Abstract
Primary brain tumors (gliomas) often present with peritumoral edema. Their ability to thrive in this osmotically altered environment prompted us to examine volume regulation in human glioma cells, specifically the relative contribution of Cl- channels and transporters to this process. After a hyposmotic challenge, cultured astrocytes, D54-MG glioma cells, and glioma cells from human patient biopsies exhibited a regulatory volume decrease (RVD). Although astrocytes were not able to completely reestablish their original prechallenge volumes, glioma cells exhibited complete volume recovery, sometimes recovering to a volume smaller than their original volumes (VPost-RVD < Vbaseline). In glioma cells, RVD was largely inhibited by treatment with a combination of Cl- channel inhibitors, 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and Cd2+ (VPost-RVD > 1.4*Vbaseline). Volume regulation was also attenuated to a lesser degree by the addition of R-(+)-[(2-n-butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]acetic acid (DIOA), a known K+-Cl- cotransporter (KCC) inhibitor. To dissect the relative contribution of channels vs. transporters in RVD, we took advantage of the comparatively high temperature dependence of transport processes vs. channel-mediated diffusion. Cooling D54-MG glioma cells to 15°C resulted in a loss of DIOA-sensitive volume regulation. Moreover, at 15°C, the channel blockers NPPB + Cd2+ completely inhibited RVD and cells behaved like perfect osmometers. The calculated osmolyte flux during RVD under these experimental conditions suggests that the relative contribution of Cl- channels vs. transporters to this process is ∼60–70% and ∼30–40%, respectively. Finally, we identified several candidate proteins that may be involved in RVD, including the Cl- channels ClC-2, ClC-3, ClC-5, ClC-6, and ClC-7 and the transporters KCC1 and KCC3a.
Keywords: voltage-gated chloride channel family, potassium-chloride cotransporters, peritumoral edema
PRIMARY BRAIN TUMORS (gliomas) often present with significant peritumoral edema signifying an enhanced water content of the surrounding brain tissue. Cells exposed to this edematous environment swell unless they actively regulate their cell volume. Cell swelling in turn can result in alterations in the concentrations of enzymes and substrates, disruption of intracellular pH, and perturbations of metabolic pathways. Some cells show growth inhibition or even cell death when osmotically challenged (7, 12, 19, 23, 27). In the brain, tissue swelling, even if localized, has the potential to cause significant damage to the entire brain because its expansion is limited by the confines of the skull. Not surprisingly, therefore, brain cells, like most cells, have established mechanisms to actively regulate and control their volume when exposed to a hypotonic environment, through a process termed regulatory volume decrease (RVD) (27). The ability of glioma cells to thrive within an edematous environment suggests that they must possess potent mechanisms to regulate their cell volume. Therefore, the major objective of this study was to examine mechanisms involved in RVD of glioma cells, including tumor cells surgically isolated from glioblastoma multiforme patients.
Although the detailed mechanisms underlying RVD have been studied in only a few model systems, it is commonly understood that a volume decrease must involve the extrusion of organic and inorganic osmolytes followed by the obligatory movement of water (14, 28, 31, 34). Cl- is the most abundant inorganic ion within the cell and, as such, is thought to be one of the major osmolytes involved in RVD. Previous studies showed that Cl- efflux during RVD may occur through ion channels or transporters. In most cell types studied, swelling results in activation of Cl- channels (2, 15, 32, 35, 39), and, particularly in mammalian cells, a swelling-activated Cl- current (ICl,swell) has been shown to be ubiquitously expressed. ICl,swell is characterized as an outwardly rectifying current with anion selectivity sequence of I- > Br- > Cl- > F- > gluconate that is inhibited by a variety of nonspecific chloride channel inhibitors including both 5-nitro-2(3-phenylpropylamino)benzoic acid (NPPB) and DIDS (5, 26, 32, 39). The molecular identity of ICl,swell, however, remains unknown. Although their contribution to RVD is unclear, other swelling-activated Cl- channels have been identified. For example, two members of the voltage-gated Cl- channel family, ClC-2 and ClC-3, have been shown to activate in response to cell swelling (11, 17). ClC-2 generates currents that are inwardly rectifying, inhibited by NPPB and Cd2+, and have an anion selectivity sequence of Cl- > Br- > I- (5, 26, 32, 42). Expression of ClC-3 generates outwardly rectifying, DIDS-sensitive currents (6, 26, 41). Although studies have shown inhibition of RVD by antisense oligonucleotides (13) and antibodies (43) generated against ClC-3, contradictory studies have shown intact volume regulation in transgenic animals with a genetic deletion of ClC-3 (38) or that expression of ClC-3 in HEK cells does not affect RVD (8). Other swelling-activated anion channels that may play a role in RVD include the yet to be identified maximal volume-sensitive anion channel current studied in astrocytes (39). Indeed, several pathways for Cl- efflux may participate during RVD, because complete inhibition of this process in astrocytes, for example, requires a combination of Cl- channel inhibitors (28).
The role of Cl- transporters in RVD has been studied most extensively in red blood cells. In these cells, volume regulation occurs via Cl--dependent K+ flux, mediated by the activity of a K+-Cl- cotransporter (KCC) (20-22). The KCC family of proteins includes four members (KCC1–4), several of which have been shown to be activated by cell swelling (9, 24). Unlike Cl- channels, which appear to be activated in almost all cell types in response to cell swelling, KCCs have been implicated mainly in volume regulation in red blood cells and a few types of epithelia, including, most recently, cervical cancer cells (36, 37).
Previous studies in astrocytes revealed that these nonmalignant cells undergo RVD aided by an increase in K+, Cl-, and free amino acid efflux. Although Cl- channel involvement in this process has been supported by inhibition of RVD (albeit to various degrees) by a variety of Cl- channel inhibitors (14, 28, 31), the contribution of transporters to RVD in astrocytes has been inconclusive. A limited number of studies have explored volume regulation in a rat glioma cell line (C6) and have demonstrated RVD that was completely inhibited by Cl- channel blockers (22, 25). Little is known regarding the properties of volume regulation in human glioma cells. Moreover, despite a wealth of previous research, the relative contribution of channels and transporters to RVD has not been clarified. In this study, we made an initial attempt to determine the mechanisms responsible for RVD in human glioma cells, with a specific emphasis on identifying the relative importance of Cl- channels vs. cation-Cl- cotransporters. Our data suggest, through various lines of experimental evidence, that ∼60–70% of Cl- efflux during RVD occurs through Cl- channels, with up to 40% occurring through a transport-mediated process.
MATERIALS AND METHODS
Cell culture
The following experiments were performed with the glioma cell line D54-MG (glioblastoma multiforme, WHO Grade IV; Dr. D. Bigner, Duke University, Durham, NC), acute cells from patient biopsies (WHO Grade IV; Dr. Y. Gillespie, University of Alabama at Birmingham), and primary cultured cortical astrocytes. Glioma cells were maintained in DMEM-F-12 (Life Technologies, Grand Island, NY) supplemented with 2 mM L-glutamine (CellGro, Herndon, VA) and 7% heat-inactivated bovine growth serum (Hy-Clone, Logan, UT) and kept at 37°C in a humidified 90% O2-10% CO2 atmosphere. Astrocytes were cultured from Sprague-Dawley rat pups according to a previously published protocol (28) that was reviewed and approved by the Animal Care and Use Committee. They were maintained in DMEM (Life Technologies) supplemented with 2 mM L-glutamine (CellGro, Herndon, VA) and 7% heat-inactivated bovine growth serum (HyClone) at 37% in a 95% O2-5% CO2 environment.
Cell volume measurements
Cell volumes were measured by electronic sizing with a Coulter Counter Multisizer 3 (Beckman-Coulter, Miami, FL) as previously described (28). The counter determines cell volume by measuring the voltage step that is created by the change in resistance that occurs when a cell displaces its volume in electrolyte solution as it passes through a small aperture. The aperture size used for these experiments was 100 μm.
To prepare the cells for volume measurements, cells were incubated for 3 min with 0.05% trypsin and 0.53 mM EDTA (Invitrogen, Carlsbad, CA). Trypsin was inactivated by the addition of an equal volume of glioma medium. After the cells were pelleted by brief centrifugation, the cells were resuspended in bath solution and passed through a 40-μm nylon cell strainer (Fisher). Cells were incubated in prewarmed or precooled bath solution for 10 min before the beginning of the first baseline measurement. The temperature of the cell suspension was maintained during the course of the experiment by pumping heated or cooled water from an external water bath through plastic tubing used to insulate the beaker and was monitored throughout the experiment. Cell volume measurements were obtained every minute, and each measurement was an average of 10,000–20,000 cells. Five or six baseline volumes were recorded before the osmotic challenge was applied.
Electrophysiology
Recordings of whole cell currents were made with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA), following standard recording techniques (12). Patch pipettes were made with thin-walled borosilicate glass (TW150F-4; World Precision Instruments, Sarasota, FL) and an upright puller (PP-830; Narishige Instruments, Tokyo, Japan) and typically had resistances of 3–5 MΩ. Current recordings were digitized online at 10 kHz and low-pass filtered at 2 kHz with a Digidata 1200 (Axon Instruments). pCLAMP 8.2 (Axon Instruments) was used to acquire and store data. Series resistance (Rs) was compensated to 80%, reducing voltage errors, and cells with a compensated Rs >10 Mω were omitted. D54 cells were plated on glass coverslips and cultured for 3 days before experiments were performed. Standard bath solution was continuously exchanged at a rate of ∼1 ml/min.
Solutions
The control NaCl bath solutions contained the following (in mM): 130 NaCl, 5.0 KCl, 10.5 glucose, 32.5 HEPES, and 1 CaCl2. The pH of each solution was adjusted to 7.4 with NaOH, and the osmolarity of each solution was confirmed with a vapor pressure osmometer (Wescor 5500; Wescor, Logan, UT) to be 310 ± 10 mosM. Pipette solutions contained the following (in mM): 145 CsCl, 1 MgCl2, 10 EGTA, 10 HEPES sodium salt, pH adjusted to 7.3 with Tris-base. CaCl2 was added directly to pipette solution on the day of use at a concentration of 0.2 mM, resulting in a free Ca2+ concentration of 1.9 nM.
Drugs were added directly to bath solutions from stock solutions. Stock solutions of NPPB and tributyltin chloride (TBT) were dissolved at 1,000× final concentration in DMSO; bumetanide, R-(+)-[(2-n-butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]acetic acid (DIOA), and DIDS were dissolved at 500× final concentration in DMSO; and CdCl2 was dissolved at 1,000× final concentration in double-distilled H2O (ddH2O). Control DMSO, at its final concentrations (0.1% and 0.2%), did not perturb cell volumes or affect volume regulation (data not shown).
Data analysis for volume regulation experiments
Coulter Counter data were collected with Multisizer 3 software, and size listings were exported to Excel. Time points were rounded to whole minutes, and mean cell volumes (MCVs) were normalized to the average baseline value for a given experiment. All data were plotted in Origin 7.0 (MicroCal, Northampton, MA) as means ± SE with the number of experiments performed (n). Similar to previous studies (25), the volume-regulatory portion of the generated curve was fit with a first-order exponential decay function, generating a unique time constant (τ) for each experiment. Post-RVD MCV was defined as the volume recorded at time = 3τ± 1 (V3τ), a time at which the volume is expected to be within 5% of its final value. Significance was determined by a Student’s t-test with an α-value of P < 0.05.
Western blots
Cells in 100-mm culture dishes were rinsed with ice-cold PBS, scraped, and collected in 0.5 ml of 1:100 protease inhibitor cocktail (Sigma) in PBS. After a brief centrifugation, the pellet was resuspended in RIPA buffer with protease inhibitors. The sample was sonicated, gently mixed for 30 min, and centrifuged at 4°C. The proteins of the supernatant were separated by electrophoresis using a 7.5% SDS-polyacrylamide gel (Bio-Rad). Proteins were transferred onto polyvinylidene difluoride membranes (Millipore), probed with anti-ClC-2 and -5 antibodies from Alomone Labs (Jerusalem, Israel) and anti-ClC-1, -3, -4, -6, -7 and KCC1-4 antibodies from Alpha Diagnostics (San Antonio, TX), and visualized with horseradish peroxidase-conjugated anti-rabbit antibodies (Bio-Rad) and the ECL system (Amersham).
RESULTS
Human glioma cells exhibit RVD in response to hyposmotic challenge
To examine the degree to which glioma cells can regulate their volume in the face of a hypotonic challenge, we obtained Coulter Counter measurements from D54-MG cells challenged with increasing ddH2O concentrations. After baseline volume measurements (Vbaseline) were collected for 5 min in the presence of normosmotic solution, D54-MG cells were exposed to a hyposmotic stress by the addition of 10%, 25%, 33%, 50%, or 66% ddH2O. The volume of the cells initially increased by a factor of 1.07, 1.24, 1.44, 1.61, and 1.72, respectively, over the average Vbaseline. In the presence of a continuous hyposmotic challenge, the volume of the cells then decreased and returned toward the baseline volume (Fig. 1A). This phenomenon has been previously described for many cell types and is known in the literature as regulatory volume decrease (RVD) (34). To compare the response of glioma cells with that of nonmalignant cells, we similarly exposed primary cultured astrocytes to a 50% or 25% osmotic challenge. In both glioma cells and astrocytes, the initial volume increase was notably less than what would be predicted if the cells behaved as perfect osmometers, suggesting that volume increase is limited by either mechanical constraint or fast-acting volume-regulatory mechanisms. However, although glioma cells exposed to a 50% addition of ddH2O recovered to 0.85*Vbaseline in the face of a continuous challenge, astrocytes were only able to recover to within 1.16*Vbaseline (Fig. 1, A and B). This signifies an enhanced ability of glioma cells to volume regulate.
Fig. 1.
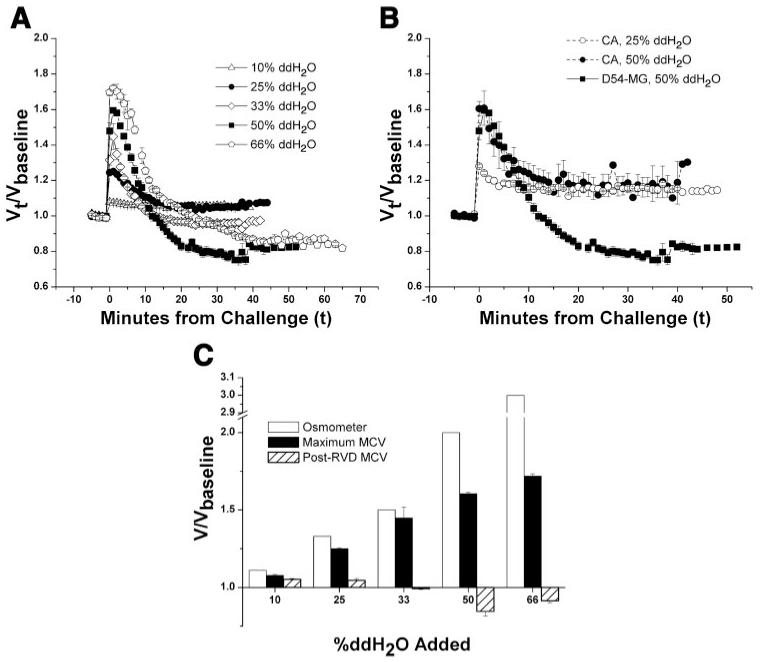
D54-MG cells exhibit regulatory volume decrease (RVD) in response to a hyposmotic challenge. A and B: normalized mean cell volumes (MCVs) during a hyposmotic challenge induced by the addition of double-distilled H2O (ddH2O) in D54-MG cells (A) and cultured astrocytes (B). Vt, volume at time t; Vbaseline, original volume. C: normalized mean peak volumes (both observed and those expected for a perfect osmometer) compared with the final volumes attained after volume regulation in D54-MG cells.
To quantitatively compare the extent of volume recovery for different experimental conditions, we determined the τ of recovery for each experiment by fitting the volume regulation data with a single-exponential curve, as previously described for C6 glioma cells (25). This allowed us to define the post-RVD MCV as the volume measured at time = 3τ, the time at which RVD is expected to be >95% complete. Comparing the post-RVD MCVs revealed that although glioma cells exposed to a less hypotonic challenge (e.g., 10%, 25%) were unable to reestablish their baseline volumes, cells exposed to a more substantial challenge (e.g., 50%, 66%) exhibited a more robust volume decrease and reestablished a volume that was in fact less than the original baseline (Fig. 1, A and C).
Cl- channel inhibitors limit RVD in human glioma cells
RVD has been shown in essentially all living cells to occur because of osmolyte release by different pathways (e.g., anion and cation channels, cotransporters, etc.). It was our objective to examine the underlying mechanisms in glioma cells. To this end, we chose to examine the robust RVD elicited by a 50% hypotonic challenge. As we were primarily interested in the role of Cl- in RVD, we first sought to determine the relative contribution of Cl- channels to RVD in these cells. We added the Cl- channel inhibitors DIDS, NPPB, and Cd2+, which have been shown to inhibit different Cl- channels (5, 32, 42), to the bath solution 10 min before measuring baseline volumes. To maintain a constant concentration, the drug was also added at the appropriate concentration to the ddH2O used to challenge the cells. Treatment with both NPPB (200 μM) and DIDS (200 μM), but not Cd2+ (250 μM), significantly inhibited RVD, as seen by comparing the mean responses in Fig. 2A and normalized post-RVD MCVs (Fig. 2B). Because Cd2+ may block different channels than DIDS or NPPB and was shown previously to synergistically inhibit volume regulation in combination with these drugs (28), we also studied the effect of Cd2+ in combination with NPPB. This combination showed a significantly greater inhibition than NPPB alone (Fig. 2, A and B), with almost complete inhibition of RVD. Similarly, whole cell recordings revealed that although NPPB was able to partially inhibit the hypotonically activated Cl- current, significantly greater inhibition of this current could be achieved by applying the combination of NPPB and Cd2+ (Fig. 2C).
Fig. 2.
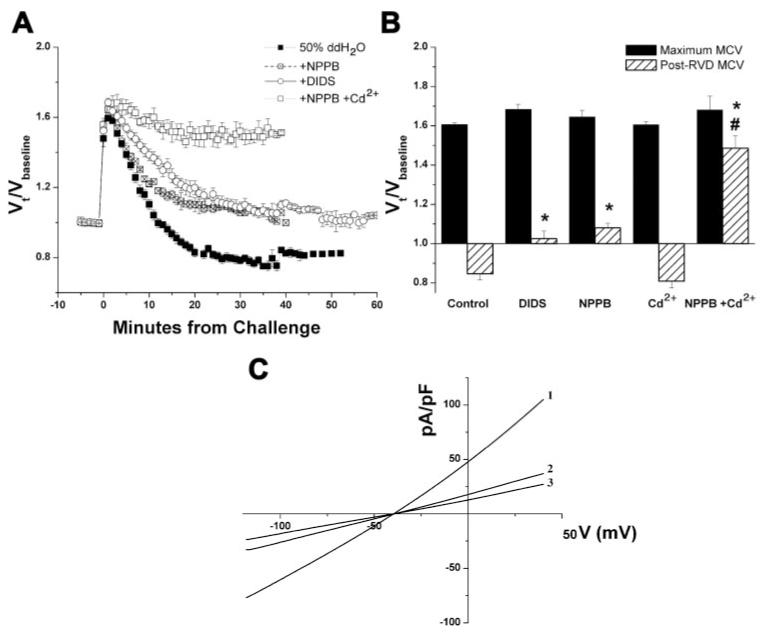
Cl- channel inhibitors limit RVD in human glioma cells. A: comparison of normalized mean volumes of D54-MG cells exposed to a 50% addition of ddH2O and treated with 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS), 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), a combination of NPPB + Cd2+, or no drug (control). B: normalized peak mean and post-RVD volumes. *P < 0.05 compared with control; #P < 0.05 compared with NPPB alone. C: current-voltage (V) relationship of whole cell recordings obtained by eliciting voltage ramps from -120 to +40 mV in cells exposed to a 50% hypotonic challenge in the presence of no drug (1), NPPB (2), or NPPB + Cd2+ (3).
It has been suggested that the channels responsible for NPPB-sensitive swelling-activated Cl- efflux may also allow for the efflux of other anions, including organic osmolytes such as taurine. It is possible that the movement of these osmolytes, independent of Cl-, may be sufficient for volume regulation. To determine whether an intact Cl- gradient was necessary for RVD, the cells were treated with the Cl- ionophore TBT (10 μM) before baseline measurements to abolish the Cl- gradient. This treatment essentially inhibited RVD similarly to treatment with the Cl- inhibitor combination NPPB + Cd2+ (Fig. 3).
Fig. 3.
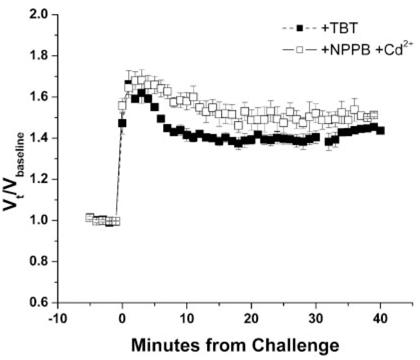
An intact Cl- gradient is necessary for volume recovery. A comparison of normalized mean volumes of D54-MG cells exposed to a 50% addition of ddH2O and treated with the Cl- channel inhibitors NPPB + Cd2+ or the Cl- ionophore tributyltin chloride (TBT).
Cl--cation transport inhibitors also limit RVD in human glioma cells
Although the above data strongly suggest a prominent role for Cl- channels in RVD, we sought to examine the contribution of Cl- transporters, some of which have overlapping pharmacological profiles. As with the Cl- channel inhibitors, the electroneutral Cl- cotransporter inhibitors bumetanide (200 μM), DIOA (40 μM), and furosemide (800 μM) were added to the normosmotic bath solution 10 min before the first volume measurement. Bumetanide, a Na+-K+-2Cl- cotransporter (NKCC)-specific inhibitor, did not have any effect on RVD (Fig. 4, A and B). In contrast, treatment of cells with the KCC inhibitors furosemide and DIOA resulted in a less rapid RVD (Fig. 4A), as evidenced by a larger time constant (τ = 15.99 and 16.70 min, respectively, compared with 8.9 min for control). Furthermore, the degree of volume recovery was significantly (P < 0.05) less than that achieved by control cells (Fig. 4B).
Fig. 4.

Cl--cation transport inhibitors also limit RVD in human glioma cells. A: comparison of normalized mean volumes of D54-MG cells exposed to a 50% addition of ddH2O and treated with bumetanide, R-(+)- [(2-n-butyl-6,7-dichloro-2-cyclopentyl-2,3- dihydro-1-oxo-1H-inden-5-yl)oxy]acetic acid (DIOA), furosemide, or no drug (control). B: normalized peak mean and post-RVD volumes. C: current-voltage relationship of whole cell recordings obtained by eliciting voltage ramps from -120 to +40 mV in cells exposed to a 50% hypotonic challenge in the presence of no drug (1), DIOA (2), or furosemide (3). D: current density values normalized to the control average at 0 mV. *P < 0.05 compared with control.
To verify that the effect of the KCC inhibitors furosemide and DIOA was due to inhibition of the KCC family of Cl- cotransporters and not of Cl- channels, we evaluated the effect of these inhibitors on the hypotonically activated Cl- currents. As Cl- movement through the cotransporters is coupled to K+ movement, it is electroneutral and, therefore, not expected to contribute to the recorded Cl- current. Surprisingly, DIOA inhibited the hypotonically activated Cl- current by ∼24%, and furosemide inhibited the current by nearly 40% (Fig. 4, C and D). We sought to investigate the inhibition of RVD by DIOA further to determine whether it was simply due to inhibition of a DIOA-sensitive Cl- channel or whether cotransporters were indeed involved.
Inhibition of Cl- channels and transporters synergistically inhibits RVD
Although the hypotonically activated Cl- current was greatly inhibited by the channel inhibitor combination of NPPB + Cd2+, the addition of DIOA was not able to provide further inhibition (Fig. 5, C and D), suggesting that the DIOA-sensitive component of this current was also sensitive to either NPPB or Cd2+. However, in the presence of the combination of the transport inhibitor DIOA and the Cl- channel inhibitors NPPB and Cd2+, cells exposed to a 50% challenge exhibited a significantly greater inhibition of RVD than that observed with channel inhibitors or DIOA alone (Fig. 5, A and B), indicating that the inhibitory effect of DIOA cannot simply be explained by a nonspecific effect on Cl- channels. Furthermore, although treatment with Cl- channel or transport inhibitors alone did not affect the magnitude of the initial volume increase, this was not the case for cells treated with a combination of channel and transporter inhibitors, in which the initial volume increase was significantly greater than control cells. Indeed, under these conditions, cells behaved more like perfect osmometers. This finding suggests that the initial volume increase is limited not only by mechanical forces but also by quickly activated volume-regulatory mechanisms.
Fig. 5.
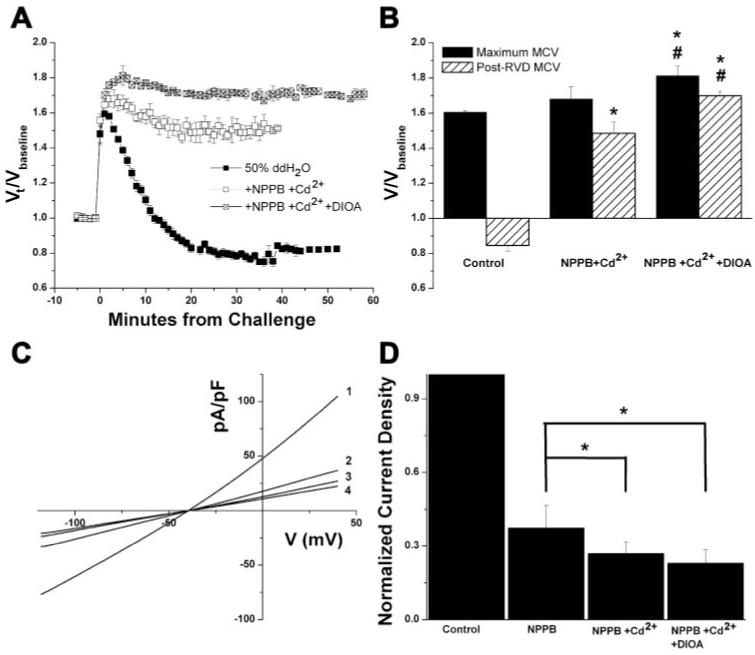
Inhibition of Cl- channels and transporters synergistically inhibits RVD. A and B: comparison of normalized mean cell volumes (A) and mean peak and post-RVD volumes (B) attained from D54-MG cells exposed to a 50% addition of ddH2O and treated with NPPB + Cd2+, NPPB + Cd2+ + DIOA or no drug (control). C: current-voltage relationship of whole cell recordings obtained by eliciting voltage ramps from -120 to +40 mV in cells exposed to a 50% hypotonic challenge in the presence of no drug (1), NPPB + Cd2+ (2), or NPPB + Cd2+ + DIOA (3). D: current density values normalized to the control average at 0 mV. *P < 0.05 compared with control (unless otherwise indicated); #P < 0.05 compared with NPPB + Cd2+.
Because the use of pharmacological inhibitors is confounded by their lack of specificity, we sought to confirm the contribution of both channels and transporters to RVD by exploiting another property of channel- and transport-mediated ion flux, their differential temperature dependence. Although both channel- and transport-mediated ion fluxes are sensitive to temperature (slowing down as the temperature is decreased) energy-dependent transporters typically show a much larger temperature dependence (Q10 > 2.0) than channel-mediated diffusion (Q10 ≈ 1.3–1.6). As demonstrated in Fig. 6A, maintaining the temperature of the solutions at 15°C resulted in a slower and less complete volume regulation than occurs in experiments performed at 25 or 37°C. Similarly, volume regulation appeared to be completely inhibited at 4°C, suggesting that all ion and water fluxes were prevented at this temperature. We predicted that, if both channels and transporters were involved in this process, volume regulation at 15°C would favor contribution by channels and hence RVD would be less sensitive or even insensitive to the transport inhibitor DIOA and yet more sensitive to the channel inhibitors NPPB and Cd2+. This is indeed what we observed. At 15°C, DIOA had no effect of volume regulation, whereas treatment with NPPB + Cd2+ resulted in an increase in the initial swell volume as well as complete inhibition of RVD, similar to the data attained by treatment with NPPB + Cd2+ + DIOA at 22°C. Hence, the temperature shifts allowed us to clearly separate the channeland transport-mediated components of RVD. Moreover, at 15°C in the presence of NPPB + Cd2+, cells finally behaved like perfect osmometers, achieving a doubling in volume with a 50% challenge (Fig. 6B).
Fig. 6.

The transport-mediated component of volume regulation can be inhibited at higher temperatures than the channel-mediated component. A: normalized mean cell volumes of D54-MG cells exposed to a 50% addition of ddH2O at 4, 10, 15, 25, and 37°C. B: normalized mean cell volumes of D54-MG cells exposed to a 50% addition of ddH2O while maintaining the temperature of the experiment at 15°C. Cells were treated with DIOA, NPPB + Cd2+, or no drug (control).
To determine the relative contribution of channels and transporters to RVD in these cells, we estimated the number of osmolytes effluxed during volume regulation under control conditions and in the presence of Cl- or transport inhibitors. To convert the volume decrease into moles effluxed, it was necessary to make the following assumptions. First, we assumed that the initial osmolarity of the cells was ∼300 mosM. We then assumed that the number of molecules in the cell was diluted because of an influx of water during the swelling phase of the experiment and that the maximum volume obtained in absence of volume regulation was at least 1.78*Vbaseline (the average maximum volume attained in the presence of the channel and transport inhibitor cocktail). After calculating the resulting osmolarity, we assumed that this osmolarity would be maintained as cells lost both solutes and water during the volume-regulatory phase of the experiment. Therefore, the number of osmolytes effluxed during any given time period would be equal to the postswell osmolarity multiplied by the volume decrease. In previous experiments with astrocytes, similar assumptions were used and the resulting calculation was shown to closely approximate the number of ions effluxed calculated from a current trace of hypotonically activated currents (30). We calculated the average flux (in pmol∙cell-1∙ min-1) over 10-min intervals after the challenge. Figure 7A shows that, under most experimental conditions, the largest efflux occurred during the first 10 min. Interestingly, at room temperature the channel inhibitor cocktail (NPPB + Cd2+) reduced the osmolyte flux during all three intervals measured, whereas the transport inhibitor DIOA only reduced the osmolyte efflux during the first 10 min of volume regulation, suggesting that the KCC plays a more important role in immediate regulation than it does in later phases of regulation. As expected, the combination of Cl- transporter and channel inhibitors greatly inhibited efflux over the entire course of regulation. At 15°C, the overall ion flux was reduced and DIOA had no effect, suggesting that the transporter was already inhibited at this temperature. At this temperature, however, NPPB + Cd2+ abolished ion flux over the entire course of the experiment (Fig. 7B). A comparison of the efflux rates calculated at 22°C suggested that 30–40% of the osmolytes effluxed during RVD were effluxed through the KCC, whereas 60–70% of the osmolytes were effluxed through NPPB- and Cd2+-sensitive Cl- channels. Consistent with this conclusion was the observation that the overall efflux of ions at 15°C, when the transporters appear to be inhibited, was only 55–60% of the efflux at 22°C and appeared to be completely sensitive to channel inhibitors.
Fig. 7.
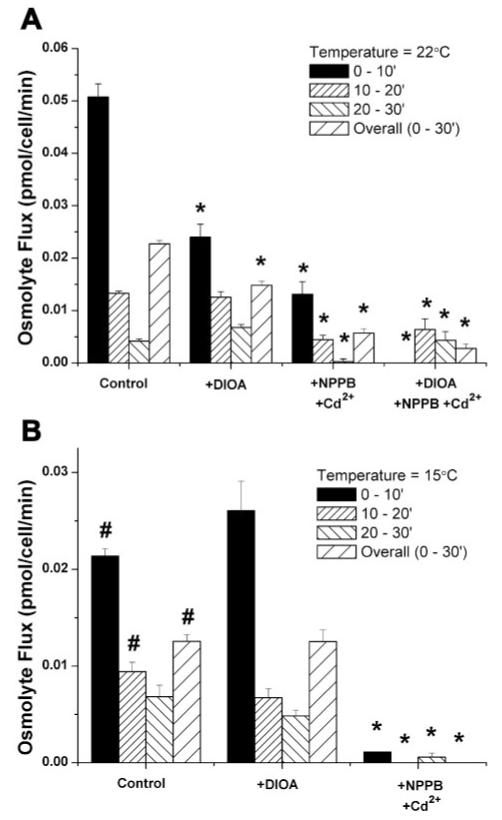
Estimated number of osmolytes effluxed from cells during 10-min intervals after the addition of ddH2O. Experiments were performed at 22°C (A) or 15°C (B) in the presence of DIOA, NPPB + Cd2+, DIOA + NPPB + Cd2+, or no drug. *P < 0.05 compared with control; #P < 0.05 compared with 22°C control.
Cells from patient biopsies are also sensitive to channel and transport inhibitors
Work with cell lines is convenient but potentially flawed by properties that cells acquire in prolonged tissue culture. To ensure that the above observations accurately reflect those of most glioma cells, we reexamined the major findings in glioma cells from acute patient biopsies. Specifically, cells were collected from two different WHO Grade IV astrocytomas (glioblastomas multiforme, or GBMs) at resection (referred to as GBM-1 and GBM-2). These cells were temporarily cultured and exposed to the same conditions as the D54 cell line. As shown in Fig. 8, these cells exhibited all of the hallmarks of RVD reported above for the glioma cell lines. They showed a robust volume regulation response after swell, which could be partially inhibited by treatment with either a combination of Cl- channel blockers (NPPB + Cd2+)or a KCC-specific inhibitor (DIOA). In both cases, the inhibition by DIOA was, in fact, even more pronounced than in the D54 cells.
Fig. 8.
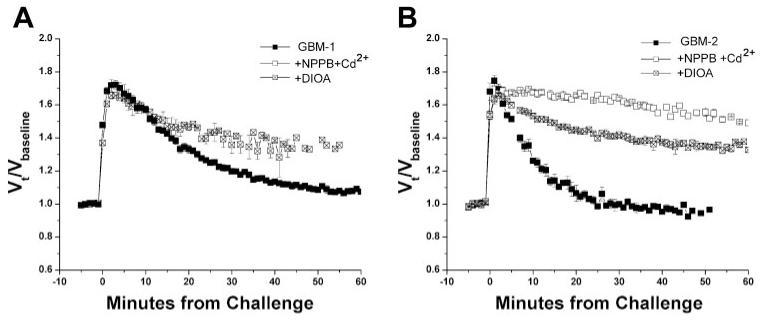
RVD in glioma cells from patient biopsies is also inhibited by both Cl- channel and transport inhibitors. A and B: comparison of normalized mean volumes of cells from patient samples [identified as GBM-1 (A) and GBM-2 (B)] exposed to a 50% addition of ddH2O and treated with DIOA, NPPB + Cd2+, or no drug (control).
Glioma cells express Cl- channels and transporters
A precise delineation of the Cl- channels that contribute to RVD in glioma cells by pharmacological means is impossible because no specific Cl- channel blockers exist. Ultimately, genetic manipulations such as antisense or small interfering RNA studies will be necessary to answer this question. In an initial attempt to identify candidates for Cl- channels and the subset of KCCs that may contribute to RVD in glioma cells, we performed Western blot analysis using whole cell lysates from D54-MG cells, the two acute patient samples, and primary cultures of cortical astrocytes. Although the identity of the channel (or channels) responsible for the Cl- efflux that occurs during RVD has yet to be elucidated, two members of the ClC family of Cl- channels (ClC-2 and ClC-3) have been suggested to participate in this process (5, 26). Furthermore, these proteins were shown previously to give rise to functional Cl- channels in the glioma cell line used in our studies (29). Therefore, we focused our efforts on this family of channels and the KCC family. Clear bands were observed when the blots were exposed to antibodies generated against ClC-2, -3, -5, -6, and -7 and KCC1 and -3a, suggesting that these proteins are indeed expressed in the glioma cells. Interestingly, the expression of KCC1 appeared to be enhanced in the two patient samples, consistent with the more pronounced inhibition by DIOA in these cells (Fig. 9). The specificity of our antibodies was confirmed by preincubation with a control peptide (data not shown).
Fig. 9.

Glioma cells show expression of Cl- channels and transporters. Whole cell lysates of D54-MG cells, patient samples GBM-1 and GBM-2, and cortical astrocytes were separated by SDS-PAGE and probed with antibodies to ClC-1–7 (A) and K+-Cl- cotransporter (KCC)1–4 (B).
DISCUSSION
This study was motivated by the fact that glioma cells, unlike normal brain cells, thrive in the vicinity of an edematous environment. We hypothesized, therefore, that glioma cells can better regulate their cell volume under conditions of changing osmolarities.
This is indeed what we observed. Unlike astrocytes, which only show partial recovery of their cell volume (28, 31), glioma cells are able to regulate their volume back to baseline levels or even lower when exposed to an osmotic challenge. We also found that Cl- channels account for 60–70% of this process, whereas Cl- transport via KCCs accounts for 30–40%, primarily within the first 10 min of a challenge. The differential temperature sensitivity of channels vs. transporters allowed us to delineate their relative contributions. Finally, although we made no attempt to unequivocally identify the underlying proteins, we describe a “cast of characters” that includes ClC-2 and ClC-3 as Cl- channels and KCC1 and KCC3b as Cl- transporters.
Inhibition of RVD in human glioma cells by DIDS and NPPB is consistent with the involvement of several Cl- channels in volume regulation. For example, ClC-2, ClC-3, and/or the protein responsible for ICl,swell among others (5, 29, 39) are all sensitive to these drugs. In our studies, however, the inhibition of RVD by either DIDS or NPPB was incomplete, but both drugs acted synergistically with Cd2+, suggesting that more than one population of Cl- channels is involved. Although similar results have been obtained in astrocytes (28), these data stand in contrast to work published by Bond et al. (2) showing that treatment with 300 μM Cd2+ had no effect on volume regulation in T84 cells. Our inhibition studies suggest that in all likelihood at least two protein populations contribute to the Cl- efflux during RVD—a Cd2+-insensitive population and a Cd2+-sensitive population. One of these populations may be mediated by ICl,swell, which is inhibited by both NPPB and DIDS (5, 39) but insensitive to 300 μM Cd2+ (2), or by ClC-3, which is also sensitive to DIDS and has been shown to produce outwardly rectifying Cl- current in D54-MG cells (29). The Cd2+-sensitive component may be mediated by ClC-2, which produces weakly rectifying currents in glioma cells (29) that are sensitive to NPPB and weakly inhibited by DIDS but, unlike ICl,swell and ClC-3, are highly sensitive to Cd2+. Although ClC-2 was previously dismissed as the protein responsible for ICl,swell because it is an inwardly rectifying current with an anion permeability sequence that differs from that reported for ICl,swell (32, 42), it may nonetheless have a role in RVD.
Although we have chosen to discuss the possibility of ClC-2 and ClC-3 involvement in RVD, we acknowledge that our data do not conclusively support the involvement of any one of these channels. Furthermore, we do not intend to make any conclusions about the involvement of ICl,swell in RVD or its identity. Clearly, the molecular identification of the proteins responsible for channel-mediated Cl- efflux during RVD is an essential next step toward a better understanding this process in glioma cells.
Our study has focused primarily on the role of Cl- movement through channels and transporters during RVD in glioma cells. However, we have not ruled out the involvement of other osmolytes, including osmoregulatory amino acids such as taurine, glutamate, and aspartate. Previous studies have shown swelling-induced efflux of these osmolytes in astrocytes and have suggested that this release is important in volume regulation (18, 31). Because several Cl- channel blockers have been shown to inhibit the efflux of these osmolytes, it has been suggested that they may move through Cl- channels or may be tightly coupled to Cl- movement (16, 18, 33). Interestingly, when our cells were treated with TBT to disrupt the Cl- gradient (to prevent passive movement through channels), RVD was essentially inhibited. Similarly, other studies have shown that swelling induced by an isotonic high-KCl medium, a condition under which the Cl- gradient is not favorable for efflux, does not result in complete volume regulation even though the release of other organic osmolytes occurs (16). These data suggest that Cl- movement is indeed necessary for RVD.
Inhibition of RVD in glioma cells by the KCC inhibitor DIOA strongly suggests that KCCs also play a role in volume regulation of these cells. Although KCCs have not been shown to play a role in the RVD of astrocytes (28), they have been shown to play a role in several other cell types studied (20–22, 40), including other malignant cells. For instance, in the rat glioma cell line C6, the protein phosphatase inhibitor okadaic acid has been shown to inhibit RVD. Although it may affect many proteins, one protein known to be indirectly inhibited through the use of okadaic acid is the KCC, suggesting that this protein may, indeed, be involved in the volume regulation of these cells (21, 22). Although this evidence is indirect, a recent study of cervical cancer cells has shown that these malignant cells express more KCCs than their nonmalignant counterparts (normal cervical epithelium) and that inhibition of KCCs by DIOA limits the volume regulatory response in these cells. Interestingly, this study also showed that inhibition of KCCs also limited cell growth and the invasion of the tumor cells into surrounding tissues (36, 37). Further studies must be done to see whether KCCs play a role in the growth and invasion of glioma cells as well.
Our assertion that KCCs are involved in volume regulation of glioma cells was initially based on the inhibitory effect of 40 μM DIOA, believed to be a relatively specific KCC inhibitor (10). However, previous studies showed that at higher concentrations, i.e., 100 μM, this drug can also partially inhibit Cl- and K+ channels (1, 3, 4). More specifically, a study showed that 100 μM DIOA was able to reduce a swelling-activated Cl- conductance in human osteoblasts (4). Indeed, our own studies confirm that DIOA, even at the lower concentration of 40 μM, was able to reduce the hypotonically activated Cl- current in our cells. We are quite confident, however, that in our studies DIOA affected primarily, if not exclusively, KCCs. This conclusion is based on our inability to observe any inhibitory effect of DIOA when ion transport was blocked by lowering temperature to 15°C, whereas at this temperature Cl- channel blockers remained effective. Moreover, when we inhibited Cl- channels with a combination of NPPB and Cd2+, DIOA had no further effect on the hypotonically activated Cl-’ current but was able to inhibit the residual volume regulation that we attribute to KCC transport. Together these data strongly suggest that the inhibitory effects of DIOA on RVD are due, primarily, to its inhibition of KCCs.
We used an additional approach to verify the relative contribution of channels vs. transporters to RVD that proved to be quite instructive. Specifically, lowering the temperature to 15°C eliminated any contribution from DIOA-sensitive Cl- transport (i.e., KCC activity). Under these conditions, the residual volume recovery was entirely blocked by NPPB + Cd2+, suggesting that this approach had isolated the channel-mediated component. Although in our cells volume regulation at 15°C appeared to have been entirely mediated by channels, this approach must be validated on a case-by-case basis as channel function may also be affected by lower temperatures. Although it is true that in general channel-mediated diffusion is less sensitive to temperature changes than energy-dependent ion transport, the gating of some Cl- channels was recently shown to have an unusually high temperature dependence (44).
Interestingly, at 15°C cells in the presence of NPPB + Cd2+ behaved like perfect osmometers, achieving a doubling in cell size when osmolarity was reduced by 50%. Similarly, inhibition of RVD in C6 glioma cells (22, 25), thymocytes (1), and human cervical cancer cells (37) by different mechanisms (inhibition of K+ channels, Cl- channels, or KCCs) each resulted in an increase in the magnitude of the initial rapid swell. These data demonstrate that cell volume is exclusively an osmotic property with no mechanical limitations.
The exciting new observation in this study is that both Cl- channels and transporters play significant roles in volume regulation in human glioma cells. Furthermore, the contribution of these two components can be dissected by taking advantage of their relative temperature dependence, offering us a new method for studying the mechanisms of volume regulation. Future studies will have to determine whether these same mechanisms are involved in other aspects of cell behavior in which volume changes may be important, such as cell proliferation, death, migration, and invasion.
ACKNOWLEDGMENTS
We thank Dr. Yancy Gillespie (University of Alabama at Birmingham) for providing cells from patient biopsies.
GRANTS
This work was supported by National Institutes of Health Grants R01-NS-36692 and P50-CA-97247.
REFERENCES
- 1.Arrazola A, Rota R, Hannaert P, Soler A, Garay RP. Cell volume regulation in rat thymocytes. J Physiol. 1993;465:403–414. doi: 10.1113/jphysiol.1993.sp019683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond TD, Ambikapathy S, Mohammad S, Valverde MA. Osmosensitive Cl- currents and their relevance to regulatory volume decrease in human intestinal T84 cells: outwardly vs. inwardly rectifying currents. J Physiol. 1998;511:45–54. doi: 10.1111/j.1469-7793.1998.045bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botchkin LM, Matthews G. Swelling activates chloride current and increases internal calcium in nonpigmented epithelial cells from the rabbit ciliary body. J Cell Physiol. 1995;164:286–294. doi: 10.1002/jcp.1041640209. [DOI] [PubMed] [Google Scholar]
- 4.Brauer M, Frei E, Claes L, Grissmer S, Jager H. Influence of K-Cl cotransporter activity on activation of volume-sensitive Cl- channels in human osteoblasts. Am J Physiol Cell Physiol. 2003;285:C22–C30. doi: 10.1152/ajpcell.00289.2002. [DOI] [PubMed] [Google Scholar]
- 5.de Tassigny A D’Anglemont, Souktani R, Ghaleh B, Henry P, Berdeaux A. Structure and pharmacology of swelling-sensitive chloride channels, ICl,swell. Fundam Clin Pharmacol. 2003;17:539–553. doi: 10.1046/j.1472-8206.2003.00197.x. [DOI] [PubMed] [Google Scholar]
- 6.Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 7.Dubois JM, Rouzaire-Dubois B. The influence of cell volume changes on tumour cell proliferation. Eur Biophys J. 2004;33:227–232. doi: 10.1007/s00249-003-0364-1. [DOI] [PubMed] [Google Scholar]
- 8.Ferrer J, Wasson J, Salkoff L, Permutt MA. Cloning of human pancreatic islet large conductance Ca2+-activated K+ channel (hSlo) cDNAs: evidence for high levels of expression in pancreatic islets and identification of a flanking genetic marker. Diabetologia. 1996;39:891–898. doi: 10.1007/BF00403907. [DOI] [PubMed] [Google Scholar]
- 9.Gamba G. Electroneutral chloride-coupled co-transporters. Curr Opin Nephrol Hypertens. 2000;9:535–540. doi: 10.1097/00041552-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Garay RP, Nazaret C, Hannaert PA, Cragoe EJ., Jr. Demonstration of a[K+,Cl-]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl-]-cotransport system. Mol Pharmacol. 1988;33:696–701. [PubMed] [Google Scholar]
- 11.Grunder S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of ClC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- 12.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 13.Haussinger D. The role of cellular hydration in the regulation of cell function. Biochem J. 1996;313:697–710. doi: 10.1042/bj3130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermoso M, Satterwhite CM, Andrade YN, Hidalgo J, Wilson SM, Horowitz B, Hume JR. ClC-3 is a fundamental molecular component of volume-sensitive outwardly rectifying Cl- channels and volume regulation in HeLa cells and Xenopus laevis oocytes. J Biol Chem. 2002;277:40066–40074. doi: 10.1074/jbc.M205132200. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann EK. Cell swelling and volume regulation. Can J Physiol Pharmacol. 1992;70(Suppl):S310–S313. doi: 10.1139/y92-277. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Tatsumi H, Katayama Y. Mechanosensitive chloride channels on the growth cones of cultured rat dorsal root ganglion neurons. Neuroscience. 2000;97:347–355. doi: 10.1016/s0306-4522(00)00018-x. [DOI] [PubMed] [Google Scholar]
- 17.Jackson PS, Madsen JR. Cerebral edema, cell volume regulation, and the role of ion channels in organic osmolyte transport. Pediatr Neurosurg. 1997;27:279–285. doi: 10.1159/000121271. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki M, Uchida S, Monkawa T, Miyawaki A, Mikoshiba K, Marumo F, Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994;12:597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 19.Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang F, Ritter M, Gamper N, Huber S, Fillon S, Tanneur V, Lepple-Wienhues A, Szabo I, Bulbins E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol Biochem. 2000;10:417–428. doi: 10.1159/000016367. [DOI] [PubMed] [Google Scholar]
- 21.Lauf PK. K:Cl cotransport: emerging molecular aspects of a ouabain-resistant, volume-responsive transport system in red blood cells. Renal Physiol Biochem. 1988;11:248–259. doi: 10.1159/000173165. [DOI] [PubMed] [Google Scholar]
- 22.Lauf PK, Adragna NC. K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem. 2000;10:341–354. doi: 10.1159/000016357. [DOI] [PubMed] [Google Scholar]
- 23.Lauf PK, Bauer J, Adragna NC, Fujise H, Zade-Oppen AM, Ryu KH, Delpire E. Erythrocyte K-Cl cotransport: properties and regulation. Am J Physiol Cell Physiol. 1992;263:C917–C932. doi: 10.1152/ajpcell.1992.263.5.C917. [DOI] [PubMed] [Google Scholar]
- 24.McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–1266. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- 25.Mercado A, Mount DB, Gamba G. Electroneutral cation-chloride cotransporters in the central nervous system. Neurochem Res. 2004;29:17–25. doi: 10.1023/b:nere.0000010432.44566.21. [DOI] [PubMed] [Google Scholar]
- 26.Mountian I, Declercq PE, Van Driessche W. Volume regulation in rat brain glial cells: lack of a substantial contribution of free amino acids. Am J Physiol Cell Physiol. 1996;270:C1319–C1325. doi: 10.1152/ajpcell.1996.270.5.C1319. [DOI] [PubMed] [Google Scholar]
- 27.Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 28.Olsen ML, Schade S, Lyons SA, Amarillo MD, Sontheimer H. Expression of voltage-gated chloride channels in human glioma cells. J Neurosci. 2003;23:5572–5582. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill WC. Physiological significance of volume-regulatory transporters. Am J Physiol Cell Physiol. 1999;276:C995–C1011. doi: 10.1152/ajpcell.1999.276.5.C995. [DOI] [PubMed] [Google Scholar]
- 30.Parkerson KA, Sontheimer H. Contribution of chloride channels to volume regulation of cortical astrocytes. Am J Physiol Cell Physiol. 2003;284:C1460–C1467. doi: 10.1152/ajpcell.00603.2002. [DOI] [PubMed] [Google Scholar]
- 31.Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia. 2004;46:419–436. doi: 10.1002/glia.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasantes-Morales H, Alavez S, Sanchez OR, Moran J. Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochem Res. 1993;18:445–452. doi: 10.1007/BF00967248. [DOI] [PubMed] [Google Scholar]
- 33.Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF. Cell volume regulation and swelling-activated chloride channels. Biochim Biophys Acta. 2003;1618:153–162. doi: 10.1016/j.bbamem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Olea R, Pena C, Moran J, Pasantes-Morales H. Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl- transport in cultured astrocytes. Neurosci Lett. 1993;156:141–144. doi: 10.1016/0304-3940(93)90458-w. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Nielsen B. Comparative physiology of cellular ion and volume regulation. J Exp Zool. 1975;194:207–219. doi: 10.1002/jez.1401940114. [DOI] [PubMed] [Google Scholar]
- 36.Schwiebert EM, Mills JW, Stanton BA. Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. J Biol Chem. 1994;269:7081–7089. [PubMed] [Google Scholar]
- 37.Shen MR, Chou CY, Ellory JC. Volume-sensitive KCl cotransport associated with human cervical carcinogenesis. Pflügers Arch. 2000;440:751–760. doi: 10.1007/s004240000338. [DOI] [PubMed] [Google Scholar]
- 38.Shen MR, Chou CY, Hsu KF, Hsu YM, Chiu WT, Tang MJ, Alper SL, Ellory JC. KCl cotransport is an important modulator of human cervical cancer growth and invasion. J Biol Chem. 2003;278:39941–39950. doi: 10.1074/jbc.M308232200. [DOI] [PubMed] [Google Scholar]
- 39.Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 40.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 41.Taouil K, Hannaert P. Evidence for the involvement of K+ channels and K+-Cl- cotransport in the regulatory volume decrease of newborn rat cardiomyocytes. Pflügers Arch. 1999;439:56–66. doi: 10.1007/s004249900117. [DOI] [PubMed] [Google Scholar]
- 42.Waldegger S, Jentsch TJ. From tonus to tonicity: physiology of ClC chloride channels. J Am Soc Nephrol. 2000;11:1331–1339. doi: 10.1681/ASN.V1171331. [DOI] [PubMed] [Google Scholar]
- 43.Walz W. Chloride/anion channels in glial cell membranes. Glia. 2002;40:1–10. doi: 10.1002/glia.10125. [DOI] [PubMed] [Google Scholar]
- 44.Wang GX, Hatton WJ, Wang GL, Zhong J, Yamboliev I, Duan D, Hume JR. Functional effects of novel anti-ClC-3 antibodies on native volume-sensitive osmolyte and anion channels in cardiac and smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1453–H1463. doi: 10.1152/ajpheart.00244.2003. [DOI] [PubMed] [Google Scholar]
- 45.Zuniga L, Niemeyer MI, Varela D, Catalan M, Cid LP, Sepulveda FV. The voltage-dependent ClC-2 chloride channel has a dual gating mechanism. J Physiol. 2004;555:671–682. doi: 10.1113/jphysiol.2003.060046. [DOI] [PMC free article] [PubMed] [Google Scholar]


