Abstract
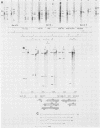
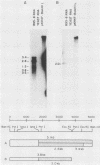
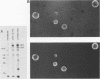
Two major outer envelope glycoproteins of Epstein-Barr virus, gp350 and gp220, are known to be encoded by 3.2- and 2.5-kilobase RNAs which map to the same DNA fragment (M. Hummel, D. Thorley-Lawson, and E. Kieff, J. Virol. 49:413-417). These RNAs have the same 5' and 3' ends. The larger RNA is encoded by a 2,777-base DNA segment which is preceded by TATTAAA, has AATAAA near its 3' end, and contains a 2,721-base open reading frame. The smaller RNA has one internal splice which maintains the same open reading frame. Translation of the 3.2- and 2.5-kilobase RNAs yielded proteins of 135 and 100 kilodaltons (Hummel et al., J. Virol. 49:413-417). The discrepancy between the 907 codons of the open reading frame and the 135-kilodalton size of the gp350 precursor is due to anomalous behavior of the protein in gel electrophoresis, since a protein translated from most of the Epstein-Barr virus open reading frame in Escherichia coli had similar properties. Antisera raised in rabbits to the protein expressed in E. coli specifically immunoprecipitated gp350 and gp220, confirming the mapping and sequencing results and the translational reading frame. The rabbit antisera also reacted with the plasma membranes of cells that were replicating virus and neutralized virus, particularly after the addition of complement. This is the first demonstration that the primary amino acid sequence of gp350 and gp220 has epitopes which can induce neutralizing antibody. We propose a model for the gp350 protein based on the theoretical analysis of its primary sequence.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolyniuk M., Wolff E., Kieff E. Proteins of Epstein-Barr Virus. II. Electrophoretic analysis of the polypeptides of the nucleocapsid and the glucosamine- and polysaccharide-containing components of enveloped virus. J Virol. 1976 Apr;18(1):289–297. doi: 10.1128/jvi.18.1.289-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson C. M., Thorley-Lawson D. A. Epstein-Barr virus membrane antigens: characterization, distribution, and strain differences. J Virol. 1981 Jul;39(1):172–184. doi: 10.1128/jvi.39.1.172-184.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson C. M., Thorley-Lawson D. A. Synthesis and processing of the three major envelope glycoproteins of Epstein-Barr virus. J Virol. 1983 May;46(2):547–556. doi: 10.1128/jvi.46.2.547-556.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Deininger P. L., Bankier A., Barrell B. Homologous upstream sequences near Epstein-Barr virus promoters. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1565–1569. doi: 10.1073/pnas.80.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hayashi K. A cloning vehicle suitable for strand separation. Gene. 1980 Oct;11(1-2):109–115. doi: 10.1016/0378-1119(80)90091-8. [DOI] [PubMed] [Google Scholar]
- Hoffman G. J., Lazarowitz S. G., Hayward S. D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci U S A. 1980 May;77(5):2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Thorley-Lawson D., Kieff E. An Epstein-Barr virus DNA fragment encodes messages for the two major envelope glycoproteins (gp350/300 and gp220/200). J Virol. 1984 Feb;49(2):413–417. doi: 10.1128/jvi.49.2.413-417.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono K. Computer programs to analyze DNA and amino acid sequence data. Nucleic Acids Res. 1982 Jan 11;10(1):85–89. doi: 10.1093/nar/10.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R. K., Murthy S. N., Reddy A. G., Steck T. L., Kohler H. Amino acid sequence of the N alpha-terminal 201 residues of human erythrocyte membrane band 3. J Biol Chem. 1983 Jul 10;258(13):7981–7990. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Levitt M. A simplified representation of protein conformations for rapid simulation of protein folding. J Mol Biol. 1976 Jun 14;104(1):59–107. doi: 10.1016/0022-2836(76)90004-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Purtilo D. T., Sakamoto K., Saemundsen A. K., Sullivan J. L., Synnerholm A. C., Anvret M., Pritchard J., Sloper C., Sieff C., Pincott J. Documentation of Epstein-Barr virus infection in immunodeficient patients with life-threatening lymphoproliferative diseases by clinical, virological, and immunopathological studies. Cancer Res. 1981 Nov;41(11 Pt 1):4226–4236. [PubMed] [Google Scholar]
- Qualtiere L. F., Chase R., Vroman B., Pearson G. R. Identification of Epstein-Barr virus strain differences with monoclonal antibody to a membrane glycoprotein. Proc Natl Acad Sci U S A. 1982 Jan;79(2):616–620. doi: 10.1073/pnas.79.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtiere L. F., Pearson G. R. Radioimmune precipitation study comparing the Epstein-Barr virus membrane antigens expressed on P3HR-1 virus-superinfected Raji cells to those expressed on cells in a B-95 virus-transformed producer culture activated with tumor-promoting agent (TPA). Virology. 1980 Apr 30;102(2):360–369. doi: 10.1016/0042-6822(80)90103-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schaffer H. E., Sederoff R. R. Improved estimation of DNA fragment lengths from Agarose gels. Anal Biochem. 1981 Jul 15;115(1):113–122. doi: 10.1016/0003-2697(81)90533-9. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Adams M. R., Rabin H. Glycosylation pathways of two major Epstein-Barr virus membrane antigens. Virology. 1983 May;127(1):168–176. doi: 10.1016/0042-6822(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Neubauer R. H., Rabin H., Mazur R. A. Correlation between Epstein-Barr virus membrane antigen and three large cell surface glycoproteins. J Virol. 1979 Dec;32(3):885–894. doi: 10.1128/jvi.32.3.885-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T., Klein R., Hopkins R. F., 3rd, Witmer T., Neubauer R. H., Rabin H. Production and characterization of monoclonal antibodies against the Epstein-Barr virus membrane antigen. J Virol. 1982 Jan;41(1):258–264. doi: 10.1128/jvi.41.1.258-264.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Chess L., Strominger J. L. Suppression of in vitro Epstein-Barr virus infection. A new role for adult human T lymphocytes. J Exp Med. 1977 Aug 1;146(2):495–508. doi: 10.1084/jem.146.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Geilinger K. Monoclonal antibodies against the major glycoprotein (gp350/220) of Epstein-Barr virus neutralize infectivity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5307–5311. doi: 10.1073/pnas.77.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Poodry C. A. Identification and isolation of the main component (gp350-gp220) of Epstein-Barr virus responsible for generating neutralizing antibodies in vivo. J Virol. 1982 Aug;43(2):730–736. doi: 10.1128/jvi.43.2.730-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A., Koide N., Klein G. Two large virion envelope glycoproteins mediate Epstein-Barr virus binding to receptor-positive cells. J Virol. 1982 Jan;41(1):286–297. doi: 10.1128/jvi.41.1.286-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]