Abstract
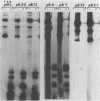
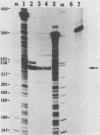
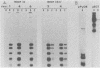
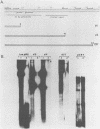
We constructed and analyzed a series of deletion mutants in the noncoding regulatory region of tsa polyomavirus DNA to identify some of the sequences critical to the DNA replication origin and to the expression of the viral early genes in vivo. By using both transient and long-term assays under conditions where the influence of large T antigen (T-Ag) in replication or autoregulation was minimized, we observed no more than a 30% reduction in early gene expression upon removal of the CAAT or TATA elements or both. These assays demonstrated a predominant effect of upstream promoter or enhancer elements and indicated that removal of the CAAT or TATA boxes did not significantly affect viral early gene expression. Studies on the replicative ability of these mutants in mouse cells constitutively expressing the polyoma early proteins revealed that the removal of DNA sequences contained within a previously identified T-Ag high-affinity binding site (nucleotides 39 to 64) abolished viral DNA replication, whereas removal of two other high-affinity sites, closer to the early mRNA cap sites, did not. Furthermore, a deletion including this same high-affinity site plus a low-affinity binding site within the 32-base-pair palindrome of the origin core sequences eliminated the ability of the viral large T-Ag to efficiently repress early gene transcription. It is thus possible that the origin-proximal high-affinity T-Ag binding site is involved in both of the functions of large T-Ag, i.e., the initiation of viral DNA replication and the autoregulation of early gene transcription.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Products of complementation between temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):127–136. doi: 10.1128/jvi.15.1.127-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen B. Virus-specific early RNA in 3T6 cells infected by a tsA mutant of polyoma virus. Virology. 1978 Mar;85(1):222–230. doi: 10.1016/0042-6822(78)90426-9. [DOI] [PubMed] [Google Scholar]
- Colantuoni V., Dailey L., Valle G. D., Basilico C. Requirements for excision and amplification of integrated viral DNA molecules in polyoma virus-transformed cells. J Virol. 1982 Aug;43(2):617–628. doi: 10.1128/jvi.43.2.617-628.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie A., Kamen R. Multiple binding sites for polyomavirus large T antigen within regulatory sequences of polyomavirus DNA. J Virol. 1984 Dec;52(3):750–760. doi: 10.1128/jvi.52.3.750-760.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L., Pellegrini S., Basilico C. Deletion of the origin of replication impairs the ability of polyomavirus DNA to transform cells and to form tandem insertions. J Virol. 1984 Mar;49(3):984–987. doi: 10.1128/jvi.49.3.984-987.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deninger P. L., Esty A., LaPorte P., Hsu H., Friedmann T. The nucleotide sequence and restriction enzyme sites of the polyoma genome. Nucleic Acids Res. 1980 Feb 25;8(4):855–860. [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Baty D., Chambon P. The repeated GC-rich motifs upstream from the TATA box are important elements of the SV40 early promoter. Nucleic Acids Res. 1983 Apr 25;11(8):2447–2464. doi: 10.1093/nar/11.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fenton R. G., Basilico C. Changes in the topography of early region transcription during polyoma virus lytic infection. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7142–7146. doi: 10.1073/pnas.79.23.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton R. G., Basilico C. Regulation of polyoma virus early transcription in transformed cells by large T-antigen. Virology. 1982 Sep;121(2):384–392. doi: 10.1016/0042-6822(82)90176-3. [DOI] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Tyndall C., Kamen R., Cuzin F. The high affinity binding site on polyoma virus DNA for the viral large-T protein. Nucleic Acids Res. 1981 Nov 11;9(21):5697–5710. doi: 10.1093/nar/9.21.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino J., Bastia D. Interaction of the plasmid R6K-encoded replication initiator protein with its binding sites on DNA. Cell. 1983 Aug;34(1):125–134. doi: 10.1016/0092-8674(83)90142-3. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grosveld G. C., Rosenthal A., Flavell R. A. Sequence requirements for the transcription of the rabbit beta-globin gene in vivo: the -80 region. Nucleic Acids Res. 1982 Aug 25;10(16):4951–4971. doi: 10.1093/nar/10.16.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen U., Tenen D. G., Livingston D. M., Sharp P. A. T antigen repression of SV40 early transcription from two promoters. Cell. 1981 Dec;27(3 Pt 2):603–613. doi: 10.1016/0092-8674(81)90402-5. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Saragosti S., Blangy D., Yaniv M. Fine structure of the origin-proximal DNAase I-hypersensitive region in wild-type and EC mutant polyoma. Cell. 1981 Sep;25(3):651–658. doi: 10.1016/0092-8674(81)90172-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jat P., Novak U., Cowie A., Tyndall C., Kamen R. DNA sequences required for specific and efficient initiation of transcription at the polyoma virus early promoter. Mol Cell Biol. 1982 Jul;2(7):737–751. doi: 10.1128/mcb.2.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Tjian R. Essential contact residues within SV40 large T antigen binding sites I and II identified by alkylation-interference. Cell. 1984 Jan;36(1):155–162. doi: 10.1016/0092-8674(84)90084-9. [DOI] [PubMed] [Google Scholar]
- Kamen R., Jat P., Treisman R., Favaloro J., Folk W. R. 5' termini of polyoma virus early region transcripts synthesized in vivo by wild-type virus and viable deletion mutants. J Mol Biol. 1982 Aug 5;159(2):189–224. doi: 10.1016/0022-2836(82)90493-4. [DOI] [PubMed] [Google Scholar]
- Katinka M., Yaniv M. DNA replication origin of polyoma virus: early proximal boundary. J Virol. 1983 Jul;47(1):244–248. doi: 10.1128/jvi.47.1.244-248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Activation of the SV40 late promoter: direct effects of T antigen in the absence of viral DNA replication. Cell. 1984 Feb;36(2):381–389. doi: 10.1016/0092-8674(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Luthman H., Nilsson M. G., Magnusson G. Non-contiguous segments of the polyoma genome required in cis for DNA replication. J Mol Biol. 1982 Nov 15;161(4):533–550. doi: 10.1016/0022-2836(82)90406-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKay R., DiMaio D. Binding of an SV40 T antigen-related protein to the DNA of SV40 regulatory mutants. Nature. 1981 Feb 26;289(5800):810–813. doi: 10.1038/289810a0. [DOI] [PubMed] [Google Scholar]
- Muller W. J., Mueller C. R., Mes A. M., Hassell J. A. Polyomavirus origin for DNA replication comprises multiple genetic elements. J Virol. 1983 Sep;47(3):586–599. doi: 10.1128/jvi.47.3.586-599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Rio D. C., Robbins A. K., Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981 Aug;25(2):373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. doi: 10.1073/pnas.77.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K. Replication of polyoma plasmid recombinants in mouse cells. J Mol Biol. 1981 Sep 5;151(1):203–210. doi: 10.1016/0022-2836(81)90229-1. [DOI] [PubMed] [Google Scholar]
- Pellegrini S., Dailey L., Basilico C. Amplification and excision of integrated polyoma DNA sequences require a functional origin of replication. Cell. 1984 Apr;36(4):943–949. doi: 10.1016/0092-8674(84)90044-8. [DOI] [PubMed] [Google Scholar]
- Pomerantz B. J., Mueller C. R., Hassell J. A. Polyomavirus large T antigen binds independently to multiple, unique regions on the viral genome. J Virol. 1983 Sep;47(3):600–610. doi: 10.1128/jvi.47.3.600-610.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C., Tjian R. SV40 T antigen binding site mutations that affect autoregulation. Cell. 1983 Apr;32(4):1227–1240. doi: 10.1016/0092-8674(83)90305-7. [DOI] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Shenk T. Definition of the boundaries of the origin of DNA replication in simian virus 40. Nucleic Acids Res. 1978 Oct;5(10):3635–3642. doi: 10.1093/nar/5.10.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Lewton B. A., DeLucia A. L., Wilson V. G., Ryder K. Topography of simian virus 40 A protein-DNA complexes: arrangement of protein bound to the origin of replication. J Virol. 1983 Apr;46(1):151–161. doi: 10.1128/jvi.46.1.151-161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Folk W. R. Essential nucleotides in the polyomavirus origin region. J Virol. 1984 Aug;51(2):437–444. doi: 10.1128/jvi.51.2.437-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]