Abstract
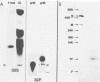
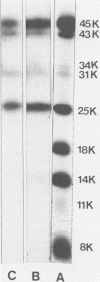
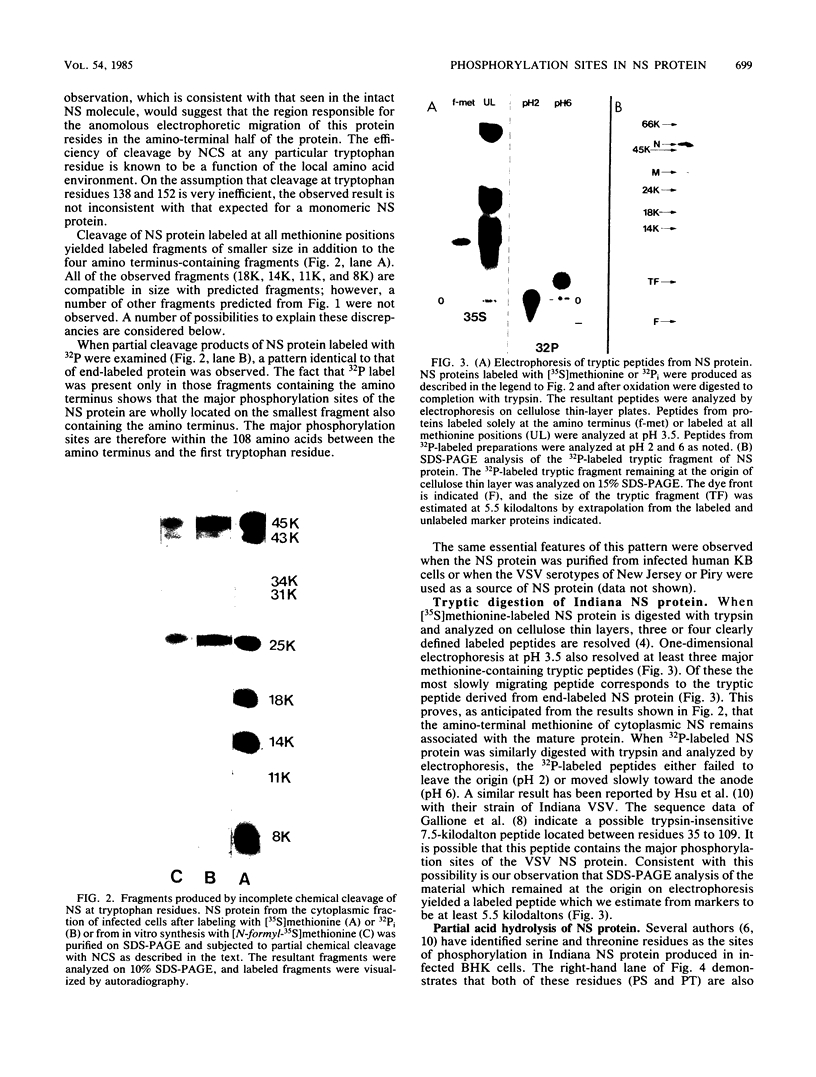
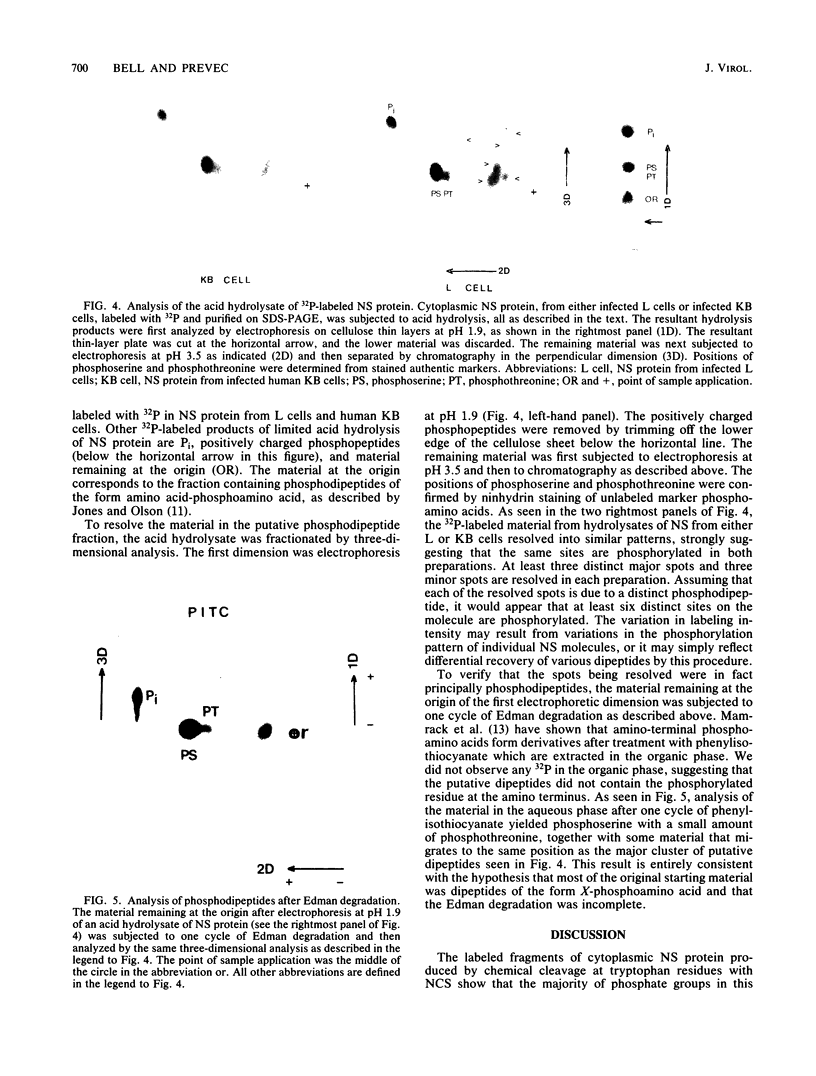
The phosphoprotein NS of vesicular stomatitis virus which accumulates within the infected cell cytoplasm is phosphorylated at multiple serine and threonine residues (G. M. Clinton and A. S. Huang, Virology 108:510-514, 1981; Hsu et al., J. Virol. 43:104-112, 1982). Using incomplete chemical cleavage at tryptophan residues, we mapped the major phosphorylation sites to the amino-terminal half of the protein. Analysis of phosphate-labeled tryptic peptides suggests that essentially all of the label is within the large trypsin-resistant fragment predicted from the sequence of Gallione et al. (J. Virol. 39:52-529, 1981). A similar result has been obtained for NS protein isolated from the virus particle by C.-H. Hsu and D. W. Kingsbury (J. Biol. Chem., in press). Analysis of phosphodipeptides utilizing the procedures of C. E. Jones and M. O. J. Olson (Int. J. Pept. Protein Res. 16:135-142, 1980) enabled us to detect as many as six distinct phosphate-containing dipeptides. From these studies, together with the known sequence data, we conclude that the major phosphate residues on cytoplasmic NS protein are located in the amino third of the NS molecule and most probably between residues 35 and 106, inclusive. The studies also provide formal chemical proof that NS protein has a structure consistent with a monomer of the sequence of Gallione et al. as modified by J. K. Rose (personal communication). The low electrophoretic mobility of this protein on sodium dodecyl sulfate-polyacrylamide gel electrophoresis is not therefore due to dimerization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. C., Brown E. G., Takayesu D., Prevec L. Protein kinase activity associated with immunoprecipitates of the vesicular stomatitis virus phosphoprotein NS. Virology. 1984 Jan 30;132(2):229–238. doi: 10.1016/0042-6822(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Brown E. G., Prevec L. Comparative analyses of vesiculovirus proteins utilizing partial cleavage fragments at tryptophan residues. Virology. 1979 May;95(1):244–248. doi: 10.1016/0042-6822(79)90424-0. [DOI] [PubMed] [Google Scholar]
- Brown E., Prevec L. Linear mapping of tryptophan residues in Vesiculovirus M and N proteins by partial chemical cleavage. J Virol. 1982 Apr;42(1):311–316. doi: 10.1128/jvi.42.1.311-316.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Prevec L. Proteins of vesicular stomatitis virus. IV. A comparison of tryptic peptides of the vesicular stomatitis group of rhabdoviruses. Virology. 1978 Aug;89(1):7–21. doi: 10.1016/0042-6822(78)90035-1. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Huang A. S. Distribution of phosphoserine, phosphothreonine and phosphotyrosine in proteins of vesicular stomatitis virus. Virology. 1981 Jan 30;108(2):510–514. doi: 10.1016/0042-6822(81)90459-1. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W., Murti K. G. Assembly of vesicular stomatitis virus nucleocapsids in vivo: a kinetic analysis. J Virol. 1979 Oct;32(1):304–313. doi: 10.1128/jvi.32.1.304-313.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Morgan E. M., Kingsbury D. W. Site-specific phosphorylation regulates the transcriptive activity of vesicular stomatitis virus NS protein. J Virol. 1982 Jul;43(1):104–112. doi: 10.1128/jvi.43.1.104-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. E., Olson M. O. Phosphodipeptide analysis of nonhistone nuclear proteins from Novikoff hepatoma ascites cells. Int J Pept Protein Res. 1980 Aug;16(2):135–142. doi: 10.1111/j.1399-3011.1980.tb02946.x. [DOI] [PubMed] [Google Scholar]
- Kingsford L., Emerson S. U. Transcriptional activities of different phosphorylated species of NS protein purified from vesicular stomatitis virions and cytoplasm of infected cells. J Virol. 1980 Mar;33(3):1097–1105. doi: 10.1128/jvi.33.3.1097-1105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamrack M. D., Olson M. O., Busch H. Amino acid sequence and sites of phosphorylation in a highly acidic region of nucleolar nonhistone protein C23. Biochemistry. 1979 Jul 24;18(15):3381–3386. doi: 10.1021/bi00582a026. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Patchornik A., Burstein Y. Selective chemical cleavage of tryptophanyl peptide bonds by oxidative chlorination with N-chlorosuccinimide. Biochemistry. 1976 Nov 16;15(23):5071–5075. doi: 10.1021/bi00668a019. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. In vitro synthesis of vesicular stomatitis virus membrane glycoprotein and insertion into membranes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):715–719. doi: 10.1073/pnas.75.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]