Abstract
In complex with FKBP12, the immunosuppressant rapamycin binds to and inhibits the yeast TOR1 and TOR2 proteins and the mammalian homologue mTOR/FRAP/RAFT1. The TOR proteins promote cell cycle progression in yeast and human cells by regulating translation and polarization of the actin cytoskeleton. A C-terminal domain of the TOR proteins shares identity with protein and lipid kinases, but only one substrate (PHAS-I), and no regulators of the TOR-signaling cascade have been identified. We report here that yeast TOR1 has an intrinsic protein kinase activity capable of phosphorylating PHAS-1, and this activity is abolished by an active site mutation and inhibited by FKBP12-rapamycin or wortmannin. We find that an intact TOR1 kinase domain is essential for TOR1 functions in yeast. Overexpression of a TOR1 kinase-inactive mutant, or of a central region of the TOR proteins distinct from the FRB and kinase domains, was toxic in yeast, and overexpression of wild-type TOR1 suppressed this toxic effect. Expression of the TOR-toxic domain leads to a G1 cell cycle arrest, consistent with an inhibition of TOR function in translation. Overexpression of the PLC1 gene, which encodes the yeast phospholipase C homologue, suppressed growth inhibition by the TOR-toxic domains. In conclusion, our findings identify a toxic effector domain of the TOR proteins that may interact with substrates or regulators of the TOR kinase cascade and that shares sequence identity with other PIK family members, including ATR, Rad3, Mei-41, and ATM.
INTRODUCTION
The immunosuppressants, cyclosporin A, FK506, and rapamycin, form complexes with either cyclophilin A or FKBP12 that inhibit components of signal-transduction pathways conserved from yeast to man (Heitman et al., 1992; Schreiber and Crabtree, 1992; Cardenas et al., 1994b). Rapamycin has attracted much attention because of its potent immunosuppressive, antifungal, and antineoplastic activities, and late-phase III clinical trials are in progress.
Rapamycin binds with high affinity to the prolyl isomerase FKBP12, which is highly conserved from yeast and other microorganisms to man. The active intracellular toxin is the resulting FKBP12–rapamycin complex, and yeast mutants lacking FKBP12 are viable and rapamycin resistant (Heitman et al., 1991; Koltin et al., 1991). The targets of the FKBP12-rapamycin complex were first identified in yeast as the TOR1 and TOR2 proteins, which share 67% overall identity, have a C-terminal domain with similarity to both protein and lipid (PI-3 and PI-4) kinases, and directly interact with FKBP12-rapamycin (Heitman et al., 1991; Cafferkey et al., 1993; Kunz et al., 1993; Helliwell et al., 1994; Cardenas and Heitman, 1995; Zheng et al., 1995). Subsequently, the mammalian homologue of the yeast TOR proteins (mTOR, FRAP, RAFT1, SEP, or RAPT1) was identified as an FKBP12-rapamycin–binding protein and found to share ∼50% identity with TOR1 and TOR2 (Brown et al., 1994; Chiu et al., 1994; Sabatini et al., 1994; Sabers et al., 1995).
Gene deletion experiments revealed that the yeast TOR2 protein is essential for viability, whereas in most genetic backgrounds the yeast TOR1 protein is not (Cafferkey et al., 1993; Kunz et al., 1993; Helliwell et al., 1994). Yeast cells exposed to rapamycin or depleted of both TOR proteins arrest as large unbudded cells in the G1 or G0 phase of the cell cycle, indicating a specific role for the TOR proteins in cell-cycle progression and as the targets of rapamycin (Heitman et al., 1991; Kunz et al., 1993). TOR mutants altered in any of three conserved residues (S1975, W2042, and F2049 in TOR2) fail to bind FKBP12–rapamycin and, as a consequence, confer dominant rapamycin resistance (Stan et al., 1994; Chen et al., 1995; Lorenz and Heitman, 1995).
The yeast and mammalian TOR proteins share sequence similarity with both protein and lipid kinases. The mammalian TOR protein exhibits an autophosphorylation activity that is dependent on integrity of the kinase domain and can be inhibited by FKBP12–rapamycin, suggesting that the TOR proteins might be unusual protein kinases (Brown et al., 1995; Brunn et al., 1996; Hara et al., 1997; Withers et al., 1997). Consistent with this view, recent studies reveal that the mammalian TOR protein phosphorylates PHAS-I (Brunn et al., 1997; Burnett et al., 1998), which had been implicated in earlier studies as a component functioning downstream of TOR and involved in the regulation of translational initiation (Lin et al., 1995; von Manteuffel et al., 1996).
Genetic studies reveal that integrity of the TOR protein kinase domains is also essential for TOR in vivo functions in yeast, further supporting the hypothesis that activity of the kinase domain is important for TOR function (Cardenas and Heitman, 1995; Zheng et al., 1995; Schmidt et al., 1996). The TOR proteins have been implicated in two intracellular functions. First, yeast TOR1 and TOR2 and their mammalian homologue, mTOR/FRAP/RAFT1, regulate initiation of translation (Graves et al., 1995; Barbet et al., 1996; Di Como and Arndt, 1996). Rapamycin or TOR depletion results in an inhibition of translation in yeast, and the mammalian TOR protein plays an analogous role in regulating translation, directly or indirectly, via P70 S6 kinase and the PHAS-I regulator of the CAP-binding protein eIF-4E (for reviews see Brown and Schreiber[1996] and Lawrence and Abraham [1997]). A second distinct role has been defined for the yeast TOR2 protein in regulating polarization of the actin cytoskeleton (Schmidt et al., 1996, 1997).
The TOR proteins have been implicated as components of signal transduction cascades that regulate cell-cycle progression and proliferation in response to growth factors, such as IL-2, in mammalian cells and in response to nutrients in yeast cells. For example, the TOR proteins function in nutrient-sensing pathways that regulate autophagy and sporulation in Saccharomyces cerevisiae (Zheng and Schreiber, 1997; Noda and Ohsumi, 1998) and during mating and sporulation in Saccharomyces pombe (Weisman et al., 1997). However, the mechanisms by which the TOR proteins are activated, and the target substrates altered by the TOR proteins to exert their regulatory influences on translation, cell polarity, and cell cycle progression are largely unknown. In previous studies, we established that the TOR kinase domain has been functionally conserved from yeast to man, indicating that TOR is a highly conserved regulator of cell growth (Alarcon et al., 1996). Here we have sought to identify functional domains of the TOR proteins as a starting point to identify other interacting components of these cascades.
In this report we demonstrate that TOR1 exhibits an intrinsic protein kinase activity capable of phosphorylating PHAS-I. This activity is stimulated by Mn2+ ions, abolished by an active site mutation, and inhibited by FKBP12–rapamycin or wortmannin. We find that integrity of the yeast TOR1 kinase domain is required for TOR1 function in yeast and for a rapamycin-resistant TOR1 mutant to confer drug resistance. These findings are in accord with previous reports on TOR1 and TOR2 kinase-inactive mutants (Cardenas and Heitman, 1995; Zheng et al., 1995; Schmidt et al., 1996). Moreover, we also find that overexpression of the TOR1 kinase-inactive mutant is toxic to the cell, possibly by sequestering substrates or regulators of TOR1. Interestingly, deletion of the TOR1 FRB domain, the kinase domain, or both domains, results in TOR1-truncation derivatives that are toxic to the cell. Overexpression of different truncated forms of TOR1 and TOR2 identifies a central-toxic-effector domain of the TOR proteins, distinct from the FRB and kinase domains, which may interact with substrates or regulators of the TOR- signaling cascade. Toxicity of the TOR1 kinase-inactive mutant or the TOR-toxic effector domains was mitigated by overexpression of wild-type TOR1, indicating a specific inhibition of TOR function. Overexpression of the yeast phospholipase C homologue PLC1 also suppressed growth inhibition by the TOR1-toxic domain, suggesting a link between the TOR- and PI-signaling cascades. The yeast PLC1 gene was recently independently identified as a multicopy suppressor of certain TOR2 conditional mutants (Helliwell et al., 1998), further supporting a connection between the TOR- and PI-signaling cascades in the regulation of cell function.
The TOR-toxic domain is conserved between yeast TOR1 and TOR2 and their mammalian counterpart, mTOR/RAFT/FRAP. In addition, a BLAST search with the TOR-toxic domain reveals limited identity over an ∼240-amino acid region with the PIK-related family member ATR from humans, and other PIK family members, including S. pombe Rad3, Drosophila mei-41, and the human ATM protein. Notably, ATR is most closely related to ATM, the protein mutated in patients with ataxia-telangiectasia, and both ATR and ATM have been implicated in signaling cascades regulating cell checkpoint responses to DNA damage and may also play roles in meiotic recombination (Barlow et al., 1996; Xu et al., 1996; Xu and Baltimore, 1996; Morgan et al., 1997; Cliby et al., 1998). That the TOR-toxic domain shares identity with ATR and other PIK family members suggests that, like the kinase domain, the toxic domain has been conserved and could have a role in interactions with PIK-effector proteins.
MATERIALS AND METHODS
Media and Strains
Yeast media were prepared as previously described (Sherman, 1991). Minimal galactose medium is identical to SD medium, except that the carbon source is 2% galactose and 3% glycerol. Where indicated, medium was supplemented with different concentrations of rapamycin. Yeast transformations were performed using the lithium acetate method (Schiestl et al., 1993).
Yeast strain CAY7 was derived from strain CAY1 (MATa tor1::leu2::hisG Δsrk1::G418 TRP+) (Alarcon et al., 1996) by one-step gene disruption with the trp1::hisG-URA3-hisG::trp1-integrating plasmid pNKY1009 and rendered ura3- by selection on 5-FOA, as previously described (Alani et al., 1987). Strain CAY6 was derived from strain BJ5459 (MATa ura3–52 trp1 lys2–801 leu2Δ1 his3Δ200 can1 pep4::HIS3 prb1Δ1.6R) by one-step gene disruption with a PCR product bearing Δtor1::G418 obtained with primers 676 (5′-TTGGAGAAAATTTTCCGCGAATTAACCAGTGATTACAAGGCAG -CTGAAGCTTCGTACGC-3′) and 677 (5′-TTGCGCCCTTTCTTAATAATTCACTAGGATTAATCAACGGGCATAGGCCACTAGT -TGGATCTG-3′) and template plasmid pFA6-kanMX2 (Wach et al., 1994).
TOR1 Mutant and Truncation Plasmid Constructions
The wild-type TOR1 and TOR2 proteins and their mutant or truncated derivatives were expressed from the regulatable GAL1 promoter with an amino-terminal hemagglutinin (HA) epitope tag. The HA-tagged wild-type TOR1 (GAL1 2μ TRP1) plasmid was provided by David Fiorentino (plasmid pYDF72). The TOR1 kinase-inactive mutation D2275A was introduced by PCR overlap mutagenesis (Ho et al., 1989) using primers 522 (5′-CTGGGACTAGGTGCTCGCCATCCAAGC-3′) and 523 (5′-GCTTGGATGGCGAGCACCTAGTCCCAG-3′) (mutations in bold) and flanking primers 734 (5′-GATATCCAACAATACCCGGCTATTCCAT-3′) (upstream of the unique KpnI site in TOR1 gene) and 558 (5′-CCGCGTCGACATACCTATTGTGAAAAGTACCGATG-3′) (SalI site shown in bold). The PCR product was digested with KpnI and SalI and subcloned into plasmid pYDF72 (HA-tagged TOR1 construct) that had been cleaved with KpnI and SalI.
The TOR1 rapamycin-resistance mutations, S1972I and S1972R, were constructed by gap repair of genomic TOR1 mutations. The HA-tagged wild-type TOR1 plasmid was cleaved with KpnI and NcoI, and 1 μg of this gapped plasmid DNA was transformed into either strain RR1 or R20, which are isogenic derivatives of strain Y190OY JK9–3d isolated as spontaneous rapamycin-resistant mutants. By DNA sequence analysis, RR1 is a TOR1 S1972I mutation, and R20 is a TOR1 S1972R mutation. Transformants were selected on SD-TRP medium, and the gap-repaired plasmid was isolated. The complete NcoI–KpnI region was sequenced, revealing that the expected S1972I or S1972R mutations, and no other extraneous mutations, were now present. These mutant NcoI–KpnI fragments were subcloned into the HA-tagged TOR1 and kinase-inactive TOR1 plasmids between the NcoI and KpnI sites.
Carboxy-terminal TOR1 deletion mutants were generated by restriction endonuclease digestion. The HA-tagged TOR1 plasmid was digested with either KpnI–SalI or NcoI–SalI, blunted, and ligated. Deletion 290-1682 was generated by digesting and ligating pCNTOR1(wild-type TOR1 in pRS316 CEN, URA3) or pT1DA (kinase-inactive TOR1 in pRS316 CEN, URA3) with BamHI. This TOR1-truncated plasmid was used as template for a PCR reaction using primers 430 (5′-GGCCAGTATTTCATTATTTG-3′) and 536 (5′-CTTTTCTCTTAAGATGGCAGC-3′).
All other internal deletions were generated by PCR overlap mutagenesis using the TaKaRa long-range PCR system from Intergen (Purchase, NY). Deletion 1962–2051 was generated with overlapping primers 752 (5′-GCCGTTCTATGGCACATACCACAGTTACAAACCTTAGACTTACAG-3′) and 751 (5′-GTGCCATAGAAC-GGCTACTCTGAT-3′) (overlap in bold) and flanking primers 536 and 519 (5′-GAATCAAACGGATGCTGCAATTGG-3′). Deletion 1775–2157 was generated with overlapping primers 734 (5′-AAGCTCAACGGAGATATCCAACAATACCCGC-TATTCCA-3′) and 733 (5′-TCCGTTGAGCTTAGTCTCTTCCTG-3′) and flanking primers 536 and 519. Deletion 731-1682 was generated with overlapping primers 707 (5′-AAGGAAGAAACTAATAATATGATCG-CGCAAAGTGTCAAACTC-3′) and 706 (5′-AGTTTCTTCCTTTTCTCGAGA-3′) and flanking primers 536 and 430. Deletion 1207–1682 was generated with overlapping primers 791 (5′GCAGCAGATGCTAATAATATGATCGCGCAAAGTGTCAAAC-TC-3′) and 790 (5′-AGCATCTGCTGCCTCCATTTG-3′) and flanking primers 536 and 430. Deletion 1483–1682 was generated with overlapping primers 801 (5′-GAATATATCAGCAATAATATGAT-CGCGCAAAGTGTCAAACTC-3′) and 800 (5′-GCTGATATATTCGTCAAGCAT-3′) and flanking primers 536 and 430. The two PCR fragments generated were isolated and used as templates for a second PCR reaction using the flanking primers. The HA-tagged TOR1 and the HA-tagged kinase-inactive TOR1 constructs were digested with NcoI and KpnI and the 12-kilobase (kb) fragment was isolated using gene-clean (BIO 101, Vista, CA). Gapped plasmid DNA (1 μg) was cotransformed with the PCR products described above into yeast strain CAY6, and transformants were selected on SD-TRP medium. Homologous recombination between TOR1 DNA sequences at each end of the PCR fragment and the TOR1 gene on the plasmid results in targeted integration into the plasmid (Orr-Weaver et al., 1981). The truncated forms of TOR1 were identified by PCR, using primers 536 and 430.
To clone TOR1 and TOR2 fragments into the same plasmid background, a URA3 cassette was generated containing the HA epitope and NotI sites with the following primers: 5′-CGATAC-CCATACGACGTCCCAGACTACGCTAGCTC-GACGGTATCGG-CGGCCGCCTTATTCTTTTTTTTGATTTCGG-3′ (no. 628) (the HA epitope is underlined and the NotI site in bold) and 5′-GTCGACGG-TATCGATAAGCTTGATATCGAATTCCTGCAGCCCGGGGCGGC-CGCGCTTTTTCTTTCCAATTTTTTTTTTTTCG-TC-3′(no. 629) (NotI site in bold). This PCR product was cotransformed with the gapped HA-tagged TOR1 plasmid into yeast strain CAY6, and transformants were selected on SD-URA medium. Homologous recombination between the vector sequences at each end of the PCR product and the plasmid replaced the TOR1 sequence. This plasmid was digested with NotI to remove the URA3 sequence, and TOR1 and TOR2 fragments were cloned as NotI cassettes. A TOR1 fragment containing residues 1207–1774 was PCR amplified with primers 911 (5′-CCGCTC-GAGGCGGCCGCCGGGGTCGCAAAATTACCTATAAACCAATC-A-3′) and 886 (5′-CGCGGATCCGCGGCCGCCTCCGTTGAGCT-TAGTCTCTTCCTGAACCAT-3′) (NotI sites in bold). A TOR1 fragment containing residues 1207–1961 was PCR amplified with primers 911 and 912 (5′-AAACTGCAGGCGGCCGCCGTGCCATAGAACGGCTACTCTGATCAACTC-3′). A TOR1 fragment containing residues 1207–2340 was PCR amplified with primers 911 and 913 (5′-AAACTGCAGGCGGCCGCCGACATTTTCACAAGTAATTCG-GAAACTGCC-3′), and the template was either the wild-type TOR1 gene, the kinase-inactive TOR1 gene, or the double-mutant TOR1 gene. A TOR2 fragment containing residues 1216–1782 was PCR amplified with primers 1094 (5′-AAGGAAAAAGCGGCCGCCGTAACGAAATTACCGGTAAACCAAAATATC-3′) and 1095 (5′-AAGCTGCAGGCGGCCGCCCTGTTTCTTTTTAGAGACAGATGTTAGCAT-3′). A TOR2 fragment containing residues 1216–1964 was PCR amplified with primers 1094 and 1096 (5′-AAGCTGCAGGCGGCCGCCATGCCAAAGCACCGCCATACGTATCAATTC-3′). A TOR2 fragment containing residues 1216–2345 was PCR amplified with primers 1094 and 1097 (5′-AAGCTGCAGGCGGCCGCCAACATTCTCACAAGTAATACGGAAGCTACC-3′). All constructs were transformed into yeast strain CAY6. Several independent constructs and from 5 to 10 transformants for each construct were tested for their ability to grow on galactose medium. In addition, the integrity of these PCR-generated constructs was verified in the majority of cases by DNA sequence analysis with synthetic primers corresponding to the known sequences of the yeast TOR1 and TOR2 genes.
PLC1, STT4, and MSS4 Overexpression Plasmids
To create pGMSS4, the MSS4 gene was amplified with primers 1227 (5′-CGGGATCCATGTCAGTCTTGCGATCAC-3′) and 1228 (5′-GCGGATCCTCAGTCTTTATAATTTTTCTGC-3′) using pMSS4 (Yoshida et al., 1994b) as template. This PCR product was digested with BamHI and cloned in pYeF1 at the BamHI site to place expression of the MSS4 gene under the control of the GAL10-CYC1 promoter. The his-c-myc epitope-tagged PLC1 overexpression plasmid pJF137 has been described previously (Flick and Thorner, 1993). The plasmid pGALHA-STT4 employed to overexpress the HA-tagged STT4 protein was as previously described (Cutler et al., 1997).
Protein Extracts for Immunoprecipitates and Protein Kinase Assays
Yeast cells CAY6 transformed with the 2μ, GAL1 plasmid pYDF72 (encoding the H6 tagged full-length TOR1 gene or different mutant forms under control of the GAL1 promoter) induced with galactose as indicated above were spheroplasted in 1.2 M sorbitol, 100 mM KH2PO4, pH 7.5, and 3500 U/ml lyticase for 20 min at 30°C. The spheroplasts were washed twice in the same buffer without lyticase and lysed by resuspending in lysis buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 0.2% Tween 20, 25 mM β-glycerophosphate, 25 mM NaF, 100 μM Na3VO4, 0.5 mM PMSF, 1 μg/ml pepstatin, 100 μM leupeptin, 1 mM benzamidine, and 1% trasylol), for 30 min at 4°C. Cell lysates were centrifuged at 15,000 rpm for 15 min at 4°C. HA-TOR1 polypeptides were precipitated from these supernatants with 30 μl/ml HA.11 monoclonal antibody immobilized on sepharose beads (Babco). After incubation for 1 h at 4°C, the beads were recovered by brief centrifugation, and immunoprecipitates were washed three times with lysis buffer containing 400 mM NaCl and twice with kinase buffer consisting of 10 mM HEPES, pH 7.4, 50 mM NaCl, 10 mM MnCl2, 1 mM DTT. For inhibition studies, immunobeads were preincubated for 15 min at 4°C in 15 μl of kinase buffer alone or containing FKBP12–FK506 or FKBP12–rapamycin complexes or wortmannin at the concentration indicated in the legend to Figure 1. Kinase reactions were started by addition of 15 μl of reaction mix consisting of kinase buffer containing 20 μM ATP, 10 μCi γ32P-ATP, and 1 μg recombinant PHAS-I (Stratagene, La Jolla, CA) and incubated at 30°C for 40 min. FKBP12–FK506 or FKBP12–rapamycin complexes were formed by mixing 1 μg of recombinant yeast H6-FKBP12 with 1 μM FK506 or 1 μM rapamycin (added from 1 mM stocks in methanol) in kinase buffer followed by incubation for 1 h at 4°C.
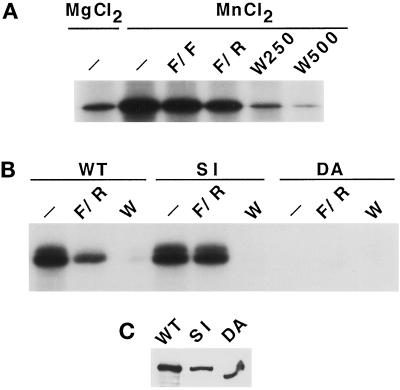
Figure 1.
TOR1 has an intrinsic protein kinase activity. (A) TOR1 was immunoprecipitated from protein extracts prepared from spheroplasts of CAY6 yeast cells (MATa ura3–52 trp1 lys2–801 leu2Δ1 his3Δ200 can1 pep4::HIS3 prb1Δ1.6R Δtor1::G418) overexpressing HA-TOR1 from the GAL1 promoter. TOR1 immunoprecipitates were preincubated for 15 min at 4°C with kinase buffer in the absence (−) or presence of FKBP12-FK506 (F/F), FKBP12-rapamycin (F/R), or 250 nM or 500 nM Wortmannin (W250 and W500, respectively). Phosphorylation reactions were started by addition of a radioactive mix containing 10 μM ATP, 10 μCi γ32P-ATP, 1 μg PHAS-I, and 10 mM MgCl2 or MnCl2 as indicated. (B) Immunoprecipitates of the wild-type (WT) TOR1, the S1972I (SI), or the D2275A (DA) TOR1 mutant enzymes were assayed for protein kinase activity with PHAS-I as substrate and MnCl2 as cofactor. Phosphorylation reactions were performed in the absence (−) or presence of FKBP12-rapamycin (F/R), or 500 nM wortmannin (W) as indicated in panel A. (C) TOR1 Western blot of the protein extracts employed for the immunoprecipitates performed in panel B.
Yeast Cell Extracts for Western Blot Analysis
Yeast cells CAY6 transformed with the 2μ, GAL1 plasmid pYDF72 (encoding the full-length TOR1 gene or different mutant forms under control of the GAL1 promoter) were grown in minimal medium, with 2% raffinose as the carbon source. When cultures reached an OD600 ∼1, 2% galactose was added for 2 h to induce TOR1 expression from the GAL promoter. Cells were harvested by centrifugation, and protein extracts and Western blot analysis were performed as previously described (Cardenas et al., 1994a).
RESULTS
TOR1 Has an Intrinsic Protein Kinase Activity In Vitro
The yeast targets of rapamycin, the TOR1 and TOR2 proteins, have a carboxy-terminal domain with similarity to both lipid and protein kinases. Despite this sequence similarity, no intrinsic kinase activity has yet been detected for either yeast TOR protein. We have therefore taken biochemical and genetic approaches to determine the enzymatic activity and to define residues and domains important for TOR in vivo functions.
The mammalian TOR homologue protein (mTOR) has an intrinsic protein kinase activity capable of phosphorylating the eIF-4E–binding protein PHAS-I (Brunn et al., 1997; Burnett et al., 1998). We therefore tested whether yeast TOR1 can phosphorylate PHAS-I. For these experiments, the TOR1 protein was overexpressed from the GAL promoter as an N-terminally HA epitope-tagged protein. Immunoprecipitated HA-TOR1 readily phosphorylated PHAS-I in vitro (Figure 1A). As previously observed with the mammalian TOR enzyme, phosphorylation of PHAS-I by yeast TOR1 was stimulated by including MnCl2 instead of MgCl2 in the reaction mix (Figure 1A). Phosphorylation of PHAS-I by the yeast TOR1 enzyme was fully inhibited by wortmannin and partially inhibited by FKBP12–rapamycin (∼50%), but not by FKBP12–FK506 (Figure 1A).
TOR mutants altered in a conserved serine residue (S1972 in TOR1, S1975 in TOR2, and S2035 in mTOR) fail to interact with the FKBP12–rapamycin complex and thus confer resistance to rapamycin (Helliwell et al., 1994; Stan et al., 1994; Brown et al., 1995; Lorenz and Heitman, 1995; Zheng et al., 1995). Furthermore, conserved active site residues of protein kinases required for ATP binding and phosphate transfer have been previously identified (Taylor et al., 1993). These active site residues are also conserved in lipid kinases, including the yeast PI-3 kinase VPS34 and the mammalian PI-3 kinase, and are required for in vitro activity and in vivo functions of these kinases (Schu et al., 1993; Dhand et al., 1994). We therefore introduced both a rapamycin-resistant mutation (S1972I) and a kinase-inactivating substitution at a conserved residue in the presumptive TOR1-active site, aspartic acid 2275 to alanine (D2275A).
To determine whether the kinase activity detected with the immunoprecipitated TOR1 is intrinsic to the TOR1 protein, HA epitope-tagged forms of wild-type TOR1, the rapamycin-resistant TOR1 (S1972I), and the putative kinase-inactive TOR1 (D2275A) mutant proteins were overexpressed, immunoprecipitated, and tested for their ability to phosphorylate PHAS-I. Both the wild-type TOR1 and the rapamycin-resistant (S1972I) mutant enzyme robustly phosphorylated PHAS-I (Figure 1B). Wild-type TOR1 was sensitive to inhibition by the FKBP12-rapamycin complex, whereas the S1972I TOR1 mutant enzyme was completely resistant to FKBP12-rapamycin (Figure 1B). Both wild-type TOR1 and the S1972I mutant enzyme were sensitive to inhibition by wortmannin (Figure 1B). As expected, the D2775A-active site mutation abolished phosphorylation of PHAS-I by TOR1 (Figure 1B). Taken together, these results establish that the yeast TOR1 protein possesses an intrinsic protein kinase activity.
TOR1 Kinase Domain Is Essential for TOR1 Function
We next assessed the contribution of the kinase domain for TOR1 cellular functions in vivo by two assays. First, we tested the ability of the wild-type, and the S1972I, D2275A, and S1972I/D2275A mutant TOR1 genes to complement a previously described conditional synthetic lethal interaction between mutations in the TOR1 and SRK1/SSD1 genes (Alarcon et al., 1996). As shown in Figure 2A, both wild-type TOR1 and the rapamycin-resistant S1972I TOR1 mutant complemented the tor1 mutation and restored growth of a tor1 srk1 mutant strain at 39°C. In contrast, neither the kinase-inactive D2275A TOR1 mutant nor the S1972I/D2275A TOR1 double mutant complemented the tor1 mutation, indicating that integrity of the TOR1 kinase domain is essential for TOR1 in vivo function.
Figure 2.
TOR1 kinase domain is essential for TOR1 function and rapamycin resistance, and overexpression of TOR1 kinase-inactive mutant inhibits growth. (A) A tor1 Δsrk1 yeast strain (CAY7) was transformed with 2μ plasmids expressing wild-type TOR1, the S1972I rapamycin-resistant TOR1 mutant, the D2275A kinase-inactive TOR1 mutant, or the S1972I/D2275A double-mutant TOR1 protein. Plasmids expressing TOR1 function complement the tor1 srk1 conditional lethal phenotype and rescue cell viability at 39°C, whereas plasmids lacking TOR1 function do not. Cells were grown for 72 h at 30 and 39°C. (B) Wild-type strain JK9–3da was transformed with 2μ plasmids expressing wild-type TOR1, the kinase-inactive D2275A mutant TOR1, four independent rapamycin-resistant TOR1 mutant isolates (S1972I), and two independent isolates of the S1972I/D2275A TOR1 double mutant. Cells were grown on medium containing 1 μg/ml rapamycin for 72 h at 30°C. (C) Yeast strain CAY6 expressing wild-type TOR1 (TOR1), the TOR1 kinase-inactive mutant (TOR1 D2275A), the TOR1 rapamycin-resistant mutant (TOR1 S1972I), or the TOR1 kinase-inactive rapamycin-resistant double mutant (TOR1 S1972I/D2275A) from the galactose-inducible GAL1 promoter were grown on glucose or galactose medium lacking tryptophan for 96 h at 30°C.
As a second measure of TOR1 function, we tested the ability of the wild-type and mutant TOR1 genes to confer rapamycin resistance. Previous studies established that the TOR1 S1972I mutation confers dominant rapamycin resistance by preventing FKBP12–rapamycin binding to and inhibition of TOR1 function (Lorenz and Heitman, 1995; Zheng et al., 1995). As expected, the S1972I TOR1 mutant protein conferred rapamycin resistance (Figure 2B). In contrast, introduction of the kinase-inactive mutation D2275A into the S1972I TOR1 mutant gene, to result in the S1972I/D2275A double-mutant TOR1 protein, abolished the ability of the S1972I mutation to confer rapamycin resistance (Figure 2B), in accord with an earlier report (Zheng et al., 1995). Thus, activity of the TOR kinase domain is required both for TOR1 in vivo function and for the S1972I TOR1 mutant protein to confer rapamycin resistance.
Overexpressed TOR1 Kinase-inactive Mutant Inhibits Cell Growth
Catalytically inactive kinases often exhibit dominant negative effects on cell growth or function by titrating factors or substrates that normally associate with the wild-type kinase. We engineered genes encoding wild-type TOR1, a kinase-inactive TOR1 mutant (D2275A), a rapamycin-resistant TOR1 mutant (S1972I or S1972R), and a double-mutant TOR1 (D2275A/S1972I) under the control of the inducible GAL1 promoter. The effects of these TOR1 proteins were assessed in cells grown on galactose medium (SG-trp) to induce expression. As shown in Figure 2C, overexpression of wild-type TOR1, or of the rapamycin-resistant TOR1 mutant (S1972I or S1972R), had no effect on growth. In contrast, overexpression of the kinase-inactive TOR1 mutant (D2275A), or of the TOR1 double-mutant TOR1 (D2275A/S1972I), inhibited growth. This growth- inhibitory effect was observed on solid medium but not in liquid culture. These results are in accord with a previous study in which overexpression of other kinase-inactive TOR1 mutants (R2276P, D2294E) inhibited yeast cell growth on solid, but not in liquid, medium (Zheng et al., 1995). However, our findings disagree with the observation in the same report that overexpression of the rapamycin-resistant TOR1 mutant (S1972I) results in a dominant growth-inhibitory effect, and that introduction of the kinase-inactive mutations (R2276P or D2294E) into the TOR1 S1972I mutant suppressed this effect (Zheng et al., 1995).
Our results clearly demonstrate that overexpression of the kinase-inactive mutant (D2275) dominantly inhibits yeast cell growth. The kinase-inactive mutant was toxic in the context of both wild-type TOR1 and the S1972I rapamycin-resistant TOR1 mutant protein. The growth-inhibitory phenotype conferred by overexpression of the kinase-inactive D2275A TOR1 mutant protein was suppressed by overproduction of wild-type TOR1 (our unpublished results), indicating that the toxicity of the kinase-inactive protein is a specific effect attributable to interference with TOR function.
The FKBP12-rapamycin complex binds to and inhibits the yeast TOR1 and TOR2 proteins. We tested whether growth inhibition by the overexpressed TOR1 kinase-inactive mutant could be overcome by inhibiting TOR1 with FKBP12–rapamycin. To this end, a plasmid expressing the TOR2–1 mutant protein (S1975I) was introduced to render cells resistant to rapamycin, and cells were exposed to rapamycin. Treatment with 0.1 or 1 μg/ml rapamycin did not block the toxic effect of the kinase-inactive TOR1, indicating that the TOR1 FRB domain is not required for this effect (our unpublished results). This conclusion is also supported by deletion analysis of the TOR1 FRB domain, as described below.
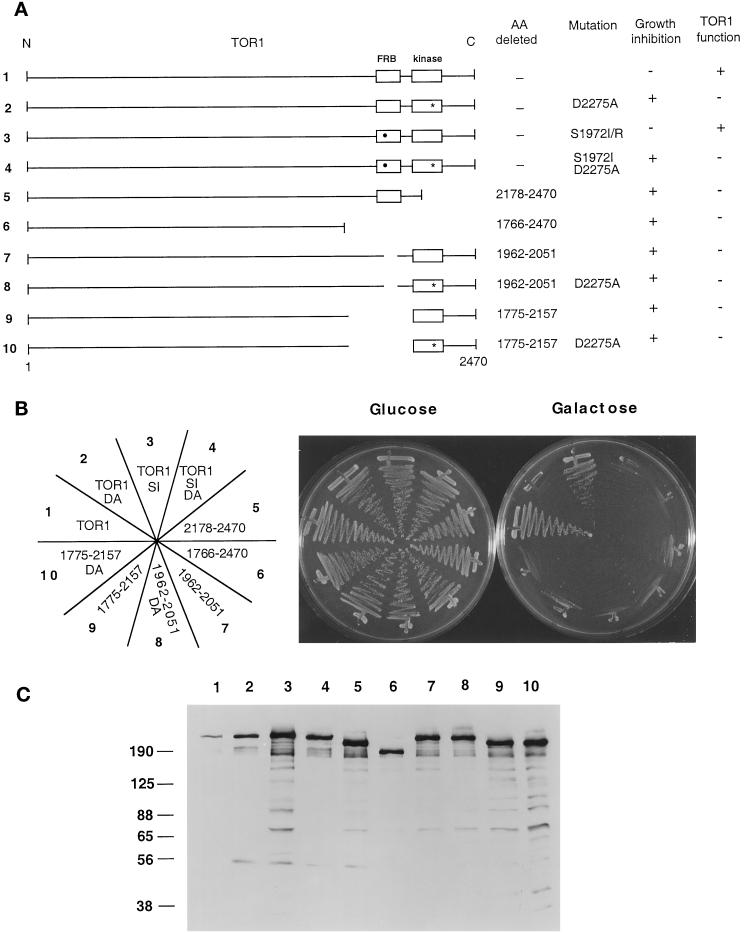
Mapping the Domain Required for TOR1 Toxicity
To identify regions of TOR1 responsible for the toxic effect of the kinase-inactive mutant, deletions or truncation variants of the TOR1 protein were fused to the HA epitope tag and expressed from the inducible GAL1 promoter (Figure 3A). A 45-kDa region of TOR1 (amino acids 1775–2157) is sufficient to bind the FKBP12-rapamycin complex (Zheng et al., 1995). To determine whether this FKBP12-rapamycin–binding domain was required for toxicity of the kinase-inactive TOR1 mutant, we deleted the region from amino acid 1775 to 2157 in wild-type TOR1 and in the TOR1 kinase-inactive mutant. Western blot analysis with an anti-HA antibody confirmed that proteins of the expected molecular weights were expressed in all cases (Figure 3C). The ability of these TOR1 deletion mutants to inhibit growth on galactose medium was then tested.
Figure 3.
TOR1 structural features required for in vivo function and overexpression toxicity. (A) Diagram of full-length TOR1 with the FRB and kinase domains depicted by open boxes. The S1972I rapamycin- resistant mutation is indicated by a black circle, and the kinase-inactive mutation D2275A by a star. The specific amino acids (AA) mutated or deleted in each mutant, and the ability of these TOR1 derivatives to inhibit growth when overexpressed, or to provide TOR1 function and complement the synthetic lethal growth defect at 39°C in a tor1 Δsrk1 mutant strain (CAY7) are indicated. N and C indicate the amino- and carboxy termini of TOR1, and 1 and 2470 indicate the positions of the first and last amino acids, respectively, of the TOR1 protein. (B) TOR1 mutants and deletion derivates were expressed in the Δtor1 strainCAY6, and the ability to inhibit growth on galactose media was tested. Cells were grown on glucose or galactose medium lacking tryptophan for 96 h at 30°C. DA and SI indicate the D2275A kinase-inactive and the S1972I rapamycin resistance mutations, respectively. The nubers on the plate designation correspond to the construct number indicated to the left in panel A. (C) TOR1 deletion mutant proteins were expressed in the Δtor1 strain CAY6 and detected by Western blot with an α-HA monoclonal antibody. Numbers above the Western blot correspond to the construct numbers listed to the left in panel A. Numbers at left indicate molecular mass in kilodaltons of marker proteins.
As shown in Figure 3B, overexpression of the TOR1 FRB-deletion mutant conferred a growth-inhibitory effect, indicating that the FRB domain is not required for toxicity of the TOR1 kinase-inactive mutant. To our surprise, the TOR1 FRB deletion mutant with a wild-type kinase domain was also toxic (Figure 3B), indicating not only that the FRB is not required for the toxic effect, but that deleting the FRB domain from wild-type TOR1 is in itself toxic. This finding suggests that the kinase domain itself may not be directly responsible for toxicity of the kinase-inactive TOR1. Instead, one possible model is that effector molecule(s) bind to other regions of the TOR1 protein, the kinase domain transiently interacts with these molecules in an enzyme–substrate interaction, and this fails to occur with the kinase-inactive mutant, leading to a dominant loss of TOR function. In support of this interpretation, truncation mutants lacking the kinase domain (from amino acids 2178 to 2470), or lacking both the FRB and the kinase domains (from amino acids 1766 to 2470), inhibited growth when overexpressed on galactose medium (Figure 3B). These findings indicate that a region of the protein responsible for the toxic effect lies in the amino-terminal or central region of the protein (amino acids 1–1766) distinct from the C-terminal FRB and kinase domains.
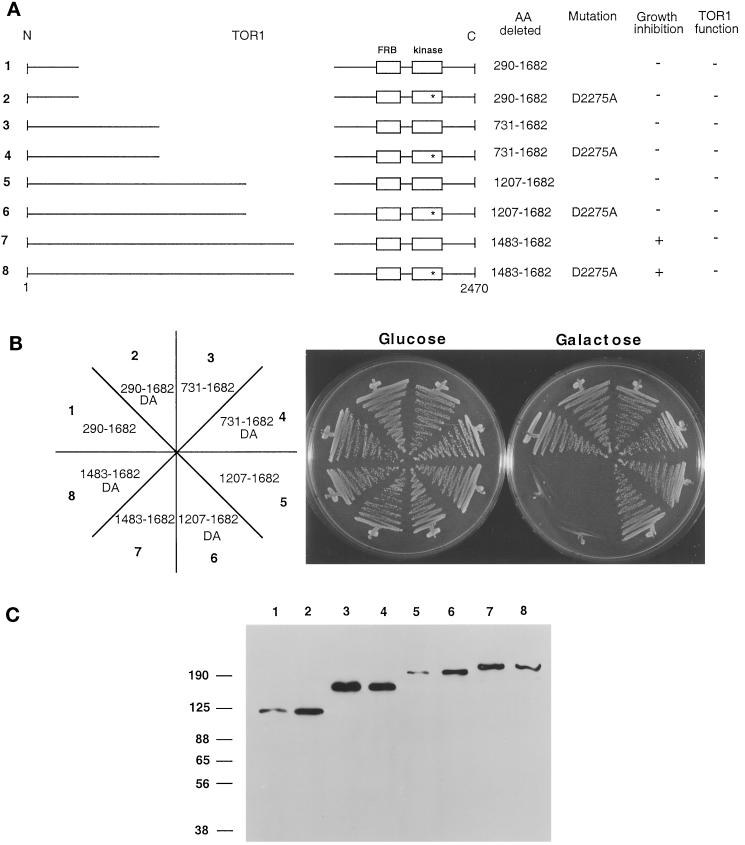
To identify the domain of the TOR1 protein required for growth inhibition when overexpressed, a series of TOR1 and TOR1 kinase-inactive deletion mutants, which are represented schematically in Figure 4A, were tested for growth-inhibitory effects on galactose medium (Figure 4B). These mutants were constructed by sequentially adding sequences back to a large internal deletion mutant that had no effect on growth when overexpressed (amino acids 290-1682). By this approach we identified a region, from amino acids 1208 to 1482, that, when reintroduced into the TOR1 deletion mutant Δ1207–1682, restored the growth-inhibitory effect. Importantly, toxicity of this TOR1 mutant protein was observed with either a wild-type or an inactive kinase domain (Figure 4B). Western blot analysis with an HA monoclonal antibody again confirmed that TOR1 derivatives of the expected molecular weights were stably expressed in all cases (Figure 4C).
Figure 4.
A central region of TOR1 is required for toxicity of TOR1 deletion derivatives. (A) Diagram of TOR1 internal deletions with the FRB and kinase domains depicted by boxes. The D2275A kinase-inactive mutation is represented by a star. The specific amino acids (AA) deleted in each mutant, the ability to inhibit growth when overexpressed, and the ability to complement and restore growth at 39°C in a synthetic lethal tor1 Δsrk1 mutant strain (CAY7) are indicated. (B) TOR1 deletion mutants were expressed in the Δtor1 strain CAY6, and ability to inhibit growth on galactose medium was assessed. Cells were grown on glucose or galactose medium lacking tryptophan for 96 h at 30°C. DA indicates the D2275A kinase-active site mutation. The numbers on the plate designation correspond to the construct number indicated to the left in panel A. (C) TOR1 deletion mutant proteins were expressed in the Δtor1 strain CAY6 and detected by Western blot with α-HA antibody. Numbers above the Western blot correspond to the construct numbers listed to the left in panel A. Numbers at the left indicate molecular mass in kilodaltons.
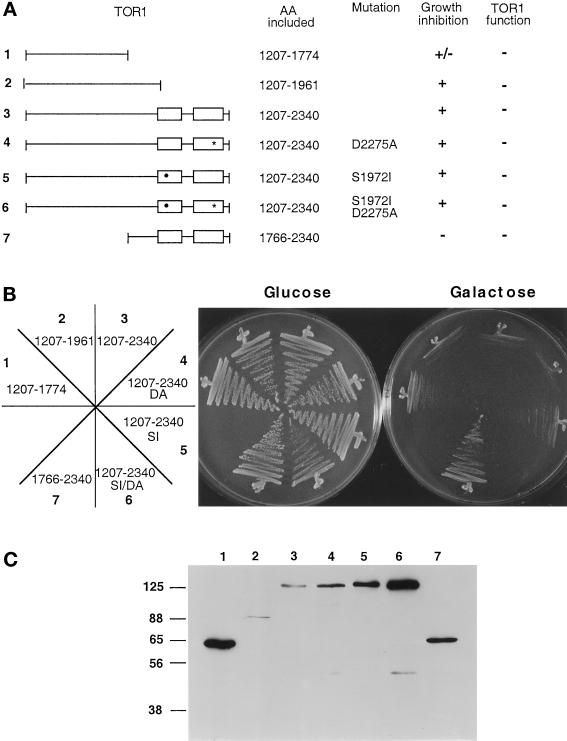
Identification of a Central Toxic Effector Domain of the TOR Proteins
Isolated HA-tagged fragments of TOR1 were expressed under the control of the GAL1 promotor to determine which region of TOR1 was sufficient to promote a dominant negative effect on growth when overexpressed (Figure 5A). Overexpression of a central domain of ∼570 amino acids of TOR1 containing residues 1207–1774, but not overexpression of the C-terminal region encompassing the FRB and kinase domains (residues 1766–2340), conferred a growth-inhibitory effect when cells were grown on galactose medium (Figure 5B). Extension of this domain by an additional ∼215 C-terminal residues resulted in an ∼785-amino acid central domain that was even more toxic to cells when overexpressed. A schematic of the regions tested and a Western blot confirming that proteins of the expected molecular weights are expressed are shown in Figure 5, A and C, respectively. Overexpression of the equivalent region of TOR2 (residues 1216–1782) also exhibited a growth-inhibitory effect (Figure 6). Overproduction of wild-type TOR1 suppressed the growth-inhibitory effect of overexpressing the TOR1 or TOR2 central domains (Figure 6). These findings indicate that overexpression of the toxic central fragment interferes with the TOR function common to both TOR1 and TOR2 (translation). In contrast, overexpression of TOR1 did not suppress the growth-inhibitory effect of the larger TOR2 toxic domain (Figure 6, AA 1216–1964), suggesting that this toxic TOR2 domain may also inhibit the unique function of TOR2 in cytoskeletal polarization that TOR1 cannot provide.
Figure 5.
Identification of a TOR1-toxic effector domain distinct from the FRB and kinase domains. (A) Diagram of TOR1 fragments with the FRB and kinase domains depicted by open boxes. The rapamycin-resistant mutation S1972I is indicated by a black circle, and the kinase-inactive mutation D2275A is indicated by a star. The specific amino acids expressed, the ability to inhibit growth when overexpressed, and the ability to complement growth at 39°C in a tor1 Δsrk1 mutant strain (CAY7) are indicated. (B) TOR1 fragments were expressed in the Δtor1 strain CAY6, and the ability to inhibit growth on galactose medium was tested. Cells were grown on glucose or galactose medium lacking tryptophan for 96 h at 30°C. DA and SI indicate the D2275A and the S1972I rapamycin resistance mutations, respectively. The numbers on the plate designation correspond to the construct number indicated to the left in panel A. (C) TOR1 fragments were expressed in the Δtor1 strain CAY6, and proteins were detected by Western blot with α-HA antibody. Numbers above the Western blot correspond to the construct numbers listed to the left in panel A. Numbers at left indicate molecular mass in kilodaltons.
Figure 6.
Overexpression of TOR1 and TOR2 effector domains inhibits growth and is suppressed by increased expression of wild-type TOR1. Yeast Δtor1 strain CAY6 expressing wild-type TOR1 or the TOR1 or TOR2 central toxic domain from the galactose-inducible GAL1 promoter was cotransformed with a 2μ URA vector alone (−) or vector expressing wild-type TOR1 (+). Cells were grown on glucose or galactose medium lacking tryptophan and uracil for 96 h at 30°C. (A) Wild-type TOR1 and vector; (B) TOR1 fragment 1207–1961 and vector; (C) TOR1 frag-ment 1207–1961 and 2μ TOR1; (D) TOR2 fragment 1216–1782 and vector; (E) TOR2 fragment 1216–1782 and 2μ TOR1; (F) TOR2 fragment 1216–1964 and vector; (G) TOR2 fragment 1216–1964 and 2μ TOR1.
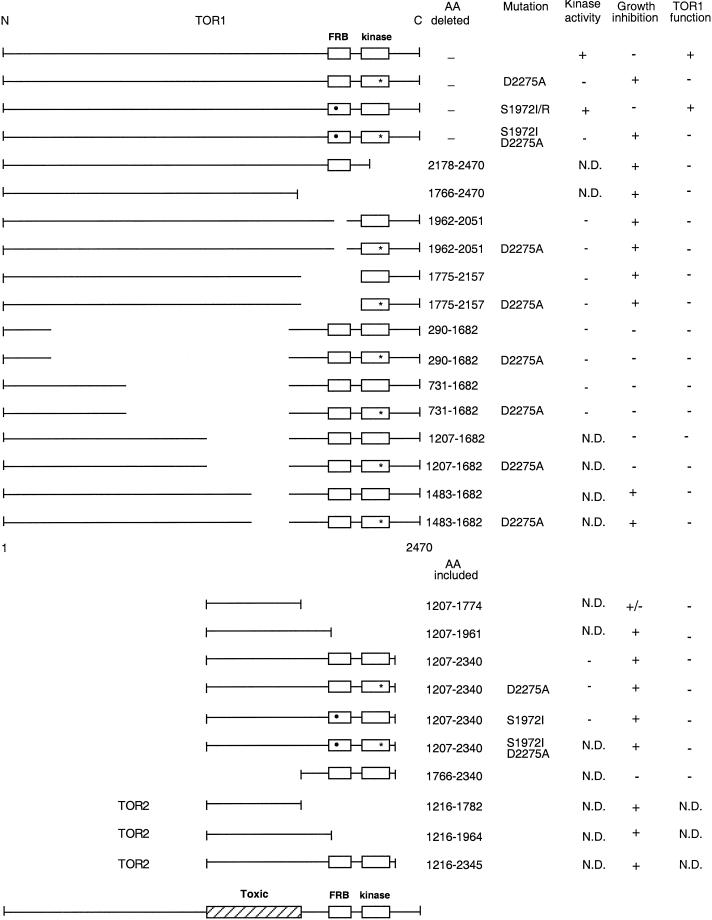
All TOR1 deletion mutants and TOR1-isolated fragments constructed for this study were tested for their ability to provide TOR1 function and complement the conditional synthetic lethal phenotype of a tor1 Δsrk1 deletion strain at 39°C. As summarized in Figure 7, only full-length TOR1 and the rapamycin-resistant TOR1 mutants (S1972I or S1972R) complemented the tor1 mutation and restored growth at 39°C. Thus, removal of any portion of TOR1, or inactivation of the kinase domain by mutation, results in a loss of TOR1 in vivo function. In agreement with these results, no protein kinase activity was detected with these TOR1 mutant polypeptides and PHAS-I as a substrate in vitro (Figure 7).
Figure 7.
Summary of the TOR deletion derivatives used to define a central toxic effector domain common to TOR1 and TOR2. Diagram of full-length TOR1, TOR1 deletion mutants, and TOR1 and TOR2 fragments. The toxic effector, FRB, and kinase domains are depicted by boxes. The rapamycin-resistant mutation S1972I is indicated by a black circle, and the kinase-inactive D2275A mutation is indicated by a star. The specific amino acids deleted in each mutant, or the amino acids included in each fragment, protein kinase activity employing PHAS-I as substrate, ability to inhibit growth, and ability to complement the conditional synthetic lethal phenotype at 39°C in a tor1 Δsrk1 deletion strain (CAY7) are indicated. N.D. indicates not determined.
In summary, this deletion analysis supports a model in which a region of the TOR proteins distinct from the FRB or kinase domains is involved in binding effector molecules. As a consequence, overexpression of this region has a growth-inhibitory effect by sequestering these effector molecules and preventing their productive interactions with TOR1 and TOR2. We term this central region of the TOR proteins the toxic effector domain (Figure 7).
The TOR1 Toxic Effector Domain Arrests Cells in G1
Inhibition of the yeast TOR proteins with FKBP12–rapamycin, or depletion of both TOR1 and TOR2 proteins, results in a G1 or G0 cell cycle arrest as large unbudded cells with dramatically enlarged vacuoles (Heitman et al., 1991; Kunz et al., 1993; Cardenas and Heitman, 1995). This specific cell cycle-arrest point is attributable to the shared function of the TOR1 and TOR2 proteins in regulating translation (Barbet et al., 1996). To ascertain whether the TOR-toxic domains might inhibit cell growth by interfering with the role of the TOR proteins in translation, we examined whether the toxic TOR domain imposes a similar cell cycle arrest. Inhibition of growth by the TOR-toxic domain was more pronounced in cells grown on solid medium compared with liquid cultures, and we thus examined the fate of cells grown on solid medium.
Overexpression of the three different TOR1-toxic domain constructs produced a high percentage, ∼60- 80%, of unbudded cells characteristic of a G1 arrest (Table 1). Moreover, overexpression of the larger TOR1-toxic domain, from amino acids 1207 to 1961, resulted in a high proportion (62%) of large unbudded cells containing enlarged vacuoles, an arrest point that is essentially indistinguishable from the arrest point of rapamycin-treated cells or cells depleted for TOR1 and TOR2 (Table 1). In comparison, overexpression of the smaller TOR1-toxic domains containing amino acids 1207–1774 or from amino acids 1483 to 1682 yielded 12 and 13%, respectively, large unbudded cells and 80% small unbudded cells (Table 1). Taken together, these observations suggest that the TOR-toxic domain inhibits the TOR function in translation.
Table 1.
Cell cycle arrest by TOR toxic domain
| Cells | Wild type full length | AA included 1207–1774 | AA included 1207–1961 | AA deleted 1483–1682 |
|---|---|---|---|---|
| Small unbudded | 60 | 80 | 29 | 80 |
| Large unbudded | 0 | 12 | 62 | 13 |
| Budded | 40 | 8 | 9 | 7 |
Overexpression of Phospholipase C Suppresses Toxicity of the TOR1-Toxic Domain
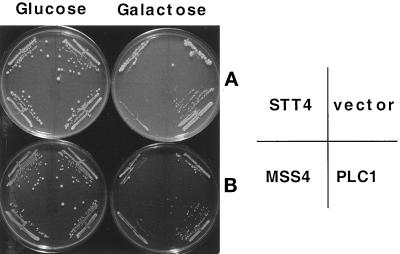
The yeast TOR1 and TOR2 proteins and their mammalian counterpart mTOR all contain a carboxy-terminal domain that shares identity with both protein and lipid kinases. We therefore examined whether the TOR-toxic domains might impinge on the yeast phosphatidylinositol metabolic pathway. The yeast tor1 mutant strain CAY6 was cotransformed with plasmids expressing the TOR1-toxic domains from amino acids 1207 to 1961, or from amino acids 1207 to 2340, together with plasmids overexpressing enzymes that function in the phosphatidylinositol metabolic pathway, including the PI-4 kinase STT4 (Yoshida et al., 1994a; Cutler et al., 1997), the PI-4 phosphate 5 kinase homologue MSS4 (Yoshida et al., 1994b), and the phospholipase C homologue PLC1 (Flick and Thorner, 1993; Flick, 1998). Interestingly, overexpression of the yeast phospholipase C homologue PLC1 suppressed the toxic effects of the TOR1-toxic domains (Figure 8). In contrast, overexpression of STT4 or MSS4 did not suppress toxicity of the TOR1-toxic domain (Figure 8); overexpression of either STT4 or MSS4 alone was not toxic under these conditions (our unpublished results). Although overexpression of yeast PLC1 suppressed the TOR1-toxic domain effects, PLC1 did not restore viability to cells overexpressing either the full-length TOR1 kinase dead mutant (TOR1 D2275A) or the TOR2-toxic domains (our unpublished results). With the caveat of indirect effects due to overexpression, these results indicate that these toxic TOR variants may inhibit cell growth by more than one pathway, only one of which is PLC1 dependent.
Figure 8.
Overexpression of phospholipase C suppresses the toxicity of the TOR1 toxic domain. Yeast strain CAY6 was cotransformed with plasmids expressing the TOR1-toxic domains (AA 1207–1961 in panel A and AA 1207–2340 in panel B), and plasmids overexpressing the STT4 (PGALHA-STT4), MSS4 (pGMSS4), and PLC1 (pJF137), proteins, respectively, under the control of the galactose promoter, were grown in minimal medium containing glucose or galactose for 4 d at 30°C.
DISCUSSION
The yeast TOR1 and TOR2 proteins were first identified as the targets of the immunosuppressive natural product rapamycin bound to the cellular protein FKBP12. Subsequently, a mammalian FKBP12-rapamycin–binding protein was identified that shares ∼50% sequence identity with the yeast TOR proteins. Two C-terminal domains that are highly conserved between yeast and mammalian TOR have been identified. The first, the FKBP12-rapamycin–binding or FRB domain, was identified as the target for rapamycin binding to both yeast and mammalian TOR, based on both biochemical and genetic approaches (Chen et al., 1995; Lorenz and Heitman, 1995), and the x-ray crystal structure of the FKBP12–rapamycin–FRB domain ternary complex has been solved (Choi et al., 1996). The second domain is the C-terminal kinase domain that also shares identity with other members of the PI kinase-related kinase superfamily, including PI-3 and PI-4 kinases from yeast and man, DNA-dependent protein kinase, and the yeast and human checkpoint control proteins MEC1, TEL1, ATM, and ATR.
We have taken biochemical and genetic approaches to determine the catalytic activity and to define functional domains in the yeast TOR proteins. Our studies show that TOR1 possess a robust protein kinase activity capable of phosphorylating PHAS-I, the only known substrate of the mammalian TOR kinase homologue. Although we do not detect autophosphorylation activity, the TOR1 kinase activity shares several other hallmarks exhibited by the mammalian TOR kinase in that the TOR1 kinase activity is enhanced by the presence of MnCl2 ions and is inhibited by FKBP12–rapamycin and wortmannin (Brunn et al., 1996, 1997). TOR1 kinase activity was also abolished by the D2275A-active site mutation and rendered resistant to rapamycin by a mutation in the FRB domain (S1972I) that interferes with FKBP12–rapamycin binding. In contrast to the yeast TOR2 and mammalian TOR homologues, FRAP and RAFT1, we found no PI-4 kinase activity associated with the yeast TOR1 enzyme. Taken together, our findings reveal that yeast TOR1, like the mammalian mTOR homologue, has protein kinase activity.
We find that the integrity of the TOR1 kinase domain is required for TOR1 functions in yeast, and for a dominant rapamycin-resistant TOR1 mutant to confer rapamycin resistance. In addition, we find that overexpression of a kinase-inactive TOR1 mutant is toxic to yeast cells. These findings are in accord with observations previously reported by Zheng et al. (1995). The toxicity of the overexpressed kinase-inactive TOR1 mutant could be overcome by overexpressing wild-type TOR1, illustrating that this is a specific toxic effect resulting from interference with TOR function in vivo. Toxicity of the TOR1 kinase-inactive mutant was not inhibited by FKBP12–rapamycin, indicating that the FRB domain is not responsible for this toxic effect. Finally, in contrast to the previous report of Zheng et al. (1995), we did not observe any toxic effect upon overexpression of two different TOR1 rapamycin-resistant mutants bearing single amino substitutions in the FRB domain. Our sequence analysis of the region rescued by gap repair in the S1972I or S1972R mutants revealed no extraneous mutations. Thus, either subtle differences in experimental conditions or additional mutations in the studies of Zheng et al. must explain this discrepancy.
We proceeded to map regions of the TOR1 protein that are required for the toxic effect of the TOR1 kinase-inactive mutant. To our surprise, simply deleting the entire kinase domain, the FRB domain, or both the kinase domain and the FRB domain, resulted in C-terminally or internally truncated forms of the TOR1 protein that were also toxic when overexpressed. Thus, inactivation of the kinase activity of TOR1 by either a point mutation or a large deletion resulted in a toxic TOR1 protein, suggesting that regions of the protein other than the kinase domain itself are responsible for the toxicity of the TOR1 kinase-inactive mutant.
To map regions of the TOR1 protein responsible for this toxic effect, we took two approaches. First, a large central region of TOR1 was deleted, after which progressively larger and larger portions of the missing section were replaced. This analysis revealed that readdition of residues 290-1207 was not sufficient to render that attached kinase-inactive domain toxic, but that readdition of residues 290-1682 was sufficient to restore toxicity, implicating residues 1207–1682 in the toxic effect. In the second approach, short defined central regions of the TOR1 protein were overexpressed and tested for toxic effects. By this approach, a 567-amino acid segment of TOR1 from residue 1207 to 1774 was found to be partially toxic, and addition of another 200 C-terminal residues rendered this domain fully toxic (754 amino acids, 1207–1961). These studies therefore defined a central domain of TOR1 that, when overexpressed on its own, was toxic to the cell. Importantly, this domain is amino-terminal to, and completely distinct from, the FRB and kinase domains that have been previously defined. The same domain derived from TOR2 was also toxic when overexpressed in yeast. We term this novel TOR domain, common to the yeast TOR1 and TOR2 proteins, the toxic central effector domain.
What might be the function of this TOR effector domain? One plausible explanation for the dominant negative toxic effect observed upon overexpression of the TOR effector domain is that this domain is involved in mediating dimerization between TOR1 and itself, or between TOR1 and TOR2. However, we have been unable to find any evidence of coprecipitation between the overexpressed toxic TOR1 domain (detected with the fused HA epitope) and endogenous yeast TOR1 or TOR2 (detected with polyclonal sera against TOR1 or TOR2) (our unpublished results). We therefore propose that the TOR effector domain interacts with substrates or regulators of the TOR-dependent, rapamycin-sensitive signaling cascade. For example, the effector domain might dock substrates onto TOR for subsequent phosphorylation by the adjacent kinase domain. In this model, the TOR proteins could serve a function as a scaffold upon which to assemble other interacting proteins for appropriate interaction with the TOR kinase domain. An alternative possibility is that this domain mediates localization to membranes and competes with the endogenous TOR1 for localization.
What role does the toxic effector domain play in known TOR functions in yeast? The TOR proteins have two defined functions in yeast. One TOR function is essential and unique to TOR2 and involves polarization of the yeast actin cytoskeleton though an effect of TOR2 on the Rho-like GTPases, RHO1 and RHO2, via the exchange factor ROM2 (Schmidt et al., 1996, 1997). The second TOR function is also essential but shared by both TOR1 and TOR2 and involves regulation of translational initiation (Barbet et al., 1996). Thus far, no direct target of the TOR1 or TOR2 protein involved in either function in yeast has been identified, and the only protein implicated as a direct target of the mammalian TOR protein is PHAS-I, but the domains with which PHAS-I interacts have not yet been identified. Because we find that the toxic effector domain is common to both TOR1 and TOR2, and toxicity of this domain is overcome by TOR1 overexpression (which does not provide the TOR2 unique function), these findings implicate the toxic effector domain in the translational regulatory function common to both TOR1 and TOR2. Consistent with this interpretation, overexpression of the most potent TOR1-toxic domain yields a G1 cell cycle arrest that is indistinguishable from rapamycin inhibition of TOR function or genetic depletion of yeast TOR1 and TOR2 (Table 1). Shorter, less potent forms of the TOR-toxic domain yield a G1 arrest, but the cells are smaller than those observed with rapamycin, TOR depletion, or the larger TOR-toxic domain (Table 1). One plausible hypothesis is that these forms of the toxic domain yield a more transient cell cycle arrest from which cells escape, reenter the cell cycle, and hence do not form the large G1 arrested cells observed with rapamycin exposure. Taken together, our observations support the hypothesis that overexpression of the TOR-toxic domain interferes with the shared TOR function in the regulation of translation.
The direct physical targets of the TOR-toxic domain remain to be identified. We have thus far been unable to identify overexpression suppressors of the TOR1-toxic domain from screens of either 2μ genomic libraries or a GAL-regulated cDNA library. In addition, we have not isolated any proteins interacting with the yeast TOR1-toxic domain using the two-hybrid system. Thus, the target of the toxic domain may have multiple subunits or may not be a protein and could be an RNA or small molecule. The yeast phospholipase C gene PLC1 was identified as a multicopy suppressor of growth inhibition by the TOR-toxic domain. Interestingly, the yeast PLC1 gene was also recently identified as a multicopy suppressor of conditional lethal TOR2 mutations in yeast (Helliwell et al., 1998). The effects of PLC1 may be indirect, as we have failed to observe a direct physical interaction between the TOR-toxic domain and PLC1 in both the two-hybrid system or by coimmunoprecipation (our unpublished results). The yeast PLC1 enzyme, like its mammalian counterpart, is involved in phosphatidylinositol metabolic cascades and cleaves PI-4P and PI-4,5P2 to yield the soluble second messengers inositol diphosphate and inositol triphosphate. Although the functions and targets of these cascades remain to be fully elucidated, yeast plc1 mutants are viable but exhibit a number of phenotypes, including poor growth, temperature-sensitive growth in some strain backgrounds, sensitivity to osmotic stress, and an inability to utilize many carbon sources (Flick and Thorner, 1993). Given the homology of the TOR kinase domains with lipid kinases, previous studies that the TOR proteins are associated with a lipid kinase in yeast and mammalian cells (Brown et al., 1995; Cardenas and Heitman, 1995; Sabatini et al., 1995), the fact that one downstream element of the TOR-regulated cascade contains an essential pleckstrin homology domain (the Rho1 GTP exchange factor Rom1, which could be a target of PI-4,5 P2) (Schmidt et al., 1997), and recent studies that reveal the growth factor-activated PI-3 lipid kinase may function upstream of mTOR in mammalian cells (Gingras et al., 1998), our findings provide evidence for another interesting link between TOR function and PI metabolic cascades in the regulation of cell function. Further studies will be required to determine whether the yeast TOR proteins and PLC1 function in the same or related signaling cascades.
Similar structure–function approaches have been recently applied to the mammalian ATM and ATR proteins, which share identity with the C-terminal TOR kinase domain. Overexpression of fragments of the ATM protein containing a leucine zipper motif have a dominant negative activity in cultured mammalian cells (Morgan et al., 1997). This region of ATM may either inhibit endogenous ATM function or bind to and inhibit other members of the ATM signaling pathway. Interestingly, overexpression of the ATM kinase domain alone was sufficient to complement many of the defects in atm mutant fibroblasts, including radiosensitivity and S phase checkpoint function, indicating that the kinase domain is responsible for much of the activity of ATM (Morgan et al., 1997). In related studies, Cliby et al. (1998) demonstrated that overexpression of a kinase-inactive mutant of the mammalian ATR protein caused sensitivity to ionizing radiation, methyl methanesulfonate, and cis-platinum and also abolished the G2/M checkpoint after DNA damage with ionizing radiation. Taken together, these studies implicate the kinase domains of ATM and ATR in mediating cellular responses to DNA damage and cell cycle progression.
The TOR-toxic domain is conserved between the yeast and mammalian TOR proteins and, in BLAST searches, shares more limited identity over an ∼240- amino acid region with the PIK family members ATR, RAD3, mei-41, and ATM (Figure 9). Yeast TOR1 and mammalian ATR share 26% overall identity, and 48% similarity, in this region. This finding suggests that the TOR-toxic domain, like the more C-terminal kinase domain, has been conserved between different PIK family members and might play a role in the signaling functions of these different proteins. In general, members of the PIK-related family have been implicated in signaling cascades that regulate responses of the cell to exogenous signals (TOR, mTOR, PI-3 kinase) or endogenous signals (ATR, ATM, Rad3, Mec1, Tel1). This conserved region may interact with effectors or regulators of the TOR- and ATR-signaling pathways. Further studies will be required to address the functions of this domain in other PIK family members.
Figure 9.
The TOR toxic domain is conserved and shares identity with regions of the PIK family members, ATR, Rad3, mei-41, and ATM. The TOR1 (amino acids 1376–1614), TOR2 (amino acids 1383–1620), and mTOR/RAFT/FRAP-toxic domains (amino acids 1427–1663) were used for BLAST searches of the NCBI database, revealing sequence similarity with ATR (amino acids 1688–1923) and Rad3 (amino acids 725–950), mei-41 (amino acids 1396–1623), and ATM (amino acids 1985–2148). The alignment shown here was generated using the ClustalW program of MacVector software. Residues that are conserved in four of the seven proteins are boxed, shaded, and in bold. Similar residues are boxed.
ACKNOWLEDGMENTS
We thank Shane Cutler for comments on the manuscript and assistance with protein sequence alignments; David Fiorentino, Gerry Crabtree, Jeff Flick, Yoshi Ohya, and Mike Hall for strains and plasmids; and Kevin Peters for suggestions. This work was supported by KO1 award CA-77075 from the National Cancer Institute (to M.E.C.) and RO1 award AI-41937 from NIAID (to J.H. and M.E.C.). Joseph Heitman is an associate investigator of the Howard Hughes Medical Institute.
REFERENCES
- Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disruption yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon CM, Cardenas ME, Heitman J. Mammalian RAFT1 kinase domain provides rapamycin-sensitive TOR function in yeast. Genes Dev. 1996;10:279–288. doi: 10.1101/gad.10.3.279. [DOI] [PubMed] [Google Scholar]
- Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell. 1996;7:25–42. doi: 10.1091/mbc.7.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow C, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–759. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schrei-ber SL. Control of p70 S6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Schreiber SL. A signaling pathway to translational control. Cell. 1996;86:517–520. doi: 10.1016/s0092-8674(00)80125-7. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi J, Houghton PJ, Lawrence JC, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signal functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferkey R, Young PR, McLaughlin MM, Bergsma DJ, Koltin Y, Sathe GM, Faucette L, Eng W-K, Johnson RK, Livi GP. Dominant missense mutations in a novel yeast protein related to mammalian phosphatidylinositol 3-kinase and VPS34 abrogate rapamycin cytotoxicity. Mol Cell Biol. 1993;13:6012–6023. doi: 10.1128/mcb.13.10.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas ME, Hemenway C, Muir RS, Ye R, Fiorentino D, Heitman J. Immunophilins interact with calcineurin in the absence of exogenous immunosuppressive ligands. EMBO J. 1994a;13:5944–5957. doi: 10.1002/j.1460-2075.1994.tb06940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas ME, Heitman J. FKBP12-rapamycin target TOR2 is a vacuolar protein with an associated phosphatidylinositol-4 kinase activity. EMBO J. 1995;14:5892–5907. doi: 10.1002/j.1460-2075.1995.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas ME, Lorenz M, Hemenway C, Heitman J. Yeast as model-T cells. Perspect Drug Discov Des. 1994b;2:103–126. [Google Scholar]
- Chen J, Zheng X-F, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci USA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler NS, Heitman J, Cardenas ME. STT4 is an essential phosphatidylinositol 4-kinase that is a target of wortmannin in Saccharomyces cerevisiae. J Biol Chem. 1997;272:27671–27677. doi: 10.1074/jbc.272.44.27671. [DOI] [PubMed] [Google Scholar]
- Dhand R, Hiles I, Panayotou G, Roche S, Fry MJ, Gout I, Totty NF, Truong O, Vicendo P, Yonezawa K. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994;13:522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Como CJ, Arndt KT. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 1996;10:1904–1916. doi: 10.1101/gad.10.15.1904. [DOI] [PubMed] [Google Scholar]
- Flick JS. An essential function of a phosphoinositide-specific phospholipase C is relieved by inhibition of a cyclin-dependent protein kinase in the yeast Saccharomyces cerevisiae. Genetics. 1998;148:33–47. doi: 10.1093/genetics/148.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick JS, Thorner J. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholipase C in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:5861–5876. doi: 10.1128/mcb.13.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A-C, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LM, Bornfeldt KE, Argast GM, Krebs EG, Kong X, Lin TA, Lawrence JC., Jr cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci USA. 1995;92:7222–7226. doi: 10.1073/pnas.92.16.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, Yonezawa K, Kozlowski MT, Sugimoto T, Andrabi K, Weng Q-P, Kasuga M, Nishimoto I, Avruch J. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem. 1997;272:26457–26463. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Proline isomerases at the crossroads of protein folding, signal transduction, and immunosuppression. New Biol. 1992;4:448–460. [PubMed] [Google Scholar]
- Helliwell SB, Howald I, Barbet N, Hall MN. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics. 1998;148:99–112. doi: 10.1093/genetics/148.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell SB, Wagner P, Kunz J, Deuter-Reinhard M, Henriquez R, Hall MN. TOR1 and TOR2 are structurally and functionally similar but not identical phosphatidylinositol kinase homologues in yeast. Mol Biol Cell. 1994;5:105–118. doi: 10.1091/mbc.5.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the PCR. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Koltin Y, Faucette L, Bergsma DJ, Levy MA, Cafferkey R, Koser PL, Johnson RK, Livi GP. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol Cell Biol. 1991;11:1718–1723. doi: 10.1128/mcb.11.3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lawrence JC, Jr, Abraham RT. PHAS/4E-BPs as regulators of mRNA translation and cell proliferation. Trends Biochem Sci. 1997;22:345–349. doi: 10.1016/s0968-0004(97)01101-8. [DOI] [PubMed] [Google Scholar]
- Lin TA, Kong X, Saltiel AR, Blackshear PJ, Lawrence JC. Control of PHAS-I by insulin in 3T3–L1 adipocytes. Synthesis, degradation, and phosphorylation by a rapamycin-sensitive and mitogen-activated protein kinase-independent pathway. J Biol Chem. 1995;270:18531–18538. doi: 10.1074/jbc.270.31.18531. [DOI] [PubMed] [Google Scholar]
- Lorenz MC, Heitman J. TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J Biol Chem. 1995;270:27531–27537. doi: 10.1074/jbc.270.46.27531. [DOI] [PubMed] [Google Scholar]
- Morgan SE, Lovly C, Pandita TK, Shiloh Y, Kastan MB. Fragments of ATM which have dominant-negative or complementing activity. Mol Cell Biol. 1997;17:2020–2029. doi: 10.1128/mcb.17.4.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver TL, Szostak JW, Rothstein RJ. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci USA. 1981;78:6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent manner and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Pierchala BA, Barrow RK, Schell MJ, Snyder SH. The rapamycin and FKBP12 target (RAFT) displays phosphatidylinositol 4-kinase activity. J Biol Chem. 1995;270:20875–20878. doi: 10.1074/jbc.270.36.20875. [DOI] [PubMed] [Google Scholar]
- Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- Schiestl RH, Manivasakam P, Woods RA, Gietz RD. Introducing DNA into yeast by transformation. Methods. 1993;5:79–85. [Google Scholar]
- Schmidt A, Bickle M, Beck T, Hall MN. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell. 1997;88:531–542. doi: 10.1016/s0092-8674(00)81893-0. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Kunz J, Hall MN. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc Natl Acad Sci USA. 1996;93:13780–13785. doi: 10.1073/pnas.93.24.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. In: Guthrie C, Fink GR, editors. Methods in Enzymology. Vol. 194. San Diego, CA: Academic Press; 1991. pp. 3–21. [DOI] [PubMed] [Google Scholar]
- Stan R, McLaughlin MM, Cafferkey RT, Johnson RK, Rosenberg M, Livi GP. Interaction between FKBP12-rapamycin and TOR involves a conserved serine residue. J Biol Chem. 1994;269:32027–32030. [PubMed] [Google Scholar]
- Taylor SS, Knighton DR, Zheng J, Sowadski JM, Gibbs CS, Zoller MJ. A template for the protein kinase family. Trends Biochem Sci. 1993;18:84–89. doi: 10.1016/0968-0004(93)80001-r. [DOI] [PubMed] [Google Scholar]
- von Manteuffel SR, Gingras A-C, Ming X-F, Sonenburg N, Thomas G. 4E-BP1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1996;93:4076–4080. doi: 10.1073/pnas.93.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Weisman R, Choder M, Koltin Y. Rapamycin specifically interferes with the developmental response of fission yeast to starvation. J Bacteriol. 1997;179:6325–6334. doi: 10.1128/jb.179.20.6325-6334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DJ, Ouwens DM, Nave BT, van der Zon GC, Alarcon CM, Cardenas ME, Heitman J, Maassen JA, Shepherd PR. Expression, enzyme activity, and subcellular localization of mammalian target of rapamycin in insulin-responsive cells. Biochem Biophys Res Commun. 1997;241:704–709. doi: 10.1006/bbrc.1997.7878. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- Xu Y, Baltimore D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 1996;10:2401–2410. doi: 10.1101/gad.10.19.2401. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ohya Y, Goebl M, Nakano A, Anraku Y. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J Biol Chem. 1994a;269:1166–1171. [PubMed] [Google Scholar]
- Yoshida S, Ohya Y, Nakano A, Anraku Y. Genetic interactions among genes involved in the STT4-PKC1 pathway of Saccharomyces cerevisiae. Mol Gen Genet. 1994b;242:631–640. doi: 10.1007/BF00283416. [DOI] [PubMed] [Google Scholar]
- Zheng X-F, Fiorentino D, Chen J, Crabtree GR, Schreiber SL. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995;82:121–130. doi: 10.1016/0092-8674(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Zheng XF, Schreiber SL. Target of rapamycin proteins and their kinase activities are required for meiosis. Proc Natl Acad Sci USA. 1997;94:3070–3075. doi: 10.1073/pnas.94.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]