Abstract
Malignant astrocytic gliomas, referred to as astrocytomas, represent the most commonly diagnosed adult primary brain tumor. These tumors are characterized by unrelenting growth that is often resistant to chemotherapy and radiation therapy. Tumor expansion into the healthy surrounding brain tissue produces severe and often fatal consequences. In this study, we examine the potential for the neuregulin-1/erbB receptor signaling cascade to contribute to this process by modulating glioma cell growth. Using antibodies specific for the erbB receptors, we demonstrate the expression patterns for the erbB2, erbB3, and erbB4 receptors in human glioma biopsy samples. We then verify receptor expression in a panel of human glioma cell lines. Next, we investigate the status of the erbB2 and erbB3 receptors in the human glioma cell lines and find that they are constitutively tyrosine-phosphorylated and heterodimerized. Subsequently, we demonstrate that theses same cell lines express membrane bound and released forms of neuregulins, the erbB receptor ligands, suggesting a possible autocrine or paracrine signaling network. Furthermore, we show that exogenous activation of erbB2 and erbB3 receptors in U251 glioma cells by recombinant Nrg-1β results in enhanced glioma cell growth under conditions of serum-deprivation. This enhancement is due to an increase in cell survival rather than an increase in cell proliferation and is dependent on the activation of erbB2 and phosphatidylinositol-3 kinase (PI3K). Moreover, Nrg-1β activates an inhibitor of apoptosis, Akt, implying a possible role for this kinase in mediating Nrg-1β effects in gliomas. This data suggests that glioma cells may use autocrine or paracrine neuregulin-1/erbB receptor signaling to enhance cell survival under conditions where growth would otherwise be limited.
Keywords: erbB2, erbB3, apoptosis, PI3K, Akt
INTRODUCTION
Malignant astrocytomas are the most common type of adult primary brain tumor. These tumors are derived from glial cells of the central nervous system. However, the precise lineage relationship between glia and gliomas is unknown; it is unclear whether astrocytomas result from the transformation of differentiated astrocytes or from their undifferentiated progenitors. Moreover, it has become increasingly evident that astrocytomas exhibit many of the same characteristics as immature glial cells in the developing brain, including uncontrolled growth and enhanced migration (Noble et al., 1991; Noble and Mayer-Proschel, 1997; Holland, 2001; Wechsler-Reya and Scott, 2001). In an attempt to delineate the mechanisms involved in the formation of astrocytomas, experimental mouse models have been described that indicate a key role for signaling pathways elicited downstream of receptor tyrosine kinases (RTKs), such as platelet-derived growth factor receptor (PDGF-R) (Dai et al., 2001b) and epidermal growth factor receptor (EGF-R) (Holland et al., 1998; Holland, 2000). These mouse models were developed based on the analysis of human glioma biopsies demonstrating the overexpression, amplification, and/or mutation of PDGF-R (Nister et al., 1988) and EGF-R (Wong et al., 1987).
The erbB2 RTK has also been reported to be overex-pressed in human glioma biopsies (Kristt et al., 1993). In fact, the earliest indication that this receptor might be involved in gliomagenesis came from a rat model of primary brain tumor (Drebin et al., 1985). In this model, pregnant rats were injected with a carcinogen, ethylnitrosourea, which induced the in vivo development of a neuroglioblastoma in the offspring. Subsequent cloning and sequencing identified a single point mutation (valine to glutamic acid) in the transmembrane region of erbB2 resulting in its constitutive activation (Bargmann et al., 1986). While this mutation has not been described in humans, overexpression and / or amplification of wild-type erbB2 has been identified in a variety of human cancers, including glioma, breast, ovarian, lung, prostate, and colon (Hynes and Stern, 1994). Although the initial discovery was made using the glioma model during the mid-1980s, relatively little is known regarding the downstream consequences of erbB2 receptor activation in human glioma cells.
ErbB2 is a member of the erbB family of RTK that includes EGF-R (erbB1), erbB3, and erbB4. All family members contain an extracellular ligand binding domain, a single transmembrane domain, and an intracellular tyro-sine kinase domain (Coussens et al., 1985). Upon ligand binding, the erbB receptors hetero- or homodimerize. All 10 dimerization pairs are possible; however, erbB2 is the preferred partner of all the erbBs (Graus-Porta et al., 1997). Dimerization stimulates receptor auto- and / or transphosphorylation of tyrosine residues, creating binding sites for adaptor proteins, kinases, and phosphatases that are unique to each dimerization pair. While erbB1 binds a range of ligands, including EGF and TGFα, it shows no affinity for the neuregulins (Zhang et al., 1997). The erbB2 receptor is an orphan receptor, with no known ligand, yet it can be activated as a consequence of heterodimerization with other erbB receptors. ErbB3 and ErbB4 serve as the direct, albeit functionally distinct, receptors for the growing group of polypeptide growth factors collectively known as NRGs.
Four different genes (nrg1-4) encode the NRG family of polypeptide growth factors (Lemke, 1996; Chang et al., 1997; Carraway et al., 1997; Busfield et al., 1997; Zhang et al., 1997; Harari et al., 1999). Protein products of the nrg-1 gene are the most well studied and were first described as mitogens for glial cells (Lemke and Brockes, 1983). Alternate RNA splicing of NRG-1 results in a number of different isoforms that contain certain characteristic domains, including an extracellular N-terminal domain, an Ig-like motif, a glycosylation sequence, an EGF-like domain (with α and β isoforms), a juxtamembrane region (with five isoforms), a single transmembrane domain, and a cytoplasmic tail of varying length. The EGF-like domain alone can induce erbB receptor activation in in vitro studies. In the peripheral and central nervous system, NRG-1 can elicit a variety of effects on both neurons and glia ranging from neuronal neurotransmitter subunit induction to oligodendroglial proliferation (Adlkofer and Lai, 2000; Buonanno and Fischbach, 2001).
In this study, we were interested in the possible role of NRG-1 in glioma growth control. Within this context, erbB receptor activation by NRG-1 has been shown to modulate the growth of both undifferentiated progenitor cells and differentiated glial cells (Canoll et al., 1996; Raabe et al., 1997; Flores et al., 2000). For example, NRG-1 was found to be essential for the development of neural crest cells (Britsch et al., 1998; Bannerman et al., 2000), for the survival and proliferation of neural progenitor cells (Calaora et al., 2001), and for the development of Schwann cells (Li et al., 2001), also reviewed in Garratt et al. (2000). In addition, NRG-1 was demonstrated to provide a survival signal for differentiated astrocytes (Pinkas-Kramarski et al., 1994) and oligodendrocytes (Flores et al., 2000).
Evidence that NRG-1 / erbB2 might contribute to the transformation of glial cells comes from studies that demonstrate NRG-1 can induce the de-differentiation and proliferation of cultured oligodendrocytes (Canoll et al., 1999) and from transgenic mice that express the activated neu (Hayes et al., 1992) oncogene under the control of the myelin basic protein promoter. These mice developed tumors that exhibited pathological features that resembled that of the most aggressive form of astrocytoma, the glioblastoma multiforme. Given the considerable evidence linking NRG-1 / erbB receptors to glial growth modulation, we performed in vitro experiments specifically focused on glioma growth control. We demonstrate that glioma cells appear to have a functional NRG-1 / erbB receptor autocrine or paracrine signaling network. In addition, we show that a recombinant form of NRG-1 (Nrg-1β) can activate erbB2 and erbB3 receptors in a dose-dependent manner in U251 human glioma cells. This activation leads to an increase in glioma growth as a result of enhanced survival without enhanced proliferation. In addition, we provide evidence that this survival effect is mediated by the PI3K-dependent activation of Akt. We hypothesize that the effects elicited by the exogenous application of Nrg-1β are indicative of those that occur under autocrine or paracrine signaling in a human glioma in situ.
MATERIALS AND METHODS
Cell Culture and Reagents
Human biopsy samples were flash frozen immediately following surgical removal in liquid nitrogen and stored at -70°C until use. Samples were homogenized in ice-cold RIPA buffer containing 150 mM NaCl, 1% Nonidet, 0.5% DOC, 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris (pH 7.5) plus protease and phosphatase inhibitors (RIPA-I) and processed for Western immunoblotting as described below. MCF-7 human breast cancer cells, U87, U113, and U138 human astrocytic glioma cell lines were purchased (American Type Cell Collection [ATCC], Rockville, MD). U251, D54, and STTG-1 human glioma cell lines were a gift from the laboratory of Dr. Y. Gillespie (University of Alabama, Birmingham). All cell lines were maintained in DMEM / F12 (50 / 50), supplemented with 2 mM glutamine (MediaTech, UAB Media Preparation Facility) and 7% fetal bovine serum (FBS) (Hyclone, Logan, UT). Recombinant neuregulin-1β (Nrg-1β) was produced in the laboratory of Dr. S. Carroll (University of Alabama, at Birmingham). The inhibitors Tyrphostin AG825 (AG825) and LY294002 (Calbiochem, La Jolla, CA) were dissolved in DMSO and stored at -20°C. All chemicals and other reagents were from Sigma (St. Louis, MO) unless otherwise stated.
Immunoblot and Immunoprecipitation
Subconfluent flasks or 10-cm dishes were rinsed two to three times with sterile phosphate-buffered saline (PBS), then lysed directly with ice-cold RIPA-I. Cells were lysed for 30 min and then spun for 5 min at 10,000g at 4°C. The supernatant was saved for protein assay or stored at -70°C. Protein concentrations were determined using the Bio-Rad (Hercules, CA) Detergent Compatible protein assay kit. Samples were diluted to equal protein concentrations with RIPA-I. Concentrated Laemmeli SDS-sample buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 0.1% bromophenol blue, and 600 mM β-mercaptoethanol) was added to samples and boiled for 5 min; 20 μm of sample was loaded per well of a 4–20% SDS-PAGE gradient Tris-HCl redi-gel (Bio-Rad), separated, and transferred to PVDF membrane (Millipore, Bedford, MA). Membranes were blocked for nonspecific binding with 5% nonfat milk at room temperature for 2 h or overnight at 4°C. Primary antibodies against the erbB receptors (erbB2, sc-284; erbB3, sc-285; erbB4, sc-283) or PI3K (Santa Cruz, Santa Cruz, CA) were used at 1:200 for 2 h at room temperature or overnight at 4°C. Peroxidase-conjugated goat anti-rabbit (Sigma) and goat anti-mouse (Santa Cruz) secondary antibodies were used at 1:3,000 or 1:2,000, respectively, for 1 h at room temperature. Proteins were detected by incubating the membranes with ECL-plus (Amersham, Piscataway, NJ).
To measure released endogenous forms of neuregulin, serum-free media that had been conditioned by the glioma cell lines for at least 18 h (overnight) was removed and concentrated using Centricon Plus-20 Centrifugal Filter Devices (Millipore). The protein concentration of each media sample was determined. Samples were diluted to equal concentrations, solubilized with sample buffer, and processed by Western immunoblotting. Mouse monoclonal Heregulin Ab-1 antibody (Neomarkers, Fremont, CA) was used at 1:1,000.
Immunoprecipitation experiments were used to investigate the phosphorylation status of erbB2 and erbB3 receptors and to determine their level of heterodimerization. For these experiments, serum-starved glioma cell lysates were immunoprecipitated overnight at 4°C with 5 μl/ml erbB2 antibody, erbB3 antibody (Santa Cruz) or 30 μl/ml agarose-conjugated anti-phosphotyrosine (Santa Cruz). Immune complexes were rinsed 3 times with ice-cold RIPA-I, solubilized with sample buffer, and processed by Western immunoblotting.
To measure the effects of NRG-1β, cells were grown to 80-90% confluency in 10-cm dishes, rinsed 3 times with sterile PBS and serum starved overnight (in DMEM / F12). The following day, increasing concentrations of NRG-1β (0.01, 0.1, 1.0, 10, and 100 ng / ml) were added for 30 min (to measure a dose response). For a time response, a saturating concentration of NRG-1β (100 ng / ml) was added and cells were incubated for increasing amounts of time (1, 5, 10, 15, 30, and 60 min). Inhibitors were added 1 h prior to addition of Nrg-1β. Cells were rinsed in ice-cold PBS with protease / phosphatase inhibitors, then lysed with ice-cold RIPA-I at 4°C for 30 min. Whole cell lysates were diluted to equal concentrations using RIPA-I (1 mg total protein / ml). Thirty μl of agarose-conjugated antibodies: phosphotyrosine, PI3K, or Akt (Santa Cruz) was added. The samples were rocked overnight at 4°C. Immunoprecipitates were pelleted by low-speed centrifugation. Agarose / antibody / antigen complexes were rinsed 3 times with ice-cold RIPA-I, solubilized by adding concentrated sample buffer (2×), and boiled for 5 min. Samples were loaded and processed as for westerns. Antibodies for phosphorylated- and total-Akt (Cell Signaling, Beverly, MA) were used according to manufacturer’s specifications (1:1,000). Semiquantitative densitometry was performed by importing digitized blots into an imaging software program (AlphaEase, Alpha Innotech, San Leandro, CA).
Cell Viability Assay (MTS / PMS)
Cells were seeded at 3 × 103 cells per well in 96-well plates using media containing 7% FBS. The following day, cells were placed in 200 μl of media containing 0.1%, 1%, or 7% FBS without or with Nrg-1β. Inhibitors were added 1 h prior to the addition of Nrg-1β. Plates were returned to the incubator for 24 or 48 h. At 1 h prior to reading the plates, the cells were rinsed with serum-free media, and then a premixed solution of media and MTS / PMS reagent (Promega, Madison, WI) was added under sterile conditions and the cells were returned to the incubator. After the 1-h incubation, the plates were read in an enzyme-linked immunosorbent assay (ELISA) plate reader. The absorbance was converted into cell number using a standard curve, and cell numbers (ordinate) were plotted versus Nrg-1β or inhibitor concentration (abscissa).
Proliferation Assay
Cells were plated in 24-well plates at 15 × 103 cells per well using media containing 7% FBS. The following day, cells were treated without or with Nrg-1β in media containing 0.1% FBS. On the second day, after ∼42 h, [3H]thymidine was added to each well (final concentration 1 μCi / ml). After 6 h, cells were rinsed with PBS and solubilized with 0.3N NaOH for 30 min at 37°C. Equal amounts of lysate were aliquoted into scintillation tubes and mixed with ScintiVerse (Fisher Scientific, Pittsburgh, PA). Radioactivity was determined with a scintillation counter. The protein concentration of each well was determined using a portion of the lysate. The amount of radioactivity per well was normalized to its protein concentration. The normalized radioactivity (cpm / μg protein) was plotted versus the Nrg-1β concentration.
Apoptosis Assays
Two types of caspase assays were used. For the first, cells were plated in 96-well plates at 3 × 103 cells per well and treated without or with Nrg-1β (and inhibitors), following the protocol outlined for the MTS / PMS viability assay described above. After 48 h, 100 μl of the Apo-ONE Homogeneous Caspase-3 / 7 Reagent (Promega) was added to each well. After 30 min or 1 h, the plates were read in a fluorescent plate reader at the appropriate excitation / emission wavelengths (485 and 538, respectively). Normalized fluorescence intensity was plotted versus treatment. For the second assay, cells were plated on glass coverslips at 15 × 103 cells per well and treated as above. After ∼48 h, the FITC-VAD-FMK In Situ reagent (Promega) was added to each well (10 μM, final concentration). After 20 min, the cells were washed with PBS and fixed with 4% paraformaldehyde. Coverslips were mounted with mounting media containing DAPI (Vector Laboratories, Burlingame, CA), visualized (Leica DMRB fluorescence-microscope, Heebrugg, Switzerland), and digitally imaged (Spot RT, Diagnostic Instruments, Sterling Heights, MI). The number of FITC-positive cells per field divided by the total number of cells (DAPI) was determined in each condition (apoptotic index). At least four fields were counted on each coverslip. The experiment was performed on three different occasions (three different coverslips for each condition).
Immunocytochemical Analysis of Phosphorylated AKT
Cells were plated on glass coverslips at 15 × 103 cells per well and treated without or with Nrg-1β (and inhibitors) as described above for activation studies. After 30 min, the cells were rinsed and fixed. Cells were blocked for nonspecific binding with Blocking Buffer (BB) containing 5% normal goat serum and 0.1% Triton X-100 in PBS, for 30 min. An antibody specific for the immunocytochemical detection of the activated form of Akt (p-Akt, Cell Signaling) was diluted in BB (1:50, manufacturer’s specification) and added to each well overnight at 4°C. Cells were then rinsed with BB and incubated with an Alexa 546-conjugated goat anti-mouse secondary antibody (Molecular Probes, Eugene, OR) for 1 h at room temperature. Coverslips were rinsed and visualized as above. All images were exposed for the same amount of time.
Statistical Analysis
Raw data from experiments requiring statistical analysis was imported into MicrocalTM Origin (Northampton, MA). Graphical representation of data was performed in Origin and analyzed using a one-way analysis of variance (ANOVA) with a significance of ≥0.05. As stated in the figure legends, most graphs represent data obtained from one assay performed with replicates. Error bars are the standard error of the mean for that assay. Additional assays were performed with similar results.
RESULTS
Overexpression of the ErbB2 Receptor in Biopsy Samples From Human Astrocytic Gliomas and Expression of ErbB Receptors in Human Astrocytic Glioma Cell Lines
Overexpression of the erbB2 receptor has been reported in human gliomas (Kristt et al., 1993) and is thought to correlate with a decreased patient prognosis in a number of human malignancies including breast and ovarian carcinomas (Slamon et al., 1989). We, therefore, first attempted to replicate those results demonstrated by others by examining erbB receptor expression in a panel of human glioma biopsy samples. We used samples collected from nonmalignant brain and compared their expression levels with those collected from pathologically classified grade III anaplastic astrocytomas (AA) or grade IV glioblastoma multiforme (GBM). A total of nine different samples, chosen randomly, were used and are displayed in Figure 1. Whole-cell lysates were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted for erbB2-4. Blots were then stripped and reprobed with antibodies specific for actin to verify equal loading. Expression levels of erbB2 in these tumors (Fig. 1) were higher than levels expressed in non-neoplastic brain (between 10- and 33-fold, as determined by densitometry, data not shown). ErbB3 expression levels were similar in all samples; however, expression of erbB4 was greatest in non-neoplastic brain.
Fig. 1.
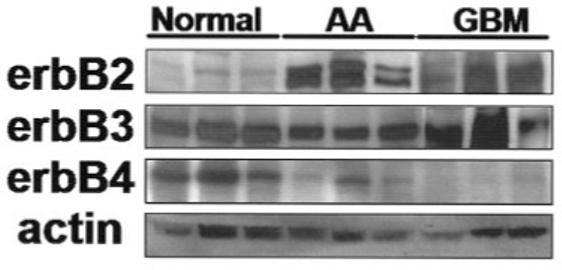
ErbB protein expression in human tissue biopsy samples. Biopsy samples were homogenized in ice-cold RIPA-I. Lysates were diluted to equal concentrations and processed by Western immunoblot. Control samples were from non-neoplastic brain tissue. AA, anaplastic astrocytoma; GBM, glioblastoma multiforme.
Next, we verified expression of erbB receptors in a panel of six human glioma cell lines. These lines were chosen because they are derived from human biopsy samples from patients diagnosed with astrocytomas and have been extensively used in the literature as model systems for glioma research. Figure 2 is a representative example of the protein expression levels detected in these cell lines.
Fig. 2.
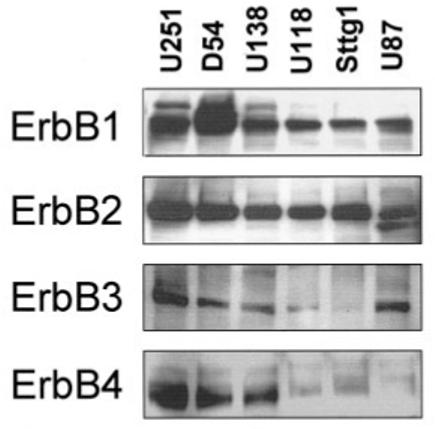
Human astrocytic glioma cell lines express erbB receptors. Twenty micrograms of protein form six different glioma cell lines were separated by SDS-PAGE, transferred to PVDF membrane, and probed for erbBs 1–4. Equal loading was confirmed by stripping each blot and reprobing for actin (data not shown). Similar results were seen on at least three occasions.
ErbB2 Receptors in Human Glioma Cells are Constitutively Tyrosine Phosphorylated
Considering the expression pattern of erbB2, erbB3, and erbB4 receptors in the biopsy samples, in which we demonstrate little expression of erbB4 in human glioma biopsis, we focused our studies on erbB2 and erbB3 receptors. As a first step in our investigation of the receptor status, we examined the level of tyrosine phosphorylation of the erbB2 receptor. The glioma cell lines were maintained in the absence of exogenous growth factors (serum) for ≥18 h prior to collecting whole-cell lysates. The erbB2 receptor was immunoprecipitated and the complexes were processed by Western immunoblotting and then probed for phosphotyrosine content. As indicated (Fig. 3A) there were several tyrosine phosphorylated proteins detected, including one at the appropriate molecular weight for erbB2 (Mr ∼185 kDa). The same blot was then stripped and reprobed for erbB2, indicating the level of total erbB2 present in the samples.
Fig. 3.
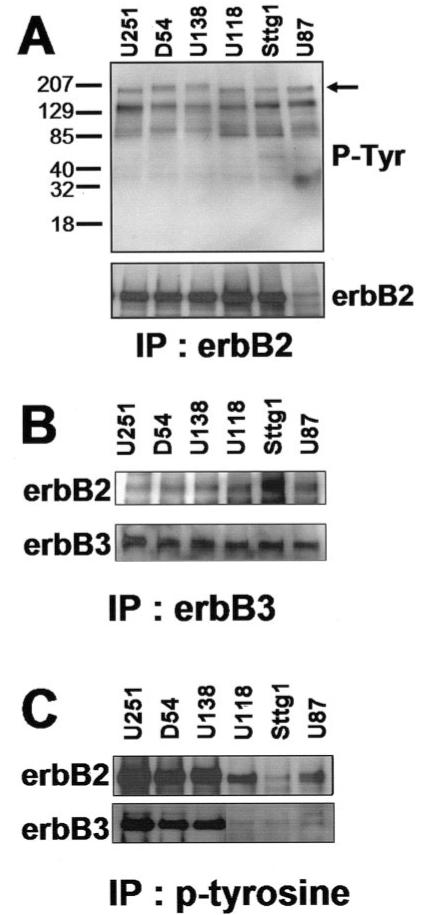
ErbB2 and ErbB3 receptor status in human glioma cells. A: ErbB2 is constitutively tyrosine phosphorylated. Equal concentrations of whole-cell lysates were immunoprecipitated with an erbB2 specific antibody. The immune complexes were separated by SDS-PAGE, transferred to a membrane, and probed with an antibody for phosphotyrosine. ErbB2 and erbB3 receptors are constitutively heterodimerized and tyrosine phosphorylated. B: ErbB2 and ErbB3 receptors are heterodimerized. Whole-cell lysates were immunoprecipitated with an anti-erbB3 specific antibody then probed for erbB2. C: Anti-phosphotyrosine immunoprecipitation reveals phosphorylated erbB2 and erbB3. Whole-cell lysates were immunoprecipitated with agarose-conjugated phosphotyrosine then probed for erbB2 and erbB3.
ErbB2 and ErbB3 Receptors Are Constitutively Heterodimerized and Tyrosine Phosphorylated
Figure 3A shows that the erbB2 receptor is constitutively tyrosine-phosphorylated. As previously mentioned, this receptor does not contain a ligand binding site. Instead, NRG-1 isoforms specifically bind erbB3 or erbB4 receptors stimulating hetero- or homodimerization and subsequent tyrosine phosphorylation. In this way, the ligand-less erbB2 receptor can be activated. Indeed, erbB2 is the preferred heterodimerization partner for the erbBs (Graus-Porta et al., 1997). The next step in our investigation was to determine whether the tyrosine phosphorylation of erbB2 (Fig. 3A) could be explained by an association with erbB3. Again, because of the lack of expression of erbB4 in human glioma biopsy samples (Fig. 1), we focused on erbB3. Serum-starved glioma cells were lysed and immunoprecipitated with an erbB3 specific antibody. The immune complexes were subjected to SDS-PAGE, transferred to a PVDF membrane, and probed for the presence of the erbB2 receptor. As demonstrated (Fig. 3B), a band at the appropriate molecular weight for erbB2 (Mr ∼185) was detected in all glioma cell lines from the erbB3 immunoprecipitates. These results suggest that the erbB2 and erbB3 receptors are heterodimerized. To complement the results shown in Figure 3A,B, we then immunoprecipitated serum-starved glioma cell lysates with a phosphotyrosine specific antibody and subsequently probed the blot for erbB2 or erbB3. The experiment demonstrated in Figure 3C revealed that both receptors were tyrosine phosphorylated. Of note, there appeared to be a difference in the level of phosphorylation between the different cell lines. This could represent the variability of the basal levels of erbB2 and erbB3 receptor expression in the cell lines.
While spontaneous erbB2 homodimerization has been shown to occur when this receptor is overexpressed (Chazin et al., 1992), there has not been any indication of spontaneous erbB2/erbB3 heterodimerization. Therefore, if an association between erbB2/erbB3 is detected, it probably reflects a ligand driven association. Our experiments were performed in the absence of serum or other exogenously applied growth factor. The fact that the receptors are heterodimerized and tyrosine phosphorylated suggests the presence of an endogenous ligand/receptor signaling cascade.
Glioma Cells Express and Secrete NRG-1 Isoforms
Thus far, our results have indicated that (1) glioma cells express erbB2 and erbB3 receptors, (2) the erbB2 and erbB3 receptors are heterodimerized, and (3) both receptors are tyrosine phosphorylated in the absence of serum or exogenously applied growth factor. We next wanted to know whether these effects were a consequence of endogenously expressed ligand, namely NRG-1. Previous studies (Westphal et al., 1997) have demonstrated that glioma cells can produce endogenous NRG-1 isoforms. Therefore, we probed serum-starved whole-cell lysates from the six glioma cell lines for expression of NRG-1 isoforms. A pan-specific NRG-1 antibody that recognizes the extracellular portion of both α and β isoforms was immunoreactive with several proteins in the glioma cell lysates (Fig. 4A). The most prominent protein was detected at an apparent molecular weight of ∼90 kDa. This is consistent with the pro-form, glycosylated, and membrane-attached, form of NRG-1 (Wang et al., 2001).
Fig. 4.
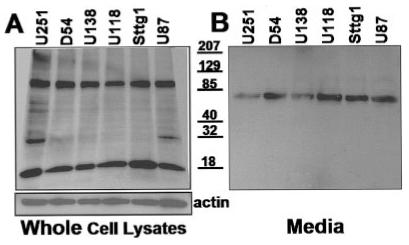
Glioma cells express and secrete Neuregulin-1. A: Whole-cell lysates from glioma cell lines were processed by Western immunoblot and probed with a pan-specific NRG-1 antibody (recognizes both β and β isoforms). B: Conditioned media from these same cell lines was concentrated, processed by Western immunoblot, and probed with the same NRG-1 antibody.
The NRG-1 ligand can activate erbB receptors while attached to the membrane (membrane-attached) or after being released or secreted (soluble). We have shown that the glioma cell lines express RIPA-soluble proteins consistent with NRG-1 isoforms (Fig. 4A). Next, we investigated whether these cell lines secrete any of the factor into the growth media. For these experiments, the cell lines were grown to 80–90% confluency and rinsed three times with PBS prior to the addition of serum-free media. The media was conditioned overnight ( ≥18 h) then was collected and examined for the presence secreted NRG-1. A faint band was detected (data not shown). In vivo, release of NRG-1 requires proteolytic cleavage of the protein in the “stalk” region by kinases such as protein kinase-C. In vitro, release of the secreted form of NRG-1 can be accelerated by incubating cells with a protein kinase-C activator such as PMA (Peles et al., 1992; Burgess et al., 1995; Loeb and Fischbach, 1995). In an attempt to increase the amount of released NRG-1 found in the media, each cell line was incubated for 30 min with 30 μM of PMA. Subsequently, the media was collected and concentrated. Equal volumes of concentrated media were diluted with sample buffer and processed by Western immunoblotting (Fig. 4B). This resulted in the presence of a prominent band at ∼70 kDa, consistent with a glycosylated, released NRG-1 peptide (Wang et al., 2001). Taken together, these results suggest that the glioma cell lines tested express and release NRG-1 isoforms, hence supporting the notion that NRG-1 may act as an autocrine or paracrine signal.
Recombinant NRG-1 (Nrg-1β) Activates erbB2 and erbB3 Receptors in U251 Human Glioma Cells
Having demonstrated the presence of the components necessary for an autocrine or paracrine signaling network in a panel of six glioma cell lines, we next wanted to investigate possible downstream consequences of the NRG-1/erbB receptor cascade. We chose to use the U251 glioma cell line for the subsequent functional experiments. This cell line exhibited high levels of the ligand/receptor pair and demonstrated a growth curve that was favorable for the subsequent in vitro models used. Using a recombinant form of NRG-1, Nrg-1β,we attempted to extend our study by focusing on pathways previously described for this most mitogenic pair of erbB receptors (Olayioye et al., 2000). We chose to use Nrg-1β as our ligand because the β isoforms are more abundant in the nervous system (Wen et al., 1994; Meyer and Birchmeier, 1994) and, in general, are more potent activators than the a isoforms (Marikovsky et al., 1995). First, we determined whether Nrg-1β could activate the receptors by monitoring the level of tyrosine phosphorylation. U251 cells were serum starved (overnight) prior to the addition of increasing concentrations of Nrg-1β. The concentrations of Nrg-1β chosen for these studies were based on those widely used in the literature. After a 30-min incubation period, whole-cell lysates were collected and diluted to equal concentrations. Subsequently, tyrosine phosphorylated proteins were immunoprecipitated and then, subjected to Western blot analysis using antibodies specific to erbB2 and erbB3. As a comparison, we also treated the MCF-7 human breast cancer cell line, a widely used line for the study of erbB2 receptors in this cancer. As demonstrated in Figure 5, Nrg-1β activated erbB2 and erbB3 receptors dose-dependently in U251 glioma cells and in MCF-7 breast cancer cells. A single concentration of EGF (50 ng/ml) was also able to activate the receptors through erbB1/erbB2 (or erbB3) heterodimer formation. Stimulation of erbB2 and erbB3 in U251 cells was time-dependent, with activation occurring within 5 min (data not shown). Of note, we continued to detect a significant amount of baseline phosphorylation of erbB2 (Fig. 5A, control), and of erbB3 (Fig. 5B, control). The results from these activation studies indicate that Nrg-1β is sufficient to effectively activate both erbB2 and erbB3 receptors in the U251 human glioma cell line.
Fig. 5.
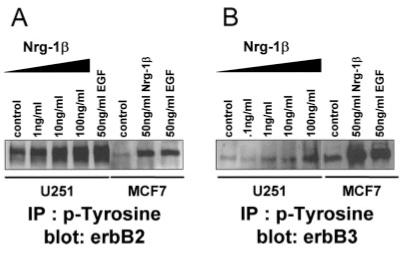
Recombinant Nrg-1β activates erbB2 and erbB3 receptors in U251 human glioma cells. Serum-starved U251 cells and MCF-7 cells were treated with increasing concentrations of Nrg-1β (as indicated) or EGF (50 ng / ml); 1 mg / ml of whole-cell lysate was immunoprecipitated using an agarose-conjugated phosphotyrosine antibody (30 μl/ml). Precipitated complexes were separated by SDS-PAGE, transferred to PVDF membrane, and probed for erbB2 (A) or erbB3 (B).
Nrg-1β Increases the Survival of U251 Human Glioma Cells
The next step in our study was to determine whether activation of erbB receptors by Nrg-1β resulted in a biological consequence, notably growth modulation. Effects on glioma cell growth were determined by quantifying the number of viable cells under a variety of experimental conditions. More specifically, equal numbers of U251 cells were plated in 96 well plates. The following day, the cells were washed and placed in media containing either high serum (7%), low serum (1%), or nominally serum-free (0.1%) media without or with increasing concentrations of Nrg-1β (0.001–100 ng / ml). After 48 h, cell viability was determined using a colorimetric MTS-based mitochondrial enzyme assay read in an ELISA plate reader. The number of viable cells was determined using a standard curve.
The most notable effect of Nrg-1β on the growth of U251 cells was detected when the cells were serum-deprived, in media with nominal serum (0.1%). As demonstrated, under these conditions, cells exhibited a dose-dependent increase in cell number (Fig. 6A, triangle symbol). The largest effect, a 2.12 ± 0.19-fold increase, P < 0.001, was seen with 100 ng/ml Nrg-1β and was maximal after 48 versus 24 h (Fig. 6B). Addition of Nrg-1β to the media of cells maintained in 7% serum resulted in a modest, but significant (at 100 ng/ml) increase in cell number, 1.30 ± 0.03-fold, P < 0.05 (Fig. 6A, square symbol). Under reduced serum conditions, 1% FBS, U251 cells exposed to Nrg-1β demonstrated similar effects (Fig. 6A, circle symbol) with a maximal increase of 1.26 ± 0.05-fold, P < 0.05.
Fig. 6.
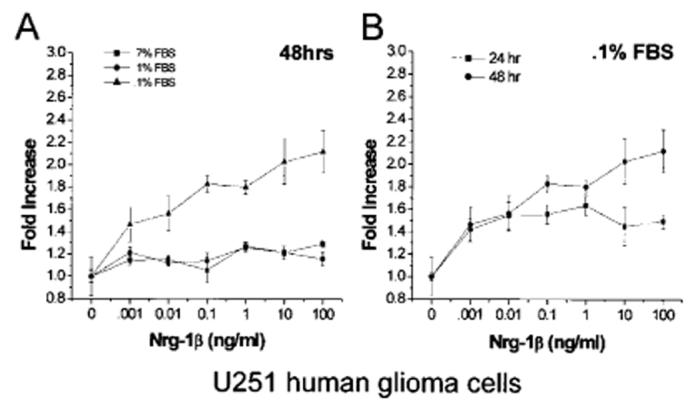
Nrg-1β increases the growth of U251 cells. A: Cells plated in 96-well plates and were treated with increasing concentrations of Nrg-1β (0.001–100 ng/ml) in the presence of 7%, 1%, or 0.1% serum. After 48 h, the number of cells was determined using a colorimetric MTS based viability assay. Data presented represents the mean ±SEM from one assay done in quadruplicate. At least four independent assays were performed with similar results. B: Viability of cells treated with increasing concentrations of Nrg-1β (0.001–100 ng/ml) in the presence of 0.1% serum was determined after 24 and 48 h. Data presented is the mean ±SEM from one assay done in quadruplicate. At least four independent assays were performed with similar results.
Nrg-1β Effects Can Be Attenuated by Inhibitors of erbB2 and of PI-3 Kinase
The results presented above indicated that Nrg-1β could activate erbB2 and erbB3 receptors and could increase glioma cell number under conditions of serum deprivation. Next, we wanted to identify more precisely the receptors and/or downstream pathways involved in this effect. To do this, we performed the following experiments.
We included an erbB2-specific inhibitor, AG825, in the media of cells treated as above: with minimal serum (0.1%) and 50 ng / ml Nrg-1β. Increasing concentrations of AG825 inhibited Nrg-1β effects dose-dependently (Fig. 7A) with an apparent IC50 of 2.51 μM. Our results demonstrated a role for the erbB2 receptor in Nrg-1β effects. As mentioned, this receptor does not contain a ligand binding domain, yet it can be activated in a ligand-dependent manner through heterodimerization with erbB3.
Fig. 7.
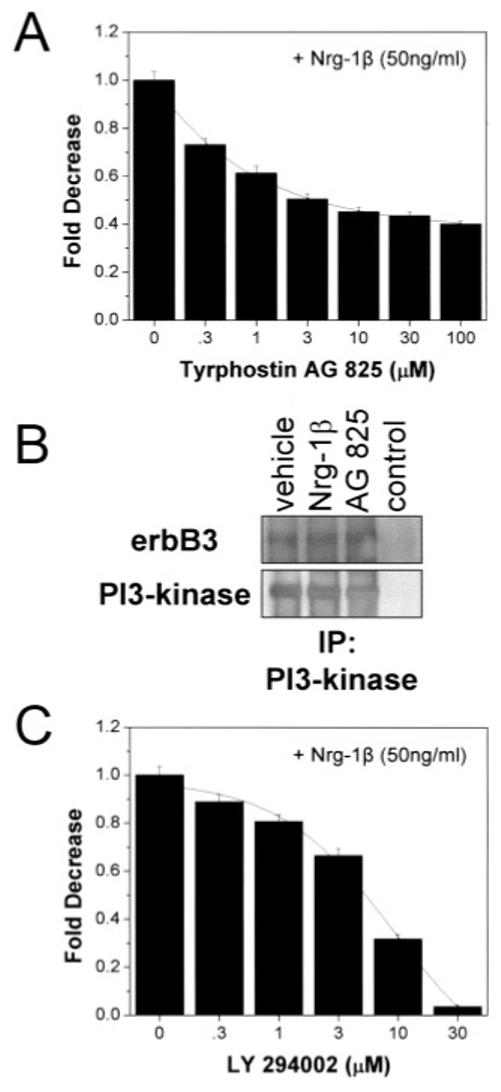
Inhibitors of erbB2 and PI3K diminish the effects of Nrg-1β. Cell viability assays performed on U251 cells maintained in 0.1% serum for 48 h treated without or with Nrg-1β (50 ng/ml) and increasing concentrations of Tyrphostin AG825 (A) or LY294002 (C) evidenced a dependence on erbB2 and PI3K. B: p85 subunit of PI3K associates with erbB3. U251 cells, treated as indicated (Nrg-1β at 50 ng/ml, and Tyrphostin AG825 at 50 μM) were lysed and immunoprecipitated with an agarose-conjugated PI3K antibody. Immune complexes analyzed by Western blot, were probed for erbB3, then stripped and reprobed for PI3K. Vehicle denotes treatment of cells with equivalent solvent (DMSO). Nrg-1β was used at 100 ng/ml. Control lane contains super-natant from the immunoprecipitation.
Activation of erbB2/erbB3 receptors has been demonstrated to stimulate the PI3K pathway in a number of cell types including glial cell (Flores et al., 2000; Li et al., 2001). Such activation occurs as a consequence of erbB3 trans-phosphorylation by erbB2 on tyrosine residues. This creates an SH2 binding site on the erbB3 cytoplasmic tail for the p85 subunit of PI3K (Soltoff et al., 1994; Hellyer et al., 1998). We wanted to know whether a similar activation could occur in human glioma cells after Nrg-1b stimulation. Thus, whole-cell lysates of treated U251 cells were immunoprecipitated with agarose-conjugated antibodies to PI3K. The immunoprecipitates were separated by SDS-PAGE, transferred, and immunoblotted for the erbB3 receptor (Fig. 7B). A protein at the appropriate molecular weight for erbB3 was detected (Mr ∼160 kDa). The same blot was stripped and reprobed using antibodies specific for the p85 subunit of PI3K. As shown, a band was detected at ∼85 kDa (Fig. 7B). With this evidence, we decided to investigate the potential effects of a PI3K inhibitor (LY 294002) on the growth of U251 cells in presence of Nrg-1β (50 ng / ml). Figure 7C shows that the inclusion of the PI3K inhibitor reduced the Nrg-1β mediated effects on cell growth dose-dependently with an apparent IC50 of 5.21 lM. The effects of the PI3K inhibitor were greater than those of the erbB2 inhibitor likely representing the contribution of other pathways that converge on PI3K in these cells.
Nrg-1β Decreases Apoptosis of U251 Human Glioma Cells
Our growth experiments (Figs. 6 and 7) demonstrated that Nrg-1β could increase the overall number of viable cells when they were subjected to serum-deprivation (0.1% serum). The balance between cell proliferation and cell death determines the growth rate of a population of cells. More specifically, an increase in proliferation or a decrease in apoptosis could each result in an overall increase in cell number. Therefore, to determine whether Nrg-1β effects were due to an increase in proliferation or to a decrease in apoptosis, we performed both proliferation and apoptosis assays.
First, to investigate an affect on proliferation, specifically on DNA synthesis, we measured the amount of [3H]thymi-dine incorporated by U251 cells in the absence or presence of increasing concentrations of Nrg-1β.Cellswereplatedin 24-well plates and treated in exactly the same way as for our viability experiments (washed, and placed in media without or with Nrg-1β). On the second day of the experiment we added [3H]thymidine to the culture media for 6 h. Subsequently, we analyzed the amount of [3H]thymidine incorporated and normalized the results to the amount of total protein in each well. We found no significant change, either decrease or increase, in the amount of [3H]thymidine incorporated in the presence of Nrg-1β (Fig. 8).
Fig. 8.
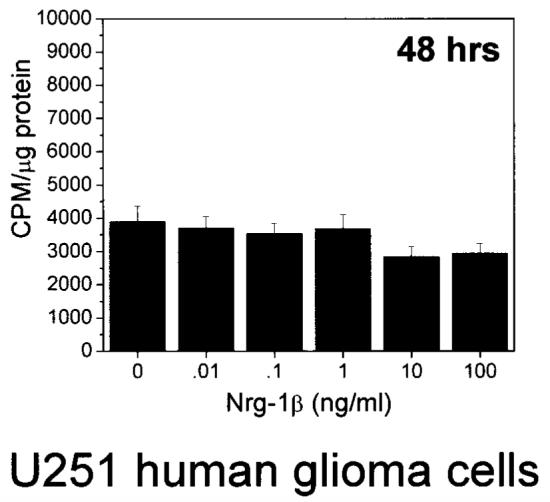
Nrg-1β does not increase DNA synthesis in U251 cells. Cells plated in 24-well plates treated with Nrg-1β (0.01–100 ng / ml) in the presence of 0.1% serum for ∼42 h were incubate with 1 μCi / ml [3H]thymidine for 6 h. Radioactivity was determined in a scintillation counter and normalized to protein concentration (μg). Results are mean ±SEM from four independent assays.
Next, we investigated Nrg-1β effects on apoptosis, by measuring the activation of the caspases known to mediate apoptosis, caspase-3 and caspase-7 (Slee et al., 2001b). Two types of caspase assays were performed. The first was performed on cells plated in 96-well plates. After treatment for 48 h without or with (1) Nrg-1β; (2) Nrg-1β plus the erbB2 inhibitor, AG825; and (3) Nrg-1β plus the PI3K inhibitor, LY 294002, a rhodamine-conjugated substrate for caspase-3 and caspase-7 was added to the wells. After 30 min or 1 h, the plates were read in a fluorescent plate reader at the appropriate excitation/emission wavelengths. These results are summarized in Figure 9A. In the presence of Nrg-1β (100 ng/ml) the amount of apoptosis, caspase-3/7 activity, was decreased (relative to control) by ∼34% ± 0.5% (mean ± SD). There was no significant difference between the control samples or the samples treated with Nrg-1β plus either AG825 or LY294002.
Fig. 9.
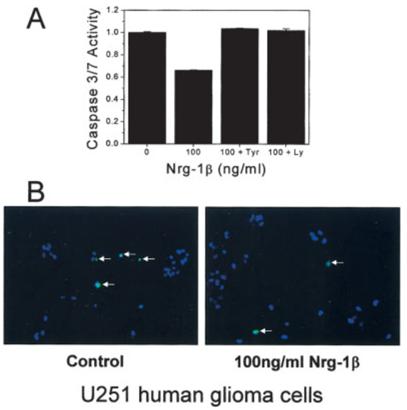
Nrg-1β protects U251 cells from apoptosis. A: Cells plated in 96-well plates were treated in the presence of 0.1% serum for 48 h as depicted. Next, they were incubated with a rhodamine-conjugated caspase-3 / 7 substrate for 30 min or 1 h. The amount of fluorescence was determined using a fluorescent plate reader. Samples were assayed in triplicate, results are mean ±SEM from one assay done after 30 min. There was no difference in the results when analyzed after 30 min or 1 h. Similar results were seen in at least four other independent assays. B: Representative images of U251 cells plated on glass coverslips treated as indicated for 48 h, incubated with an FITC-conjugated caspase-3/7 substrate for ∼20 min, fixed, mounted, and visualized.
We performed a second apoptosis assay on U251 cells that were plated on glass coverslips, treated without or with Nrg-1β (100 ng/ml) for 48 h, and then incubated with an FITC-conjugated caspase substrate. After about 20 min, the cells were washed, fixed, mounted, and visualized using a fluorescent microscope. Random fields were examined for the presence of FITC-positive cells. Representative digital images are displayed in Figure 9B. The apoptotic index was 1.4 ± 0.16% for control samples and 0.47 ± 0.07% for Nrg-1β samples (a decrease of ∼66%).
Overall, using two different caspase assays, we were able to see that Nrg-1β could decrease apoptosis. Our results for the two assays were different in magnitude, yet both demonstrated a similar trend. Taken together, these results demonstrate that Nrg-1β activation of erbB2/erbB3 decreases the rate of apoptosis in a manner that is dependent on erbB2 (as demonstrated by AG825 inhibition) and PI3K (as demonstrated by LY294002 inhibition).
Nrg-1β Stimulates Akt in U251 and U87 Human Glioma Cells
Activation of the PI3K cascade can stimulate a variety of signals, among which is the Akt-dependent survival pathway. In addition, a number of studies have shown that this pathway is stimulated in response to NRG-1 (Flores et al., 2000; Li et al., 2001). Akt is activated when it is phosphorylated, therefore its activation status can be determined by using phospho-specific antibodies. To examine this possibility, we treated U251 and U87 human glioma cells with increasing concentrations of Nrg-1β for 30 min. Whole cell lysates were collected and subjected to either (1) immunoprecipitation with an agarose-conjugated Akt antibody, followed by Western blot analysis of the immunoprecipitates for total and phosphorylated-Akt (for U251 cells; Fig. 10A) or (2) straight Western blot analysis for total and phosphorylated-Akt (for U87 cells; Fig. 10B). As shown, Akt was activated by Nrg-1β in a dose-dependent manner in both cell lines. Interestingly, there was a significant level of phosphorylated-Akt in the absence of stimulation. This result seemed to parallel the baseline level of activation detected for erbB2/erbB3 receptors in unstimulated glioma cells (Fig. 3). Nrg-1β induced phosphorylation of Akt could be completely inhibited, in U251 cells, by LY 294002 and partially inhibited by AG825 (data not shown).
Fig. 10.
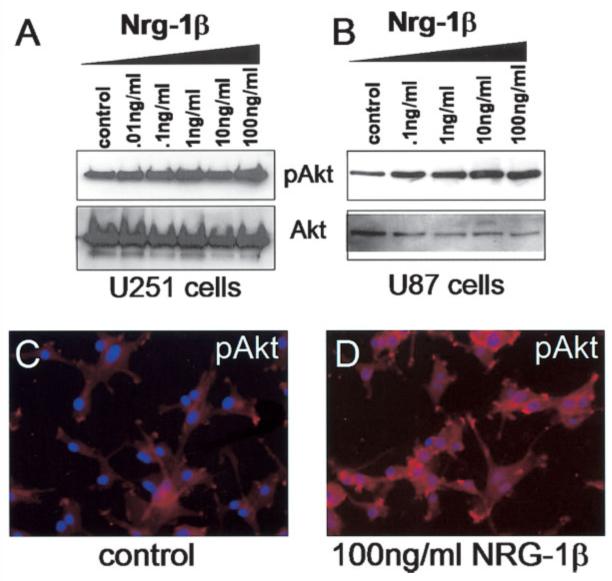
Nrg-1β stimulates Akt serine-473 phosphorylation dose-dependently. A: Whole-cell lysates of U251 cells were first immunoprecipitated with agaroseconjugated Akt antibodies then analyzed by Western blot for phospho-Akt, then stripped and reprobed for total Akt. B: Whole-cell lysates of U87 cells treated as indicated for 30 min were analyzed by Western blot. Membranes were probed for phospho-Akt, then stripped and reprobed for total Akt. C, D: U87 cells plated on glass coverslips treated without or with Nrg-1β (100 ng/ml) for 30 min were fixed and stained with an immunocytochemistry-specific antibody against phospho-Akt. Coverslips were mounted, visualized, and imaged. The shutter exposure time was the same for both images.
An additional assay was performed in the U87 cell line to measure induction of Akt. In this experiment, U87 cells were plated on glass coverslips and treated without or with Nrg-1β (100 ng/ml). After 30 min, the cells were rinsed, fixed, and stained using an immunocytochemistry specific antibody to detect phosphorylated-Akt. Again, based on fluorescence intensity, we detected a baseline level of phosphorylated Akt (Fig. 10C); however, this intensity was greatly increased in response to Nrg-1β (Fig. 10D). This experiment was also attempted in the U251 cell line, however the high level of baseline phosphorylated-Akt made comparison difficult using this assay.
DISCUSSION
The data presented in this study suggest that NRG-1/erbB receptors play a significant role in growth modulation of human astrocytic glioma cells. As previously described, the erbB2 receptor was overexpressed in human astrocytic glioma biopsy samples. In addition, the NRG-1/erbB receptors were expressed in the human glioma cell lines used as a model system. Furthermore, the erbB2 and erbB3 receptors appeared constitutively phosphorylated and heterodimerized in the absence of exogenous growth factor. One explanation for this basal level of activation was supported by the fact that the cell lines expressed and secreted NRG-1 isoforms. We used a recombinant form of NRG-1 (Nrg-1β) to monitor the physiological effects of erbB2 and erbB3 receptor activation in the U251 glioma cell line. These effects were dose-dependent and resulted in enhanced glioma cell growth, an effect that was greatest in the presence of minimal serum (0.1%). This growth effect was not a result of increased proliferation; instead, Nrg-1β increased the survival (or decreased apoptosis) of glioma cells through a PI3K/Akt-dependent pathway.
Relentless growth is one of the hallmarks of human malignant gliomas that contributes to the high morbidity of this disease. Therefore, we focused our investigation on the differential effects of Nrg-1β on the growth rate of human glioma cells. These experiments were performed in vitro on glioma cells maintained in media containing different serum concentrations. While serum is a convenient supplement to facilitate glioma cell growth in culture, it by no means simulates the growth environment encountered by tumor cells in the brain. Indeed, the levels of growth factors encountered by a tumor in vivo are unknown. However, it is likely that cells at the tumor margins experience different levels of growth factors than those at the tumor core. Sources for growth factors not only include the tumor cells themselves, but also the surrounding brain. In fact, NRG-1 transcripts and protein products have been detected in human gliomas (Westphal et al., 1997) and in glial cells and neurons throughout the brain (Pinkas-Kramarski et al., 1994; Raabe et al., 1997a; Pollock et al., 1999). NRG-1 may therefore act as an auto-crine or paracrine signal, and its availability in the brain may not be limiting. Our glioma growth assays were performed on U251 cells exposed to media with high (7%), low (1%) or nominal (0.1%) serum, the latter representing serum deprivation. Nrg-1β enhanced the growth of glioma cells under all conditions, with the most notable effect occurring when the cells were experiencing serum-deprivation (0.1% serum). We interpret these results in the following way: serum contains a variety of growth factors, which can activate downstream signaling pathways that are either parallel or anti-parallel to those activated by Nrg-1β. The growth response of cells exposed to Nrg-1β, in the presence of these other unknown factors, represents an integration of all these signals. In 7% and 1% serum, the effects of Nrg-1β on glioma growth may have been masked. However, in the absence of serum (0.1%), the effects of Nrg-1β were unmasked, leading to growth modulation via Nrg-1β that resulted in an increase in the overall cell number.
While these initial growth assays were essential for our investigation, they did not allow us to determine whether the effects of Nrg-1β were due to an increase in cellular proliferation and/or to a decrease in cellular apoptosis, both of which could lead to an overall increase in cell number. With respect to proliferation, there is evidence linking the activation of erbB receptors to an increase in proliferation under similar conditions of serum deprivation. For example, the growth stimulatory role of EGF, which signals via erbB1 / erbB2 receptors is well documented in glioma cells (Libermann et al., 1985; Westphal and Herrmann, 1989; Pollack et al., 1991) and is primarily due to cell-cycle progression downstream of the activation of the Ras signaling pathway (Feldkamp et al., 1997; Guha, 1998; Besson and Yong, 2001). Although NRG-1 receptors are part of the erbB family of RTK, NRG-1 stimulates a different set of erbB receptor heterodimers, which results in the activation of different downstream signaling cascades. Indeed, in U251 human glioma cells, we did not observe any change in DNA synthesis in response to Nrg-1β. This result is consistent with that seen by others (Westphal et al., 1997). Instead, our studies demonstrated that Nrg-1β decreased the rate of apoptosis in U251 cells as measured by a decrease in the activity of caspase-3 / 7. This effect was dependent on the activation of the erbB2 receptor and of PI3K, based on inhibitor studies (AG825 and LY294002, respectively). Interestingly, similar results have been demonstrated downstream of NRG-1 activation of erbB2 and erbB3 receptors in nontransformed glial cells (Canoll et al., 1996; Raabe et al., 1997b; Flores et al., 2000; Li et al., 2001) and in rat C6 glioma cells (Flores et al., 2000). In most cases, the survival effect is dependent on the activation of PI3K, presumably via association of its regulatory subunit (Hellyer et al., 1998) with the erbB3 receptor (Fedi et al., 1994; Prigent and Gullick, 1994). Activation of PI3K ultimately leads to the phosphorylation and stimulation of a serine / threonine kinase called Akt (Vanhaesebroeck and Alessi, 2000). While the biological functions of Akt are diverse, one critical role is to prevent programmed cell death by direct phosphorylation and inactivation of the pro-apoptotic signal Bad (Coffer et al., 1998). Consistent with these studies, we found that the p85 subunit of PI3K associated with the erbB3 receptor in U251 glioma cells and that Nrg-1β activated Akt dose-dependently in two different glioma cell lines (U251 and U 87).
Taken together, our results support the hypothesis that Nrg-1β enhances glioma cell survival through a decrease in apoptosis. These effects were particularly evident under conditions where the growth factor supply (serum) was limited. Such a condition may exist within a tumor that has grown beyond the limits of the local blood supply. While enhanced growth is often the consequence of increased cell proliferation, it represents a dynamic balance between cellular proliferation and apoptosis. Mizoguchi et al. (2000) recently demonstrated the significance of this balance in a report comparing the histopathological markers of proliferation and apoptosis in two intracranial tumors: germinomas and glioblastomas. Germinomas were characterized by high proliferation and apoptotic indices and carried a relatively good prognosis. In contrast, glioblastomas, were characterized by high proliferation but low apoptotic indices, resulting in a relatively poor prognosis.
The ability of tumor cells to survive under conditions that might ordinarily induce apoptosis allows them to escape both chemotherapy and radiation therapy, which targets actively proliferating cells. In the brain, this characteristic is particularly detrimental because there is little room within the skull to allow for tumor expansion. As a result, healthy tissue surrounding a tumor becomes damaged as a byproduct of compression. The ensuing death of peritumoral neurons, which are postmitotic and highly specialized as to function, frequently produces the first symptoms of a brain tumor and ultimately causes severe and life-threatening sequelae, including seizures, coma, and respiratory failure (Shapiro et al., 2001). A better understanding of the mechanisms that contribute to glioma cell survival and apoptosis might facilitate the development of new treatment strategies that target these pathways.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: RO1-NS36692; Grant number: P50CA97247.
REFERENCES
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bannerman PG, Puhalla S, Sahai A, Shieh A, Berman M, Pleasure D. Glial growth factor-2 promotes the survival, migration and bromodeoxyuridine incorporation of mammalian neural crest cells in caudal neural tube explant cultures. Brain Res Dev Brain Res. 2000;124:93–99. doi: 10.1016/s0165-3806(00)00090-0. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hung MC, Weinberg RA. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986;45:649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Besson A, Yong VW. Mitogenic signaling and the relationship to cell cycle regulation in astrocytomas. J Neurooncol. 2001;51:245–264. doi: 10.1023/a:1010657030494. [DOI] [PubMed] [Google Scholar]
- Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998a;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Ross SL, Qian YX, Brankow D, Hu S. Biosynthetic processing of neu differentiation factor. Glycosylation trafficking, and regulated cleavage from the cell surface. J Biol Chem. 1995;270:19188–19196. doi: 10.1074/jbc.270.32.19188. [DOI] [PubMed] [Google Scholar]
- Busfield SJ, Michnick DA, Chickering TW, Revett TL, Ma J, Woolf EA, Comrack CA, Dussault BJ, Woolf J, Goodearl AD, Gearing DP. Characterization of a neuregulin-related gene, Don-1, that is highly expressed in restricted regions of the cerebellum and hippocampus. Mol Cell Biol. 1997;17:4007–4014. doi: 10.1128/mcb.17.7.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaora V, Rogister B, Bismuth K, Murray K, Brandt H, Leprince P, Marchionni M, Dubois-Dalcq M. Neuregulin signaling regulates neural precursor growth and the generation of oligodendrocytes in vitro. J Neurosci. 2001;21:4740–4751. doi: 10.1523/JNEUROSCI.21-13-04740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF / neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Kraemer R, Teng KK, Marchionni MA, Salzer JL. GGF / neuregulin induces a phenotypic reversion of oligodendrocytes. Mol Cell Neurosci. 1999;13:79–94. doi: 10.1006/mcne.1998.0733. [DOI] [PubMed] [Google Scholar]
- Carraway KL, III, Weber JL, Unger MJ, Ledesma J, Yu N, Gassmann M, Lai C. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- Chang H, Riese DJ, Gilbert W, Stern DF, McMahan UJ. Ligands for ErbB-family receptors encoded by a neuregulin-like gene. Nature. 1997;387:509–512. doi: 10.1038/387509a0. [DOI] [PubMed] [Google Scholar]
- Chazin VR, Kaleko M, Miller AD, Slamon DJ. Transformation mediated by the human HER-2 gene independent of the epidermal growth factor receptor. Oncogene. 1992;7:1859–1866. [PubMed] [Google Scholar]
- Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335(Pt 1):1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, Seeburg PH, Libermann TA, Schlessinger J, Francke U. Tyro-sine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001a;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001b;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41:697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- Fedi P, Pierce JH, di Fiore PP, Kraus MH. Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase C gamma or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB / EGFR family members. Mol Cell Biol. 1994;14:492–500. doi: 10.1128/mcb.14.1.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkamp MM, Lau N, Guha A. Signal transduction pathways and their relevance in human astrocytomas. J Neurooncol. 1997;35:223–248. doi: 10.1023/a:1005800114912. [DOI] [PubMed] [Google Scholar]
- Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci. 2000;20:7622–7630. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a Schwann cell. BioEssays. 2000;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A. Ras activation in astrocytomas and neurofibromas. Can J Neurol Sci. 1998;25:267–281. doi: 10.1017/s0317167100034272. [DOI] [PubMed] [Google Scholar]
- Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, Yarden Y. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- Hayes C, Kelly D, Murayama S, Komiyama A, Suzuki K, Popko B. Expression of the neu oncogene under the transcriptional control of the myelin basic protein gene in transgenic mice: generation of transformed glial cells. J Neurosci Res. 1992;31:175–187. doi: 10.1002/jnr.490310123. [DOI] [PubMed] [Google Scholar]
- Hellyer NJ, Cheng K, Koland JG. ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J. 1998;333(Pt 3):757–763. doi: 10.1042/bj3330757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC. A mouse model for glioma: biology, pathology, and therapeutic opportunities. Toxicol Pathol. 2000;28:171–177. doi: 10.1177/019262330002800122. [DOI] [PubMed] [Google Scholar]
- Holland EC. Progenitor cells and glioma formation. Curr Opin Neurol. 2001;14:683–688. doi: 10.1097/00019052-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE, Stern DF. The biology of erbB-2 / neu / HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Klapper LN, Kirschbaum MH, Sela M, Yarden Y. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- Kristt DA, Reedy E, Yarden Y. Receptor tyrosine kinase expression in astrocytic lesions: similar features in gliosis and glioma. Neurosurgery. 1993;33:106–115. doi: 10.1227/00006123-199307000-00017. [DOI] [PubMed] [Google Scholar]
- Lemke G. Neuregulins in development. Mol Cell Neurosci. 1996;7:247–262. doi: 10.1006/mcne.1996.0019. [DOI] [PubMed] [Google Scholar]
- Lemke GE, Brockes JP. Glial growth factor: a mitogenic polypeptide of the brain and pituitary. Fed Proc. 1983;42:2627–2629. [PubMed] [Google Scholar]
- Li Y, Tennekoon GI, Birnbaum M, Marchionni MA, Rutkowski JL. Neuregulin signaling through a PI3K/Akt/Bad pathway in Schwann cell survival. Mol Cell Neurosci. 2001;17:761–767. doi: 10.1006/mcne.2000.0967. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Loeb JA, Fischbach GD. ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J Cell Biol. 1995;130:127–135. doi: 10.1083/jcb.130.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikovsky M, Lavi S, Pinkas-Kramarski R, Karunagaran D, Liu N, Wen D, Yarden Y. ErbB-3 mediates differential mitogenic effects of NDF/heregulin isoforms on mouse keratinocytes. Oncogene. 1995;10:1403–1411. [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Distinct isoforms of neuregulin are expressed in mesenchymal and neuronal cells during mouse development. Proc Natl Acad Sci USA. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi M, Inamura T, Shono T, Ikezaki K, Inoha S, Ohgami S, Fukui M. A comparative study of apoptosis and proliferation in germinoma and glioblastoma. Neurooncology. 2000;2:96–102. doi: 10.1093/neuonc/2.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nister M, Libermann TA, Betsholtz C, Pettersson M, Claesson-Welsh L, Heldin CH, Schlessinger J, Westermark B. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988;48:3910–3918. [PubMed] [Google Scholar]
- Noble M, Ataliotis P, Barnett SC, Bevan K, Bogler O, Groves A, Jat P, Wolswijk G, Wren D. Development, regeneration, and neoplasia of glial cells in the central nervous system. Ann NY Acad Sci. 1991;633:35–47. doi: 10.1111/j.1749-6632.1991.tb15593.x. [DOI] [PubMed] [Google Scholar]
- Noble M, Mayer-Proschel M. Growth factors, glia and gliomas. J Neurooncol. 1997;35:193–209. doi: 10.1023/a:1005898228116. [DOI] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Bacus SS, Koski RA, Lu HS, Wen D, Ogden SG, Levy RB, Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69:205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Eilam R, Spiegler O, Lavi S, Liu N, Chang D, Wen D, Schwartz M, Yarden Y. Brain neurons and glial cells express Neu differentiation factor/heregulin: a survival factor for astrocytes. Proc Natl Acad Sci USA. 1994;91:9387–9391. doi: 10.1073/pnas.91.20.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack IF, Randall MS, Kristofik MP, Kelly RH, Selker RG, Vertosick FT., Jr Response of low-passage human malignant gliomas in vitro to stimulation and selective inhibition of growth factor-mediated pathways. J Neurosurg. 1991;75:284–293. doi: 10.3171/jns.1991.75.2.0284. [DOI] [PubMed] [Google Scholar]
- Pollock GS, Franceschini IA, Graham G, Marchionni MA, Barnett SC. Neuregulin is a mitogen and survival factor for olfactory bulb ensheathing cells and an isoform is produced by astrocytes. Eur J Neurosci. 1999;11:769–780. doi: 10.1046/j.1460-9568.1999.00484.x. [DOI] [PubMed] [Google Scholar]
- Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe TD, Clive DR, Wen D, DeVries GH. Neonatal oligodendrocytes contain and secrete neuregulins in vitro. J Neurochem. 1997a;69:1859–1863. doi: 10.1046/j.1471-4159.1997.69051859.x. [DOI] [PubMed] [Google Scholar]
- Raabe TD, Suy S, Welcher A, DeVries GH. Effect of neu differentiation factor isoforms on neonatal oligodendrocyte function. J Neurosci Res. 1997b;50:755–768. doi: 10.1002/(SICI)1097-4547(19971201)50:5<755::AID-JNR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Shapiro WR, Shapiro JR, Walker RW. Central nervous system. In: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Clinical oncology. Churchill Livingstone; New York: 2001. pp. 1123–1129. [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- Soltoff SP, Carraway KL, III, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–576. [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Miller SJ, Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem. 2001;276:2841–2851. doi: 10.1074/jbc.M005700200. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- Wen D, Suggs SV, Karunagaran D, Liu N, Cupples RL, Luo Y, Janssen AM, Ben Baruch N, Trollinger DB, Jacobsen VL. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol Cell Biol. 1994;14:1909–1919. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M, Herrmann HD. Growth factor biology and oncogene activation in human gliomas and their implications for specific therapeutic concepts. Neurosurgery. 1989;25:681–694. doi: 10.1097/00006123-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Westphal M, Meima L, Szonyi E, Lofgren J, Meissner H, Hamel W, Nikolics K, Sliwkowski MX. Heregulins and the ErbB-2/3/4 receptors in gliomas. J Neurooncol. 1997;35:335–346. doi: 10.1023/a:1005837122181. [DOI] [PubMed] [Google Scholar]
- Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Sliwkowski MX, Mark M, Frantz G, Akita R, Sun Y, Hillan K, Crowley C, Brush J, Godowski PJ. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci USA. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]


