Abstract
The tumor suppressor p53 can be expressed as different isoforms because of promoter selection and mRNA editing. One isoform, “delta p53” (Δp53), results from what would be an unusual alternative splicing of exons 7/8 of the p53 gene, conserving the reading frame and generating a novel protein with proposed transcriptional activity essential for the intra S-phase checkpoint. Here, we show that the deletion of the 66 residues that correspond to strand β10 and the C-terminal helix of the core domain and the interconnecting linker to the tetramerization domain occurring in the Δp53 isoform leads to a misfolded and unstable protein, prone to form soluble aggregates, which does not bind the p21 promoter site. The complex of coexpressed Δp53 and flp53 is soluble in vitro and binds poorly to DNA. Our results provide a structural explanation for the dominant-negative effect of Δp53 and its lack of transcriptional activity.
Keywords: protein structure/folding, structure/function studies, DNA-binding domains, dominant-negative mutation
Alteration of tissue homeostasis and formation of a tumor result from deregulation of cell differentiation, death rate, and proliferation. p53 is a node in regulation of these processes, and 50% of the human cancers contain mutations and alterations in its gene (Hainaut and Hollstein 2000). The p53-related genes p63 and p73 express a large number of isoforms derived from alternative splicing of the C terminus or species lacking the N-terminal transactivation domain (Deyoung and Ellisen 2007). These can bind to p53 response elements and exert dominant-negative effects either by competing for DNA-binding sites or by direct protein–protein interactions (Harms and Chen 2005; Deyoung and Ellisen 2007). p53 isoforms arise either from differential promoter selection and/or alternative splicing (Bourdon et al. 2005; Rohaly et al. 2005). One of the proposed novel isoforms, delta p53 (Δp53), results from alternative splicing of exons 7/8, conserving the frame and generating a deletion of 66 residues (257–322) in the DNA-binding domain and part of the loop linking to the tetramerization domain of p53 (Rohaly et al. 2005). However, the splicing mechanism that would lead to Δp53 differs from that found in 99% of the genes transcribed by RNA polymerase II (Prives and Manfredi 2005; Murray-Zmijewski et al. 2006), where a 5′ donor splice site starting with GU and a 3′ acceptor splice site ending with AG are required (Nilsen 1994).
Full-length p53 (flp53) has intrinsically disordered regions (Wells et al. 2008) and two globular domains: the DNA-binding domain, which is unstable per se (Cho et al. 1994; Canadillas et al. 2006; Ho et al. 2006), and the tetramerization domain (Lee et al. 1994; Clore et al. 1995). The quaternary structure of flp53 has an elongated cross-shaped structure with a pair of loosely coupled core domain dimers at the ends, which are accessible for DNA binding (Tidow et al. 2007). Residues 257–322, spliced out in the Δp53 isoform, correspond to strand β10 and the C-terminal helix of the core domain and the interconnecting linker to the tetramerization domain (Fig. 1). Here, we show that deletion of the 66 residues from flp53 yields a misfolded protein, and we characterize its structural features. This novel protein was able to form a complex with flp53, modifying its biophysical properties in solution. Δp53 did not bind the p21 promoter site, and the hybrid complex Δp53–flp53 bound the DNA very weakly. Δp53 was mainly localized in the cytoplasm, but when coexpressed with flp53 both proteins colocalized in the nucleus.
Figure 1.
Structural elements deleted in Δp53 with respect to flp53. (A) SAXS model of p53 in solution (Tidow et al. 2007). Core domains (cyan) and tetramerization domain (blue) are shown as cartoons, and connecting linkers in semitransparent spacefill mode. Residues 257–322 that are deleted in Δp53 are colored orange. For clarity, the flexible N and C termini are not shown. (B) Close-up of p53 DNA-binding core domain with residues deleted in Δp53 shown in orange.
Results
Biophysical characterization of Δp53
Residues 94 to 292 of the p53 protein correspond to a structural domain defined as the core domain. It is a DNA-binding domain that belongs to the immunoglobulin-like fold with a central β-sandwich of two antiparallel β-sheets and contains a structural zinc ion chelated by Cys-176, His-179, Cys-238, and Cys-242 (Fig. 1B; Joerger and Fersht 2007). Deletion of residues 257–292 led to a misfolded core domain (ΔCd). Figure 2A shows the far-UV circular dichroism (CD) spectra of the native core domain and of ΔCd, indicating a clear loss in its secondary structure content. The native core domain has an apparent melting temperature of 44°C (Ang et al. 2006), while ΔCd lost all cooperativity of denaturation and displayed only a linear increase of the CD signal at 222 nm (Fig. 2B). In addition, ΔCd was unstable in solution and prone to form soluble aggregates. The analytical ultracentrifugation (AUC) sedimentation profile of the native core domain corresponded to a molecular weight of ∼24 kDa, while the AUC profile of ΔCd corresponded to an average mass of ∼204 kDa, indicating a high oligomerization state (Fig. 2C). This truncated core domain did not contain Zn as tested by the PAR/PMPS colorimetric assay (Hunt et al. 1985; data not shown). Zn-binding proteins are to be able to form high molecular soluble oligomers in the absence of the metal (Alonso et al. 2004; García-Alai et al. 2007).
Figure 2.
Biophysical characterization of p53 truncated core domain. (A) Far-UV CD spectra for p53 core domain (gray) and ΔCd (black). (B) Thermal denaturation for p53 core domain (○) and ΔCd (●). (C) AUC sedimentation profiles for p53 core domain and ΔCd show apparent molecular masses of 24 ± 0.3 kDa and 204 ± 17 kDa, respectively. Data were fitted to a single exponential model. Color code as in B.
Next we carried out structural studies with the Δp53 isoform in the context of flp53. We used small-angle X-ray scattering (SAXS) to investigate overall protein dimensions in solution. A comparison of the processed experimental patterns (scattering intensity I vs. momentum transfer s, = 4πsinθ/λ, where 2θ is the scattering angle and λ = 1.5 Å is the wavelength) is displayed in Figure 3A and the corresponding Kratky plot in Figure 3C. Δp53 showed a very different scattering pattern from flp53, with a large slope at small angles indicating the presence of soluble high molecular weight oligomers. This observation is consistent with a nonlinear Guinier plot for Δp53 (data not shown). The distance distribution function revealed that the average particle size of Δp53 was significantly larger than that of flp53 (Dmax 600 Å vs. 240 Å) (Fig. 3B).
Figure 3.
SAXS analysis of Δp53 and flp53. (A) Experimental intensities for Δp53 (black) and flp53 (gray). The scattering profiles are displaced along the ordinate for better visualization. (B) The distance distribution plots computed from the experimental data and normalized to the maximum value of unity. Color code as in A. (C) Kratky plots of SAXS data for Δp53 and flp53 normalized to the maximum value of unity. Color code as in A. The maximum at small angles indicates the presence of folded domains.
As SAXS data represent an average over all species present in solution, the existence of a minor population of tetrameric Δp53 could not be excluded although the overall parameters indicated that the majority of protein existed as higher oligomeric forms. The AUC sedimentation profile of Δp53 was consistent with the SAXS data. The absorbance of Δp53 (recorded at 280 nm) was lower than that measured for flp53 as a result of aggregation and faster sedimentation of the high molecular weight species during the experiment. The remaining species had a sedimentation coefficient that could correspond to tetramers (data not shown).
We analyzed the thermal stability for both proteins by far-UV CD. The absolute value for the molar ellipticity at 222 nm at 20°C was smaller for Δp53 than for the full-length protein, indicating its lower content of α-helical secondary structure (Fig. 4A). flp53 had a first transition (20°C–65°C) with an apparent Tm = 50 ± 0.1°C that corresponds to denaturation of the DNA-binding domain in the context of the full-length protein (Fig. 4B; Bullock et al. 2000; Ang et al. 2006). This behavior was not observed for the Δp53 isoform, which only shows a small amplitude in its curve (Fig. 4A). Both proteins had an increase in their 222-nm molar ellipticity signal ∼70°C. This second transition had an apparent Tm = 74 ± 0.2°C for Δp53 (Fig. 4C) corresponding to denaturation of the tetramerization domain (Mateu and Fersht 1998, 1999). The Δp53 isoform had only the transition corresponding to unfolding of the tetramerization domain, indicating it was still capable of oligomerization but lacked a structured DNA-binding domain.
Figure 4.
(A) Thermal denaturation curves for flp53 (gray) and Δp53 (black), molar ellipticity at 222 nm is plotted as a function of temperature. Curves are fitted to a two-state model showing denaturation of the DNA-binding domain for flp53 (B) and tetramerization domain for Δp53 (C).
The decrease in CD signal ∼220 nm is not due to an increase in the helix content of the protein but to the rearrangement of its β-sheets due to hydrophobic interactions. The core domain of p53 has a central β-sandwich of two antiparallel β-sheets. Many β-sheet-containing proteins show this behavior; they display a decrease in signal ∼217 nm as temperature is increased. This corresponds to the denaturation-aggregation of the protein.
Characterization of the Δp53–flp53 complex
Whereas flp53 was expressed as a soluble protein, Δp53 was expressed in inclusion bodies. But, when flp53 and Δp53 were coexpressed, both proteins were detected in the soluble fraction. Coexpression of flp53 and Δp53 led to the formation of a mixed Δp53–flp53 complex as revealed by analytical gel filtration. After coexpression only one peak corresponding to the Δp53–flp53 complex could be detected in the void volume (Fig. 5). No peak corresponding to tetrameric p53, which has a significantly smaller hydrodynamic radius than Δp53, could be detected. This implicated that coexpression of flp53 and Δp53 leads to the formation of mixed oligomers with a large hydrodynamic radius.
Figure 5.
Size exclusion chromatography elution profiles for flp53 and Δp53 when mixed (gray line), and Δp53–flp53 complex when coexpressed (black line). Bars denote the positions of the molecular weight standards: (left to right) thyroglobulin (669 kDa); ferritin (440 kDa); aldolase (158 kDa); conalbumin (75 kDa); ovoalbumin (43 kDa).
While ectopically expressed Δp53 was localized mainly in the cytoplasm of H1299 cells (p53 null cells) (Fig. 6B), consistent with the deletion of its nuclear localization signal (NLS, residues 316–325) (Shaulsky et al. 1990; Chan and Poon 2007), coexpression of flp53 and Δp53 causes the Δp53–flp53 complex to localize in the nucleus (Fig. 6E). In this case flp53 provided the NLS and shuttled Δp53 to the nucleus after forming a complex.
Figure 6.
Confocal sections through transiently transfected H1299 cells. GFP–p53 and HA–Δp53 were transfected alone (A,B), or cotransfected (C,D, and merged in E). HA–Δp53 is cytoplasmic but relocalized to the nucleus upon coexpression with GFP–p53.
Δp53 does not bind to the p21 response element and significantly reduces DNA binding when forming a mixed Δp53–flp53 complex
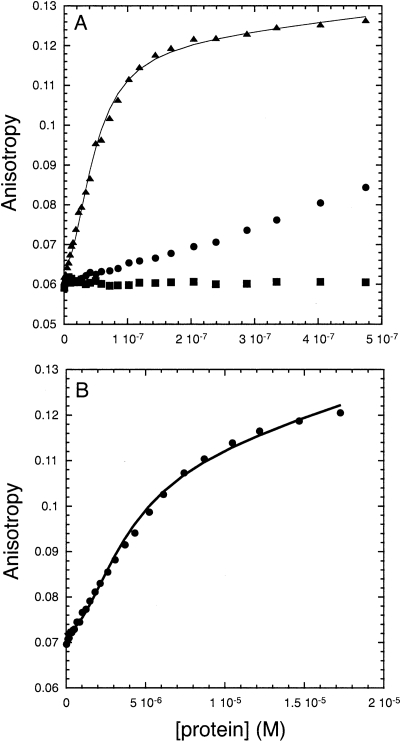
It has been proposed that Δp53 transactivates the endogenous p21 and 14-3-3σ promoters in damaged S-phase cells (Rohaly et al. 2005). In contrast, this isoform does not transactivate either the promoters of p21 and mdm2 in H1299 cells (Chan and Poon 2007). Using fluorescence anisotropy, we have previously measured the affinity of tetrameric p53 to fluorescein-labeled specific DNA (Weinberg et al. 2004; Ang et al. 2006). Here, we found that Δp53 did not bind the p21 response element (Fig. 7). flp53 bound to it with a Kd = 47 ± 0.2 nM. The complex of flp53 and Δp53 (1 μM monomer concentration, same as for flp53) had an almost linear increase in anisotropy signal over a protein concentration range of 0–500 nM, showing that the coexpressed Δp53–flp53 complex bound only weakly to the p21 promoter site. Binding was observed at μM concentrations of the complex (Fig. 7B) with a Kd = 3.7 ± 0.2 μM. The binding was most likely due to the presence of a small portion (∼1%) of homotetrameric flp53.
Figure 7.
(A) Binding of p53, Δp53, and the coexpressed complex Δp53–flp53 to the p21 DNA promoter site. One μM (monomer concentration) of flp53 (▲), Δp53 (■), and Δp53-flp53 (●) was measured at 20°C in 25 mM phosphate buffer (pH 7.2), 225 mM NaCl, and 5 mM DTT. (B) Titration using 100 μM of the complex Δp53–flp53.
Discussion
Editing of mRNA increases the coding capacity of a gene by expressing several related proteins, which introduces diversity in their functions and even antagonistic functions (Kriventseva et al. 2003). Alternative splicing is a crucial mechanism for regulating cell differentiation and developmental stages, and it has been recently shown that splicing patterns can be significantly altered in tumors (Hu and Fu 2007; Karni et al. 2007). The discovery of p53 isoforms could unveil different mechanisms in the tumor suppressor regulation, modulation of its activity, and also novel functions.
Here, we showed that the proposed Δp53 isoform (Rohaly et al. 2005) is an unfolded protein. This isoform, lacking a part of the DNA-binding domain and connecting the linker to the tetramerization domain, was unstable in solution and prone to form soluble aggregates. Deletion of residues 257–322 from flp53 to form Δp53 removes essential elements for the structure and the function of p53; the end of β-stand β9 and the entire β-stand β10, which is the longest strand within the core domain, are involved in a strong hydrogen-bond network and form an essential part of the immunoglobulin-like β-sandwich (Fig. 1). This β-sandwich is responsible for the stability of the DNA-binding core domain, and thus removal of a major strand should cause unfolding. The C-terminal helix is involved in DNA binding and further connects the core domain to the tetramerization domain via a linker, which is also entirely removed in Δp53 (Fig. 1). This would cause the remaining part of the core domain to be positioned in close proximity to the tetramerization domain, most likely introducing steric clashes, even if it were folded. Our results provide for the first time a structural explanation why Δp53 on its own is not able to bind DNA (Fig. 7), and its complex with flp53 binds only weakly to the p21 site. This lack in DNA-binding ability also occurs in the so-called p53 “hot-spot mutants,” which can be further classified into “structural mutants” and “contact mutants” (Joerger et al. 2005, 2006), both showing little or no transcriptional activity. Thus, it is unlikely that Δp53 can act as a transcription factor.
Despite the loss of a folded DNA-binding core domain, Δp53 retains its tetramerization domain (Fig. 4) and has the capacity to form mixed tetramers with p53. We coexpressed p53 and Δp53 in Escherichia coli and purified the complex. The fact that both proteins were expressed in soluble form suggests the existence of mixed oligomers (see Fig. 5), which is consistent with Δp53 lacking a NLS being imported to the nucleus when coexpressed with flp53 in H1299 cells (Fig. 6). The mixed oligomer species, however, do not show the same DNA-binding activity as tetrameric flp53 anymore. This emphasizes that Δp53 has a dominant-negative effect on flp53. In general, a similar dominant-negative effect will most likely occur for every p53 splice variant with intact tetramerization domain but defect in the DNA-binding core domain.
The detection of the mRNA encoding for Δp53 as well as the existence of the polypeptide and its transcriptional activity is the subject of debate (Murray-Zmijewski et al. 2006; Chan and Poon 2007). While Δp53 mRNA could be detected in human breast tumor samples (Baumbusch et al. 2006), it could not be detected in a large number of normal human tissues or tumors in another study (Murray-Zmijewski et al. 2006). If Δp53 is a product of alternative splicing and has been positively selected through evolution, the reason for this is most likely inhibition of transcription rather than selective transcriptional activation. There is a correlation between protein thermodynamic stability and degradation (Canadillas et al. 2006), and Δp53 could also regulate p53 turnover by making the tetramer more unstable.
Whereas the existence of Δp53 at the mRNA level is still controversial (Murray-Zmijewski et al. 2006), the structural characterization of this novel p53 isoform provides new insights into the importance of defined structural elements and gives a structural explanation for its lack of transcriptional activity. If this protein is the product of a deletion and not the result of splicing, then it could be considered as a dominant-negative mutation.
Materials and Methods
Plasmids and recombinant proteins
flp53 and core domain were expressed in E. coli and purified as described (Veprintsev et al. 2006). ΔCd was amplified by PCR with the following primers: Fw5′-GGTGGATCCTCATCTTCTGTCCC-3′ and Rev5′-TCGAATTCAGGTGTTGTTGGGCA-3′. The Δp53 was amplified by inverse PCR from the wild-type p53 clone, deleting residues 257 to 322 using the following primers: Fw5′-CTGGATGGAGAATATTTCACC-3′ and Rev5′-TGTGATGATGGTGAGGATGGG-3′. Both ΔCd and Δp53 were expressed as inclusion bodies. These were dissolved in 6 M GdmHCl, diluted in buffer A (20 mM sodium phosphate [pH 7.2], 150 mM NaCl, and 6 M urea), loaded onto a HisTrap column (GE Healthcare), and eluted in buffer A containing 250 mM imidazole. The refolding step was carried out at 4°C by dialysis against 20 mM sodium phosphate (pH 7.2), 40 μM ZnSO4, and 1 mM DTT. Further purification of both proteins was performed as for flp53 (Veprintsev et al. 2006). For the Δp53–flp53 complex, BL21 (DE3) were cotransformed with pET24–Δp53 and pRSET–flp53 and grown at 22°C in 2TY media containing 50 and 100 μg/mL kanamycin and ampicilin, respectively. Cells were harvested 18 h after induction. Both proteins were expressed as soluble proteins and purification of the complex was carried out as described for flp53 (Veprintsev et al. 2006). For cell transfections flp53 and Δp53 were subcloned into pCDNA 3.1. For immunofluorescence the following vectors were constructed: pEGFP–flp53 and pCHA–Δp53.
Small-angle X-ray scattering (SAXS)
SAXS data were collected at the X33 beamline at EMBL/DESY, Hamburg (Roessle et al. 2007), following standard procedures. Repetitive data collection on the same sample was performed to monitor possible radiation damage, and no damage was detected. The camera length was 2.7 m at X33 covering the range of scattering vectors 0.012 < s < 0.47 Å−1 at a wavelength λ = 1.5 Å. The sample concentration was 3.5 mg mL−1 in 25 mM sodium phosphate (pH 7.2), 150 mM NaCl, 5 mM DTT. The data were processed using PRIMUS (Konarev et al. 2003). The radius of gyration (R g) was evaluated using the Guinier approximation (Guinier 1939) (I(s) = I(0)exp(−s 2 R g 2/3) for sR g < 1.3) and also from the entire scattering curve with the program GNOM (Svergun 1992), the latter also provided distance distribution function p(r) and the maximum dimension Dmax. The M r of the solutes were evaluated by comparison of the forward scattering intensity with that from a BSA reference solution (M r = 66 kDa).
Circular dichroism
Measurements were performed using a Jasco J-815 spectropolarimeter; 20 μM of p53 core domain, ΔCd, flp53, or Δp53 were incubated for 4 h in 20 mM Tris (pH 7.5), 150 mM NaCl, and 1 mM DTE. Melting curves were monitored following the amplitude of negative band at 222 nm as a function of temperature with an increasing slope of 1°C/min and fitted to a two-state model as described (Neira et al. 2000).
Analytical gel filtration
A 0.5 mL of 30 μM of flp53, Δp53, and the complex p53–Δp53 were injected onto an analytical Superdex 200 HR10/30 column. Buffer conditions were 20 mM sodium phosphate buffer (pH 7.2), 150 mM KCl, 10 mM 2-mercaptoethanol, and 10% glycerol. Molecular weight standards (Pharmacia) were run under the same conditions; V0 corresponds to elution of blue-dextran.
Analytical ultracentrifugation
Equilibrium sedimentation experiments were performed with a Beckman XL-I Ultracentrifuge equipped with a Ti-60 rotor and 6-sector cells at 3000, 10,000 and 30,000 rpm, respectively. Data were collected at 10°C following absorbance at 280 nm, at a protein concentration of 20 mM. Buffer conditions were 25 mM sodium phosphate (pH 7.2), 150 mM NaCl, and 1 mM DTT. Samples were considered to be at equilibrium as judged by a comparison of the several scans of each. Data were analyzed using the UltraSpin software (http://ultraspin.mrc-cpe.cam.ac.uk/).
DNA binding
Fluorescence anisotropy measurements were recorded on a Cary Eclipse fluorescence spectrophotometer equipped with a Hamilton Microlab titrator controlled by laboratory software. The excitation and emission wavelengths were 480 nm and 530 nm, respectively, and the slit widths for excitation and emission were 20 nm with photomultiplier voltage of 600 V. The concentrations of flp53 and Δp53 were 1 μM in terms of monomer. The concentration of the flp53–Δp53 complex titrant, in terms of monomer species, was 1 μM and 100 μM, respectively. The initial concentration of DNA was 20 nM. Experiments were performed at 20°C in 25 mM phosphate buffer (pH 7.2), 225 mM NaCl, 5 mM DTT. p53 protein was titrated into a cuvette containing 1.2 mL fluorescein-labeled DNA as described in Weinberg et al. (2004). Fluorescence anisotropy was calculated from the fluorescence intensities (Lakowicz 1999) and analysis of binding was performed using a simple one-site binding isotherm with 4:1 stoichiometry (Weinberg et al. 2004).
Cell culture
H1299 cells were grown and maintained in Roswell Park Memorial Institute (RPMI) medium (Invitrogen) supplemented with 10% fetal calf serum (FCS). Cells were subcultured at a ratio of 1:6 the day before transfection in 35-mm culture wells and transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were grown on glass coverslips. The coverslips were removed 24 h after transfection and rinsed briefly with PBS. The cells were fixed for 20 min with 4% paraformaldehyde in PBS, then permeabilized by washing three times with PBS + 0.1% Triton X-100 (TX100). For blocking, the coverslips were incubated for 30 min with PBS + 0.1% TX100 + 1% BSA and incubated for 1 h with rat anti-HA (Roche) diluted 1:100 in the same solution. Coverslips were then washed three times with PBS + 0.1% TX100 and incubated for 1 h in the dark with Alexa Fluor 488 goat anti-mouse antibody (Molecular Probes) diluted 1:200 in blocking solution. Coverslips were again washed three times with PBS + 0.1% TX100, then air-dried and mounted in Fluoromount-G (SouthernBiotech). Images were collected on a Bio-Rad Radiance confocal microscope.
Acknowledgments
We thank Robert Sade and Caroline Blair for helpful discussions and technical assistance, and Efstratios Mylonas for assistance with SAXS measurements.
Footnotes
Reprint requests to: Alan R. Fersht, Medical Research Council Centre for Protein Engineering, Hills Road, Cambridge CB2 0QH, United Kingdom; e-mail: arf25@mrc-lmb.cam.ac.uk; fax: 44-1223-402140.
Abbreviations: flp53, full-length p53; Δp53, delta p53 (deletion of residues 257–322); ΔCd, delta core domain (residues 94–256); SAXS, small-angle X-ray scattering; CD, circular dichroism; AUC, analytical ultracentrifugation; GFP–flp53, flp53 fusion with green fluorescent protein; HA–Δp53, flp53 fusion with hemagglutinin.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.036996.108.
References
- Alonso, L.G., García-Alai, M.M., Smal, C., Centeno, J.M., Iacono, R., Castano, E., Gualfetti, P., de Prat-Gay, G. The HPV16 E7 viral oncoprotein self-assembles into defined spherical oligomers. Biochemistry. 2004;43:3310–3317. doi: 10.1021/bi036037o. [DOI] [PubMed] [Google Scholar]
- Ang, H.C., Joerger, A.C., Mayer, S., Fersht, A.R. Effects of common cancer mutations on stability and DNA binding of full-length p53 compared with isolated core domains. J. Biol. Chem. 2006;281:21934–21941. doi: 10.1074/jbc.M604209200. [DOI] [PubMed] [Google Scholar]
- Baumbusch, L.O., Myhre, S., Langerod, A., Bergamaschi, A., Geisler, S.B., Lonning, P.E., Deppert, W., Dornreiter, I., Borresen-Dale, A.L. Expression of full-length p53 and its isoform Δp53 in breast carcinomas in relation to mutation status and clinical parameters. Mol. Cancer. 2006;5:47. doi: 10.1186/1476-4598-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon, J.C., Fernandes, K., Murray-Zmijewski, F., Liu, G., Diot, A., Xirodimas, D.P., Saville, M.K., Lane, D.P. p53 isoforms can regulate p53 transcriptional activity. Genes & Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock, A.N., Henckel, J., Fersht, A.R. Quantitative analysis of residual folding and DNA binding in mutant p53 core domain: Definition of mutant states for rescue in cancer therapy. Oncogene. 2000;19:1245–1256. doi: 10.1038/sj.onc.1203434. [DOI] [PubMed] [Google Scholar]
- Canadillas, J.M., Tidow, H., Freund, S.M., Rutherford, T.J., Ang, H.C., Fersht, A.R. Solution structure of p53 core domain: Structural basis for its instability. Proc. Natl. Acad. Sci. 2006;103:2109–2114. doi: 10.1073/pnas.0510941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, W.M., Poon, R.Y. The p53 isoform Δp53 lacks intrinsic transcriptional activity and reveals the critical role of nuclear import in dominant-negative activity. Cancer Res. 2007;67:1959–1969. doi: 10.1158/0008-5472.CAN-06-3602. [DOI] [PubMed] [Google Scholar]
- Cho, Y., Gorina, S., Jeffrey, P.D., Pavletich, N.P. Crystal structure of a p53 tumor suppressor-DNA complex: Understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Clore, G.M., Ernst, J., Clubb, R., Omichinski, J.G., Kennedy, W.M., Sakaguchi, K., Appella, E., Gronenborn, A.M. Refined solution structure of the oligomerization domain of the tumour suppressor p53. Nat. Struct. Biol. 1995;2:321–333. doi: 10.1038/nsb0495-321. [DOI] [PubMed] [Google Scholar]
- Deyoung, M.P., Ellisen, L.W. p63 and p73 in human cancer: Defining the network. Oncogene. 2007;26:5169–5183. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- García-Alai, M.M., Dantur, K.I., Smal, C., Pietrasanta, L., de Prat-Gay, G. High-risk HPV E6 oncoproteins assemble into large oligomers that allow localization of endogenous species in prototypic HPV-transformed cell lines. Biochemistry. 2007;46:341–349. doi: 10.1021/bi602457q. [DOI] [PubMed] [Google Scholar]
- Guinier, A. La diffraction des rayons X aux tres petits angles: Application a l'etude de phenomenes ultramicroscopiques. Ann. Phys. 1939;12:161–237. [Google Scholar]
- Hainaut, P., Hollstein, M. p53 and human cancer: The first ten thousand mutations. Adv. Cancer Res. 2000;77:81–137. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]
- Harms, K.L., Chen, X. The C terminus of p53 family proteins is a cell fate determinant. Mol. Cell. Biol. 2005;25:2014–2030. doi: 10.1128/MCB.25.5.2014-2030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, W.C., Fitzgerald, M.X., Marmorstein, R. Structure of the p53 core domain dimer bound to DNA. J. Biol. Chem. 2006;281:20494–20502. doi: 10.1074/jbc.M603634200. [DOI] [PubMed] [Google Scholar]
- Hu, A., Fu, X.D. Splicing oncogenes. Nat. Struct. Mol. Biol. 2007;14:174–175. doi: 10.1038/nsmb0307-174. [DOI] [PubMed] [Google Scholar]
- Hunt, J.B., Neece, S.H., Ginsburg, A. The use of 4-(2-pyridylazo)resorcinol in studies of zinc release from Escherichia coli aspartate transcarbamoylase. Anal. Biochem. 1985;146:150–157. doi: 10.1016/0003-2697(85)90409-9. [DOI] [PubMed] [Google Scholar]
- Joerger, A.C., Fersht, A.R. Structure-function-rescue: The diverse nature of common p53 cancer mutants. Oncogene. 2007;26:2226–2242. doi: 10.1038/sj.onc.1210291. [DOI] [PubMed] [Google Scholar]
- Joerger, A.C., Ang, H.C., Veprintsev, D.B., Blair, C.M., Fersht, A.R. Structures of p53 cancer mutants and mechanism of rescue by second-site suppressor mutations. J. Biol. Chem. 2005;280:16030–16037. doi: 10.1074/jbc.M500179200. [DOI] [PubMed] [Google Scholar]
- Joerger, A.C., Ang, H.C., Fersht, A.R. Structural basis for understanding oncogenic p53 mutations and designing rescue drugs. Proc. Natl. Acad. Sci. 2006;103:15056–15061. doi: 10.1073/pnas.0607286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, R., de Stanchina, E., Lowe, S.W., Sinha, R., Mu, D., Krainer, A.R. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat. Struct. Mol. Biol. 2007;14:185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarev, P.V., Volkov, V.V., Sokolova, A.V., Koch, M.H.J., Svergun, D.I. PRIMUS: A Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 2003;36:1277–1282. [Google Scholar]
- Kriventseva, E.V., Koch, I., Apweiler, R., Vingron, M., Bork, P., Gelfand, M.S., Sunyaev, S. Increase of functional diversity by alternative splicing. Trends Genet. 2003;19:124–128. doi: 10.1016/S0168-9525(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Lakowicz, J.R. Principles of fluorescence spectroscopy. 2d ed. Kluwer Academic/Plenum Publishers; New York: 1999. [Google Scholar]
- Lee, W., Harvey, T.S., Yin, Y., Yau, P., Litchfield, D., Arrowsmith, C.H. Solution structure of the tetrameric minimum transforming domain of p53. Nat. Struct. Biol. 1994;1:877–890. doi: 10.1038/nsb1294-877. [DOI] [PubMed] [Google Scholar]
- Mateu, M.G., Fersht, A.R. Nine hydrophobic side chains are key determinants of the thermodynamic stability and oligomerization status of tumour suppressor p53 tetramerization domain. EMBO J. 1998;17:2748–2758. doi: 10.1093/emboj/17.10.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu, M.G., Fersht, A.R. Mutually compensatory mutations during evolution of the tetramerization domain of tumor suppressor p53 lead to impaired hetero-oligomerization. Proc. Natl. Acad. Sci. 1999;96:3595–3599. doi: 10.1073/pnas.96.7.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Zmijewski, F., Lane, D.P., Bourdon, J.C. p53/p63/p73 isoforms: An orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- Neira, J.L., Vazquez, E., Fersht, A.R. Stability and folding of the protein complexes of barnase. Eur. J. Biochem. 2000;267:2859–2870. doi: 10.1046/j.1432-1327.2000.01290.x. [DOI] [PubMed] [Google Scholar]
- Nilsen, T.W. RNA-RNA interactions in the spliceosome: Unraveling the ties that bind. Cell. 1994;78:1–4. doi: 10.1016/0092-8674(94)90563-0. [DOI] [PubMed] [Google Scholar]
- Prives, C., Manfredi, J.J. The continuing saga of p53—more sleepless nights ahead. Mol. Cell. 2005;19:719–721. doi: 10.1016/j.molcel.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Roessle, M.W., Klaering, R., Ristau, U., Robrahn, B., Jahn, D., Gehrmann, T., Konarev, P., Round, A., Fiedler, S., Hermes, C., et al. Upgrade of the small-angle X-ray scattering beamline X33 at the European Molecular Biology Laboratory, Hamburg. J. Appl. Crystallogr. 2007;40:S190–S194. [Google Scholar]
- Rohaly, G., Chemnitz, J., Dehde, S., Nunez, A.M., Heukeshoven, J., Deppert, W., Dornreiter, I. A novel human p53 isoform is an essential element of the ATR-intra-S phase checkpoint. Cell. 2005;122:21–32. doi: 10.1016/j.cell.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Shaulsky, G., Goldfinger, N., Ben-Ze'ev, A., Rotter, V. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol. Cell. Biol. 1990;10:6565–6577. doi: 10.1128/mcb.10.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svergun, D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992;25:495–503. [Google Scholar]
- Tidow, H., Melero, R., Mylonas, E., Freund, S.M., Grossmann, J.G., Carazo, J.M., Svergun, D.I., Valle, M., Fersht, A.R. Quaternary structures of tumor suppressor p53 and a specific p53 DNA complex. Proc. Natl. Acad. Sci. 2007;104:12324–12329. doi: 10.1073/pnas.0705069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veprintsev, D.B., Freund, S.M., Andreeva, A., Rutledge, S.E., Tidow, H., Canadillas, J.M., Blair, C.M., Fersht, A.R. Core domain interactions in full-length p53 in solution. Proc. Natl. Acad. Sci. 2006;103:2115–2119. doi: 10.1073/pnas.0511130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg, R.L., Veprintsev, D.B., Fersht, A.R. Cooperative binding of tetrameric p53 to DNA. J. Mol. Biol. 2004;341:1145–1159. doi: 10.1016/j.jmb.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Wells, M., Tidow, H., Rutherford, T.J., Markwick, P., Jensen, M.R., Mylonas, E., Svergun, D.I., Blackledge, M., Fersht, A.R. Structure of tumor suppressor p53 and its intrinsically disordered N-terminal transactivation domain. Proc. Natl. Acad. Sci. 2008;105:5762–5767. doi: 10.1073/pnas.0801353105. [DOI] [PMC free article] [PubMed] [Google Scholar]