep.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Jenny, R.J., Mann, K.G., Lundblad, R.L. A critical review of the methods for cleavage of fusion proteins with thrombin and factor Xa. Protein Expr. Purif. 2003;31:1–11. doi: 10.1016/s1046-5928(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Ladant, D. Interaction of Bordetella pertussis adenylate cyclase with calmodulin: Identification of two separated calmodulin-binding domains. J. Biol. Chem. 1988;263:2612–2618. [PubMed] [Google Scholar]
- Mao, H. A self-cleavable sortase fusion for one-step purification of free recombinant proteins. Protein Expr. Purif. 2004;37:253–263. doi: 10.1016/j.pep.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Miller, J.H. Experiments in molecular genetics. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Nilsson, J., Stahl, S., Lundeberg, J., Uhlen, M., Nygren, P.A. Affinity fusion strategies for detection, purification, and immobilization of recombinant proteins. Protein Expr. Purif. 1997;11:1–16. doi: 10.1006/prep.1997.0767. [DOI] [PubMed] [Google Scholar]
- Osicka, R., Prochazkova, K., Sulc, M., Linhartova, I., Havlicek, V., Sebo, P. A novel “clip-and-link” activity of repeat in toxin (RTX) proteins from gram-negative pathogens. Covalent protein cross-linking by an Asp-Lys isopeptide bond upon calcium-dependent processing at an Asp-Pro bond. J. Biol. Chem. 2004;279:24944–24956. doi: 10.1074/jbc.M314013200. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., Maniatis, T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Shaw, W.V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shuker, S.B., Mariani, V.L., Herger, B.E., Dennison, K.J. Understanding HTLV-I protease. Chem. Biol. 2003;10:373–380. doi: 10.1016/s1074-5521(03)00104-2. [DOI] [PubMed] [Google Scholar]