Abstract
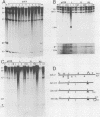
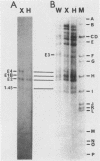
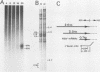
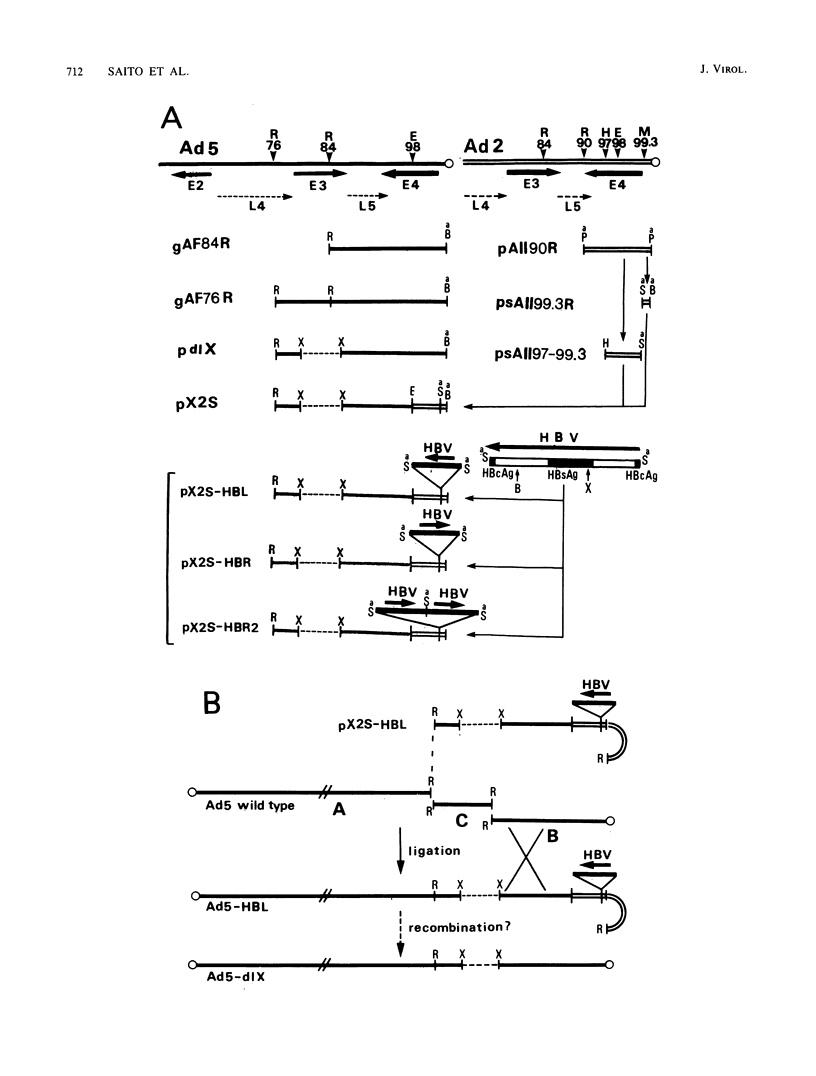
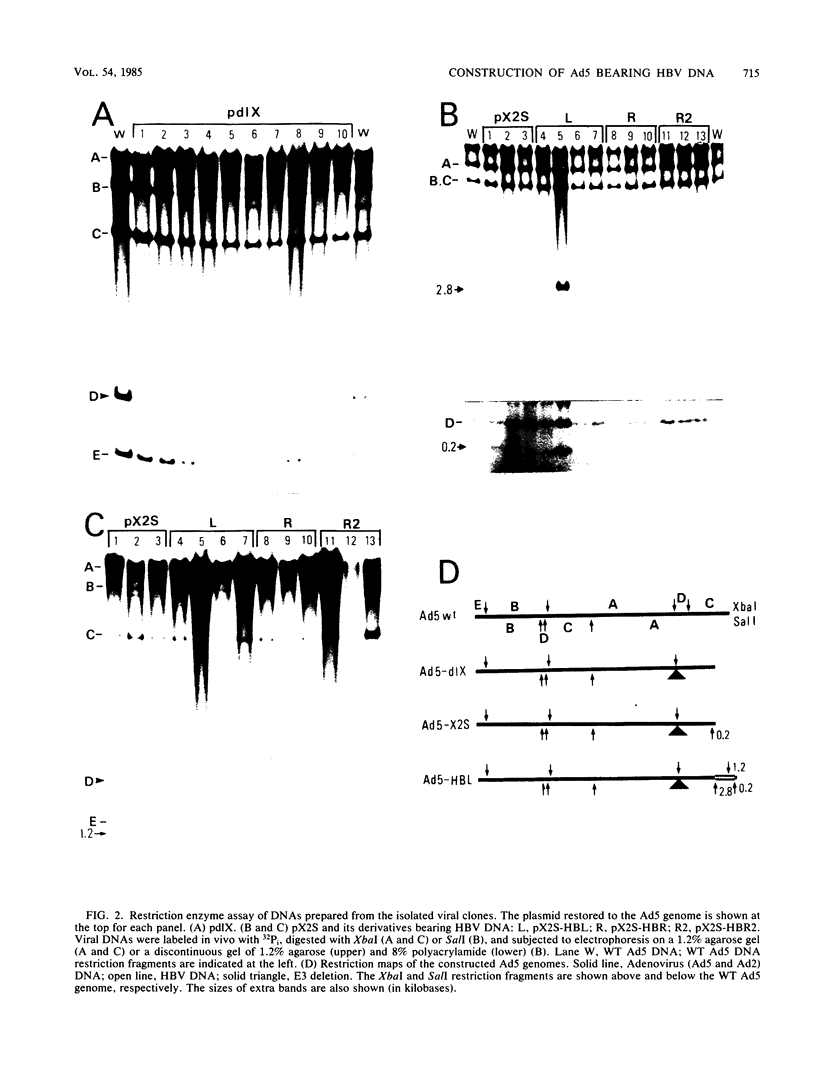
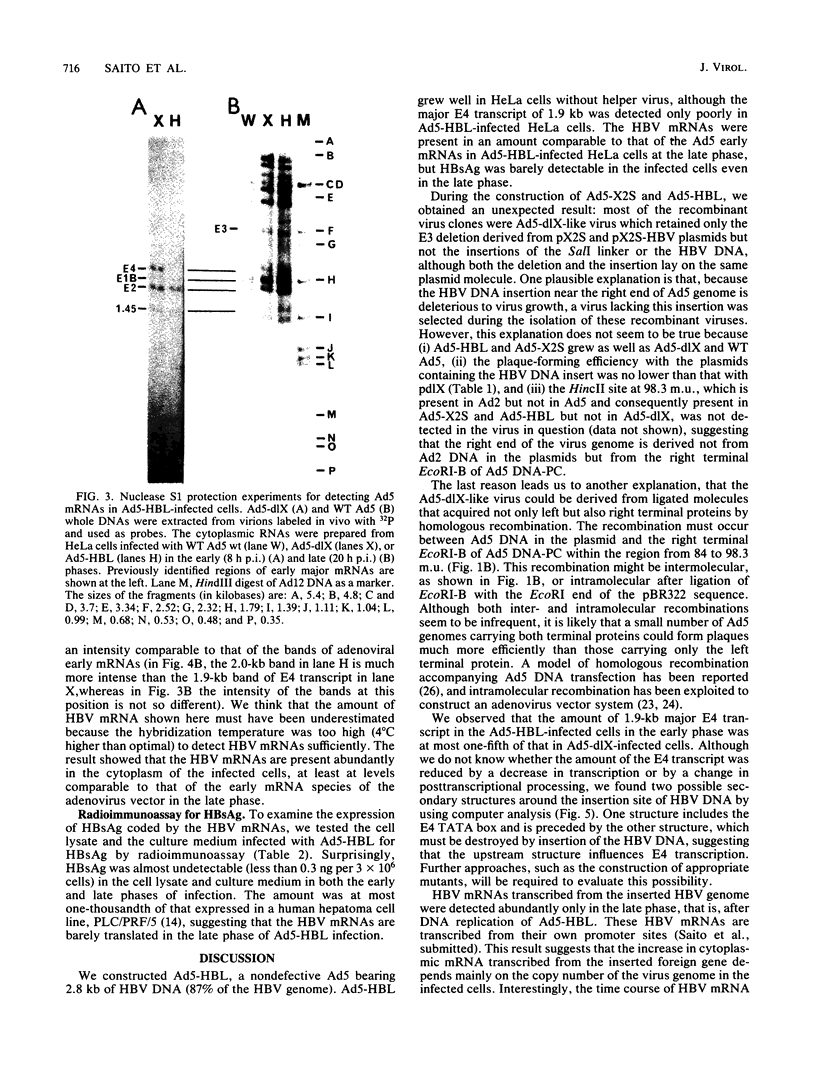
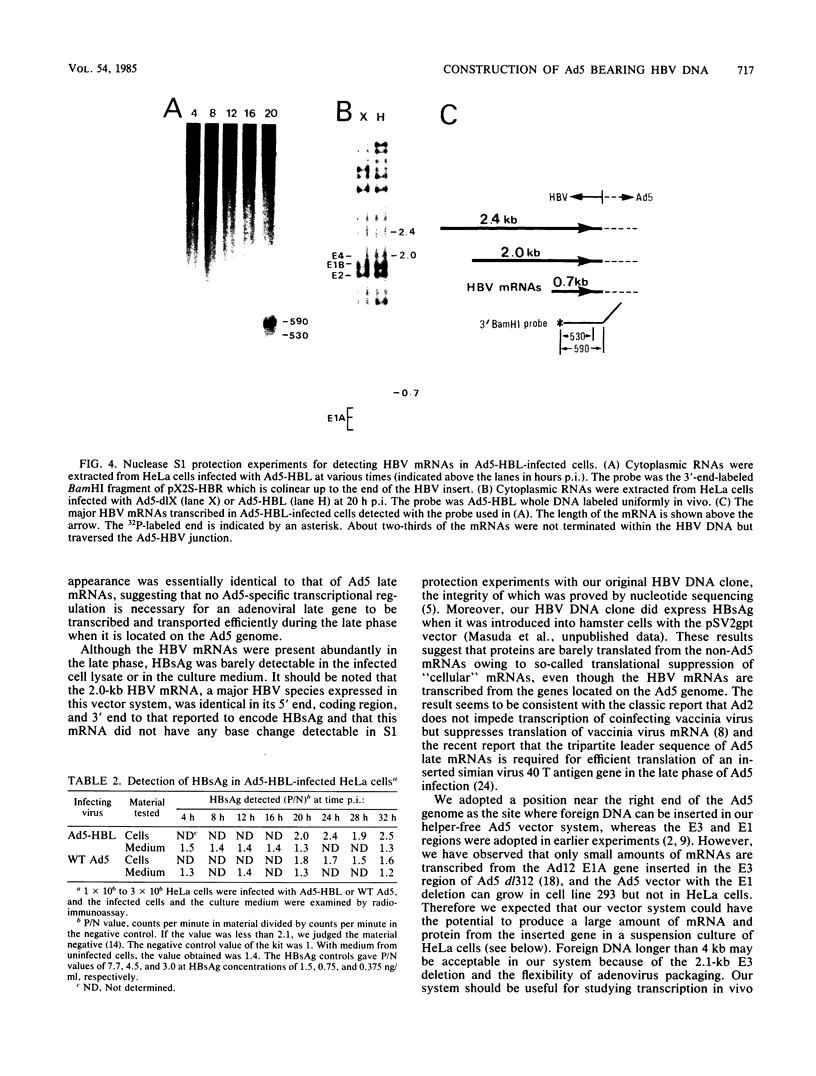
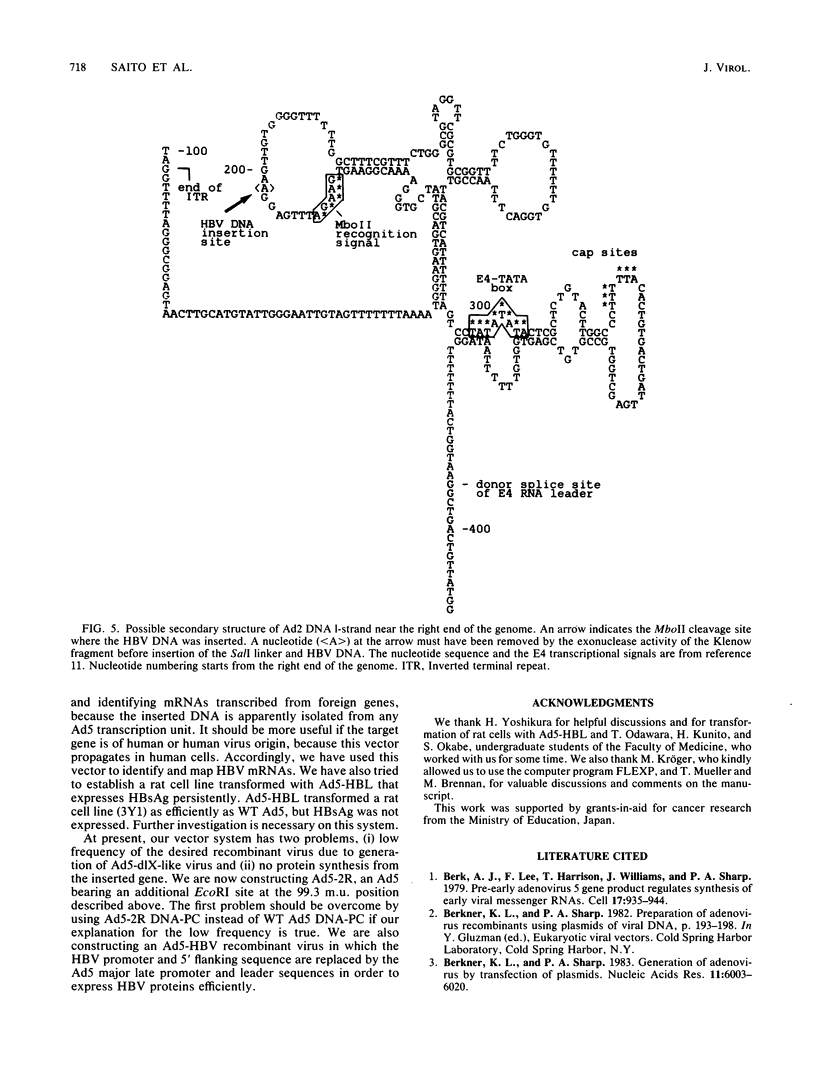
A novel helper-free adenovirus type 5 (Ad5) vector system, which utilizes a cloning site 0.2 kilobase (kb) from the right end of the genome, has been developed. To construct a nondefective Ad5 bearing the 2.8-kb DNA fragment of hepatitis B virus (HBV) at this site, we deleted the 2.1-kb nonessential E3 fragment from cloned DNA covering the right one-fourth of the Ad5 genome (76 to 100 map units), inserted the HBV DNA into this site, ligated the recombinant DNA to the rest of the Ad5 genome, and transfected the ligated DNA into human embryo kidney cells. Most of the recovered virus clones had only the E3 deletion and no HBV insertion, suggesting that a homologous recombination occurs between transfected DNAs in these cells. The isolated Ad5 virus bearing the HBV DNA (Ad5-HBL) grew without helper virus in HeLa cells as efficiently as wild-type Ad5, although the 1.9-kb major E4 transcript was detected only poorly in the early phase in the Ad5-HBL-infected cells, suggesting that the HBV DNA inserted upstream of the E4 promoter reduces the E4 transcript. HBV mRNAs transcribed from the inserted DNA were at least as abundant as Ad5 early mRNAs in the late phase of Ad5-HBL infection, but the HBV surface antigen was barely detectable in the infected-cell lysate and culture medium. This result suggests that HBV mRNAs can be transcribed from the inserted genes but no protein can be translated from the HBV mRNAs, presumably because of the translational suppression of cellular mRNAs caused by adenovirus in its late phase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Miyanohara A., Nozaki C., Yoneyama T., Ohtomo N., Matsubara K. Cloning and structural analyses of hepatitis B virus DNAs, subtype adr. Nucleic Acids Res. 1983 Jul 11;11(13):4601–4610. doi: 10.1093/nar/11.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Saito I., Shiroki K., Shimojo H. Isolation of transformation-defective, replication-nondefective early region 1B mutants of adenovirus 12. J Virol. 1984 Jan;49(1):154–161. doi: 10.1128/jvi.49.1.154-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Saito I., Shiroki K., Shimojo H., Takebe Y., Kaziro Y. The 19-kDal protein encoded by early region 1b of adenovirus type 12 is synthesized efficiently in Escherichia coli only as a fused protein. Gene. 1983 Jul;23(1):1–13. doi: 10.1016/0378-1119(83)90211-1. [DOI] [PubMed] [Google Scholar]
- Giorno R., Kates J. R. Mechanism of inhibition of vaccinia virus replication in adenovirus-infected HeLa cells. J Virol. 1971 Feb;7(2):208–213. doi: 10.1128/jvi.7.2.208-213.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hérissé J., Rigolet M., de Dinechin S. D., Galibert F. Nucleotide sequence of adenovirus 2 DNA fragment encoding for the carboxylic region of the fiber protein and the entire E4 region. Nucleic Acids Res. 1981 Aug 25;9(16):4023–4042. doi: 10.1093/nar/9.16.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Kröger M., Kröger-Block A. Extension of a flexible computer program for handling DNA sequence data. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):113–120. doi: 10.1093/nar/12.1part1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNab G. M., Alexander J. J., Lecatsas G., Bey E. M., Urbanowicz J. M. Hepatitis B surface antigen produced by a human hepatoma cell line. Br J Cancer. 1976 Nov;34(5):509–515. doi: 10.1038/bjc.1976.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Sato J., Handa H., Shiroki K., Shimojo H. Mapping of RNAs transcribed from adenovirus type 12 early and VA RNA regions. Virology. 1981 Oct 30;114(2):379–398. doi: 10.1016/0042-6822(81)90219-1. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Saito I., Maruyama K., Shimojo H. Isolation of a nondefective recombinant between adenovirus type 5 and early region 1A of adenovirus type 12. J Virol. 1983 May;46(2):632–637. doi: 10.1128/jvi.46.2.632-637.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick D. Construction of an adenovirus-SV40 recombinant producing SV40 T antigen from an adenovirus late promoter. Cell. 1981 Apr;24(1):135–143. doi: 10.1016/0092-8674(81)90509-2. [DOI] [PubMed] [Google Scholar]
- Stow N. D. Cloning of a DNA fragment from the left-hand terminus of the adenovirus type 2 genome and its use in site-directed mutagenesis. J Virol. 1981 Jan;37(1):171–180. doi: 10.1128/jvi.37.1.171-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D. The infectivity of adenovirus genomes lacking DNA sequences from their left-hand termini. Nucleic Acids Res. 1982 Sep 11;10(17):5105–5119. doi: 10.1093/nar/10.17.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Grodzicker T. Construction of adenovirus expression vectors by site-directed in vivo recombination. J Mol Appl Genet. 1982;1(5):435–446. [PubMed] [Google Scholar]
- Thummel C., Tjian R., Grodzicker T. Expression of SV40 T antigen under control of adenovirus promoters. Cell. 1981 Mar;23(3):825–836. doi: 10.1016/0092-8674(81)90447-5. [DOI] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Hu S. L., Grodzicker T. Translational control of SV40 T antigen expressed from the adenovirus late promoter. Cell. 1983 Jun;33(2):455–464. doi: 10.1016/0092-8674(83)90427-0. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Volkert F. C., Young C. S. The genetic analysis of recombination using adenovirus overlapping terminal DNA fragments. Virology. 1983 Feb;125(1):175–193. doi: 10.1016/0042-6822(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kitamura Y., Yoshikura H. Computation of statistical secondary structure of nucleic acids. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):335–346. doi: 10.1093/nar/12.1part1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]