Abstract
One of the characteristics of picornavirus infection of cells in tissue culture is a specific inhibition of utilization of host cell mRNA for protein synthesis. In this study we show that human rhinovirus 14 is similar to poliovirus in that the inhibition of host cell translation that occurs during infection correlates with the proteolytic cleavage of an Mr 220,000 subunit of the cap-binding protein complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Brown-Luedi M. L., Hershey J. W. Protein synthesis initiation factors from rabbit reticulocytes: purification, characterization, and radiochemical labeling. Methods Enzymol. 1979;60:15–35. doi: 10.1016/s0076-6879(79)60005-8. [DOI] [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Etchison D., Hansen J., Ehrenfeld E., Edery I., Sonenberg N., Milburn S., Hershey J. W. Demonstration in vitro that eucaryotic initiation factor 3 is active but that a cap-binding protein complex is inactive in poliovirus-infected HeLa cells. J Virol. 1984 Sep;51(3):832–837. doi: 10.1128/jvi.51.3.832-837.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982 Dec 25;257(24):14806–14810. [PubMed] [Google Scholar]
- Golini F., Thach S. S., Birge C. H., Safer B., Merrick W. C., Thach R. E. Competition between cellular and viral mRNAs in vitro is regulated by a messenger discriminatory initiation factor. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3040–3044. doi: 10.1073/pnas.73.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E., Brown-Luedi M. L., Hershey J. W. Alterations in initiation factor activity from poliovirus-infected HeLa cells. J Biol Chem. 1979 Nov 10;254(21):10973–10978. [PubMed] [Google Scholar]
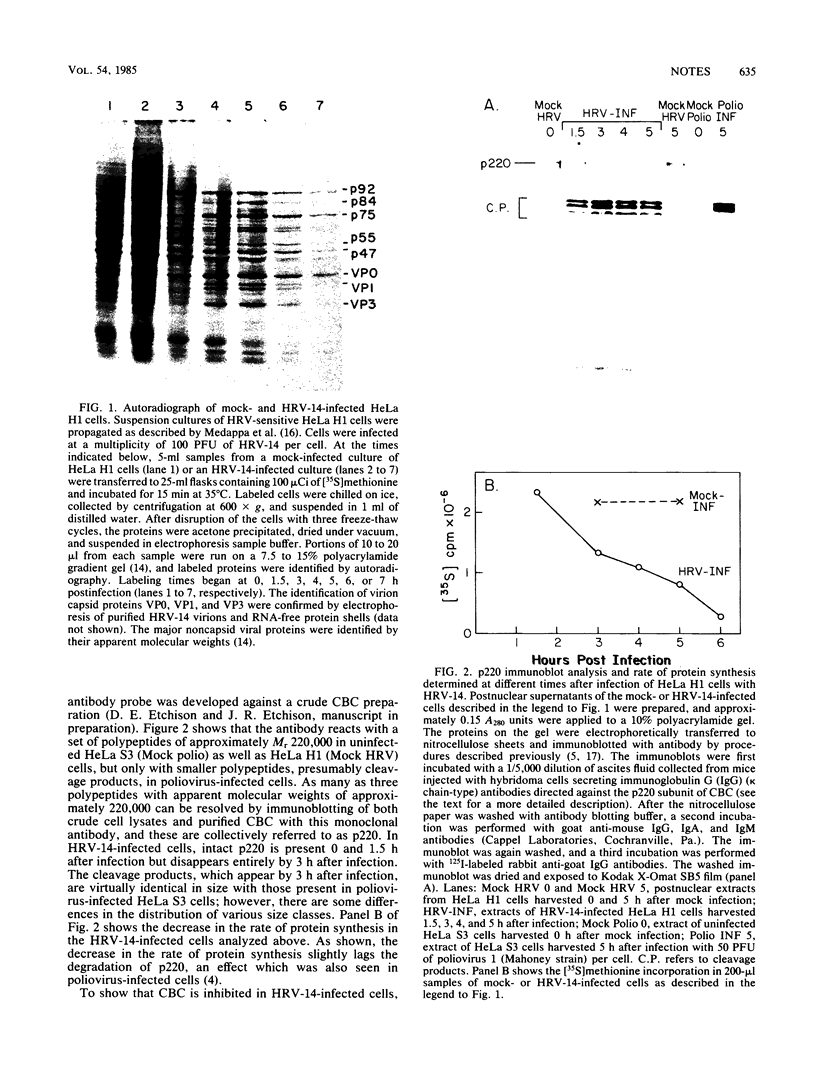
- Jen G., Birge C. H., Thach R. E. Comparison of initiation rates of encephalomyocarditis virus and host protein synthesis in infected cells. J Virol. 1978 Sep;27(3):640–647. doi: 10.1128/jvi.27.3.640-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen G., Thach R. E. Inhibition of host translation in encephalomyocarditis virus-infected L cells: a novel mechanism. J Virol. 1982 Jul;43(1):250–261. doi: 10.1128/jvi.43.1.250-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Goldstein E., Penman S. Poliovirus-induced inhibition of polypeptide initiation in vitro on native polyribosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1834–1838. doi: 10.1073/pnas.73.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Sonenberg N. Inactivation of cap-binding proteins accompanies the shut-off of host protein synthesis by poliovirus. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3447–3451. doi: 10.1073/pnas.79.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
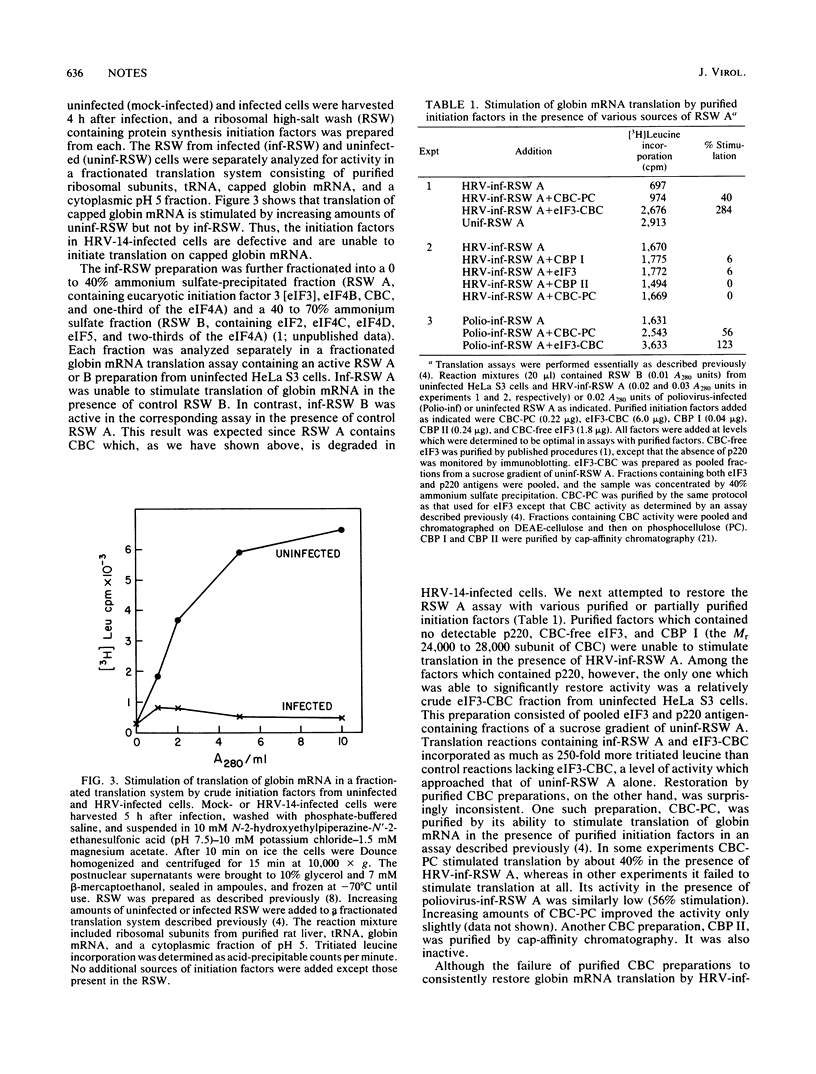
- McLean C., Rueckert R. R. Picornaviral gene order: comparison of a rhinovirus with a cardiovirus. J Virol. 1973 Feb;11(2):341–344. doi: 10.1128/jvi.11.2.341-344.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Meyer L. J., Milburn S. C., Hershey J. W. Immunochemical characterization of mammalian protein synthesis initiation factors. Biochemistry. 1982 Aug 31;21(18):4206–4212. doi: 10.1021/bi00261a003. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. ATP/Mg++-dependent cross-linking of cap binding proteins to the 5' end of eukaryotic mRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1643–1656. doi: 10.1093/nar/9.7.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]