Abstract
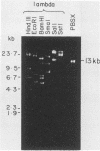
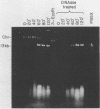
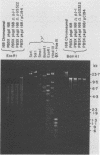
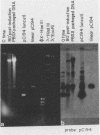
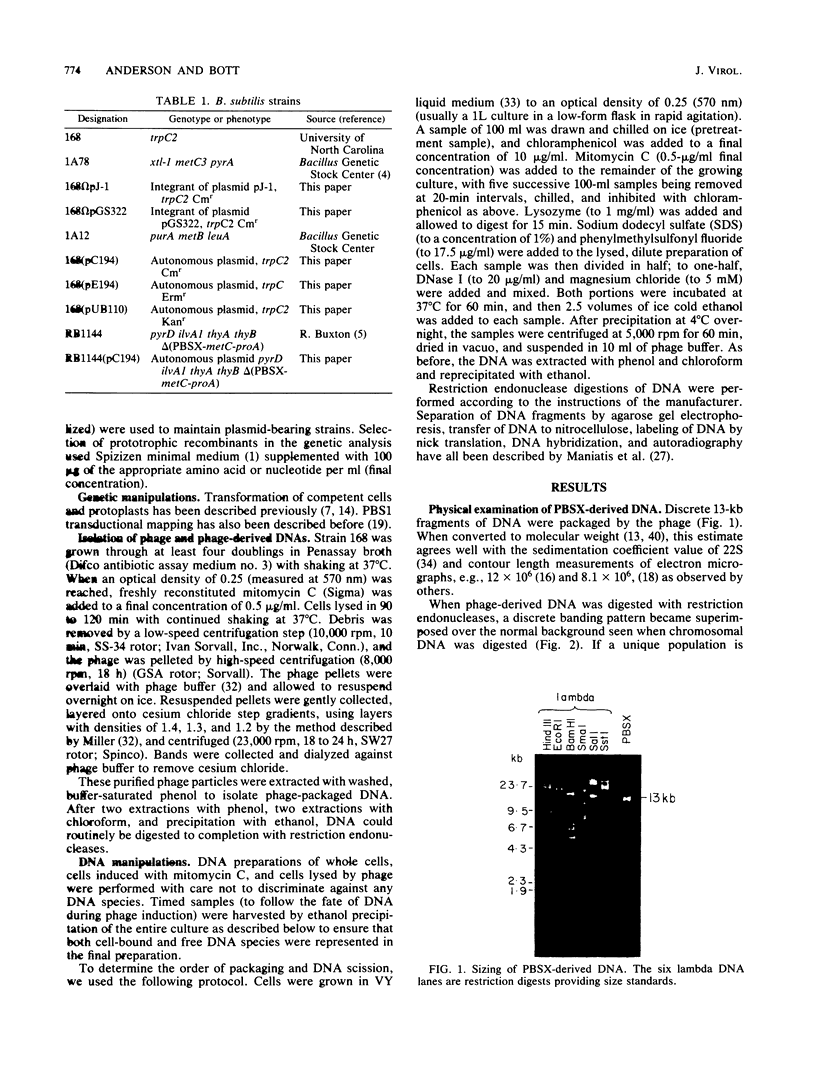
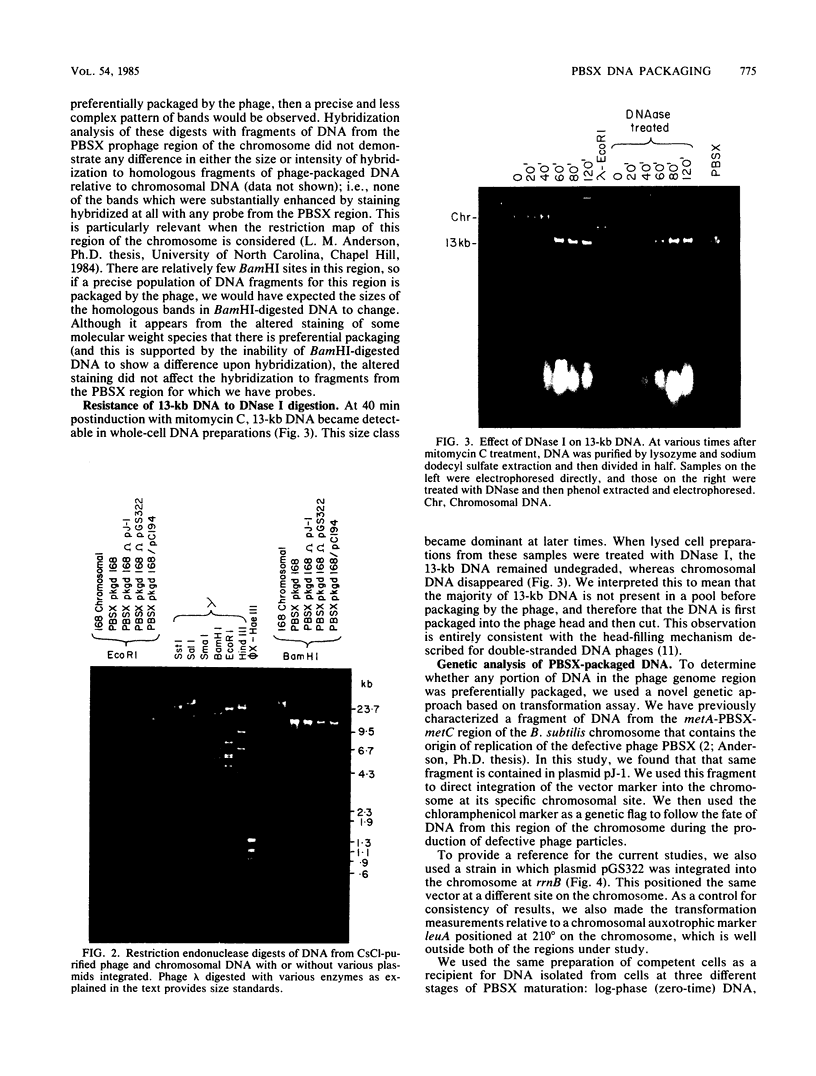
Defective bacteriophage PBSX, a resident of all Bacillus subtilis 168 chromosomes, packages fragments of DNA from all portions of the host chromosome when induced by mitomycin C. In this study, the physical process for DNA packaging of both chromosomal and plasmid DNAs was examined. Discrete 13-kilobase (kb) lengths of DNA were packaged by wild-type phage, and the process was DNase I resistant and probably occurred by a head-filling mechanism. Genetically engineered isogenic host strains having a chloramphenicol resistance determinant integrated as a genetic flag at two different regions of the chromosome were used to monitor the packaging of specific chromosomal regions. No dramatic selectivity for these regions could be documented. If the wild-type strain 168 contains autonomously replicating plasmids, especially pC194, the mitomycin C induces an increase in size of resident plasmid DNA, which is then packaged as 13-kb pieces into phage heads. In strain RB1144, which lacks substantial portions of the PBSX resident phage region, mitomycin C treatment did not affect the structure of resident plasmids. Induction of PBSX started rolling circle replication on plasmids, which then became packaged as 13-kb fragments. This alteration or cannibalization of plasmid replication resulting from mitomycin C treatment requires for its function some DNA within the prophage deletion of strain RB1144.
Full text
PDF
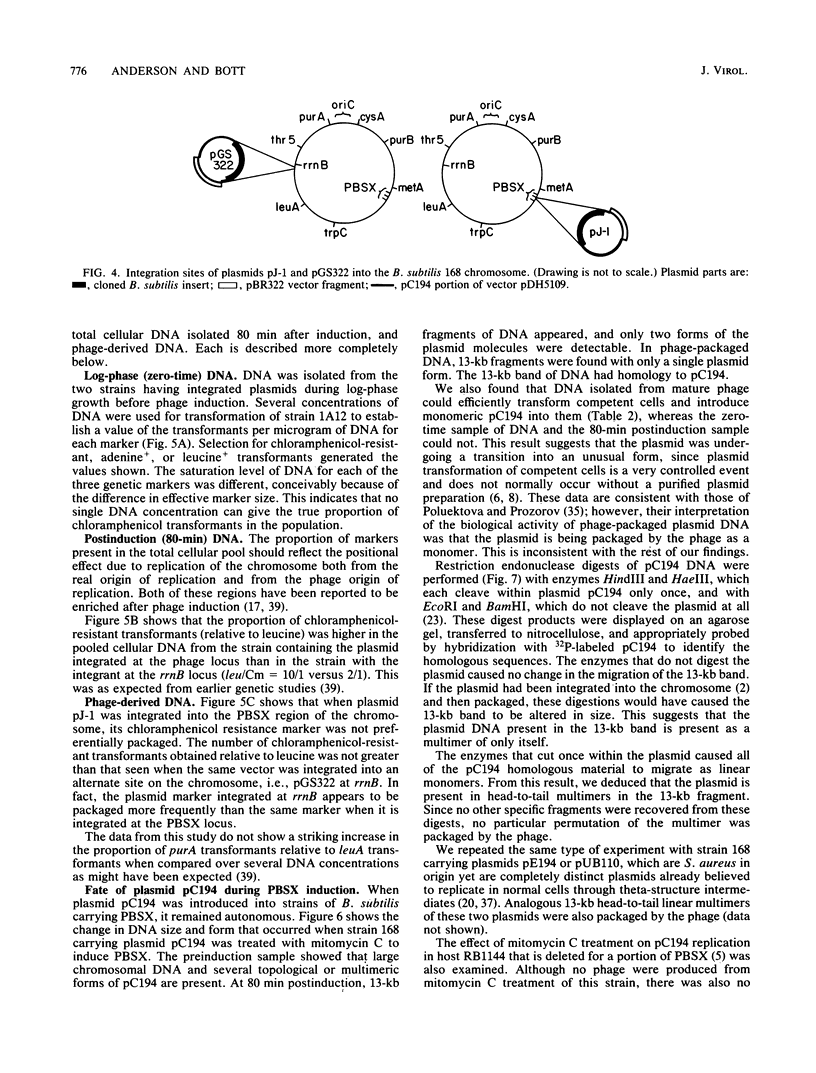
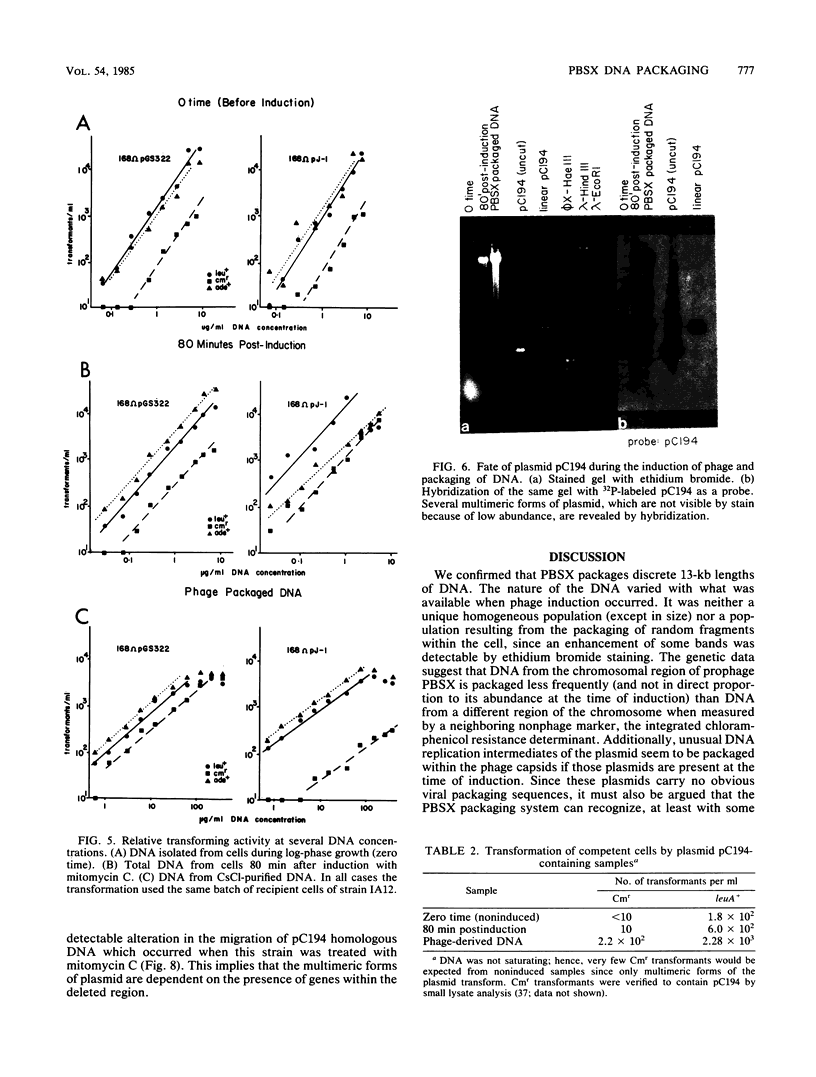



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. M., Ruley H. E., Bott K. F. Isolation of an autonomously replicating DNA fragment from the region of defective bacteriophage PBSX of Bacillus subtilis. J Bacteriol. 1982 Jun;150(3):1280–1286. doi: 10.1128/jb.150.3.1280-1286.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastia D., Sueoka N. Studies on the late replication of phage lambda: rolling-circle replication of the wild type and a partially suppressed strain, Oam29 Pam80. J Mol Biol. 1975 Oct 25;98(2):305–320. doi: 10.1016/s0022-2836(75)80120-3. [DOI] [PubMed] [Google Scholar]
- Buxton R. S. Prophage mutation causing heat inducibility of defective Bacillus subtilis bacteriophage PBSX. J Virol. 1976 Oct;20(1):22–28. doi: 10.1128/jvi.20.1.22-28.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S. Selection of Bacillus subtilis 168 mutants with deletions of the PBSX prophage. J Gen Virol. 1980 Feb;46(2):427–437. doi: 10.1099/0022-1317-46-2-427. [DOI] [PubMed] [Google Scholar]
- Canosi U., Iglesias A., Trautner T. A. Plasmid transformation in Bacillus subtilis: effects of insertion of Bacillus subtilis DNA into plasmid pC194. Mol Gen Genet. 1981;181(4):434–440. doi: 10.1007/BF00428732. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Contente S., Dubnau D. Characterization of plasmid transformation in Bacillus subtilis: kinetic properties and the effect of DNA conformation. Mol Gen Genet. 1979 Jan 2;167(3):251–258. doi: 10.1007/BF00267416. [DOI] [PubMed] [Google Scholar]
- Coughlin S. A., Green D. M. Two-dimensional zymogram analysis of nucleases in Bacillus subtilis. Anal Biochem. 1983 Sep;133(2):322–329. doi: 10.1016/0003-2697(83)90091-x. [DOI] [PubMed] [Google Scholar]
- Deichelbohrer I., Messer W., Trautner T. A. Genome of Bacillus subtilis Bacteriophage SPP1: Structure and Nucleotide Sequence of pac, the Origin of DNA Packaging. J Virol. 1982 Apr;42(1):83–90. doi: 10.1128/jvi.42.1.83-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- Erickson R. J., Copeland J. C. Structure and replication of chromosomes in competent cells of Bacillus subtilis. J Bacteriol. 1972 Mar;109(3):1075–1084. doi: 10.1128/jb.109.3.1075-1084.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Marmur J. Defective bacteriophages. J Cell Physiol. 1970 Dec;76(3):253–263. doi: 10.1002/jcp.1040760305. [DOI] [PubMed] [Google Scholar]
- Haas M., Yoshikawa H. Defective bacteriophage PBSH in Bacillus subtilis. I. Induction, purification, and physical properties of the bacteriophage and its deoxyribonucleic acid. J Virol. 1969 Feb;3(2):233–247. doi: 10.1128/jvi.3.2.233-247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Yoshikawa H. Defective bacteriophage PBSH in Bacillus subtilis. II. Intracellular development of the induced prophage. J Virol. 1969 Feb;3(2):248–260. doi: 10.1128/jvi.3.2.248-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa H., Kadlubar F. Length of deoxyribonucleic acid of PBSX-like particles of Bacillus subtilis induced by 4-nitroquinoline-1-oxide. J Virol. 1969 Feb;3(2):205–209. doi: 10.1128/jvi.3.2.205-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. DNA sequences necessary for packaging of bacteriophage lambda DNA. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7456–7460. doi: 10.1073/pnas.80.24.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M., Marmur J. The 5'-ends of the DNA of defective bacteriophages of Bacillus subtilis. J Mol Biol. 1970 Feb 14;47(3):591–593. doi: 10.1016/0022-2836(70)90326-8. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Adler G. K., Novick R. P. Functional origin of replication of pT181 plasmid DNA is contained within a 168-base-pair segment. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4580–4584. doi: 10.1073/pnas.79.15.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn R., Winston S., Tanaka T., Sueoka N. Specific in vitro binding of a plasmid to a membrane fraction of Bacillus subtilis. Proc Natl Acad Sci U S A. 1983 Jan;80(2):574–578. doi: 10.1073/pnas.80.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero R., Chiafari F. A., Lovett P. S. SP02 particles mediating transduction of a plasmid containing SP02 cohesive ends. J Bacteriol. 1981 Jul;147(1):1–8. doi: 10.1128/jb.147.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero R., Lovett P. S. Interference of plasmid pCM194 with lysogeny of bacteriophage SP02 in Bacillus subtilis. J Bacteriol. 1982 Oct;152(1):284–290. doi: 10.1128/jb.152.1.284-290.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero R., Lovett P. S. Transductional selection of cloned bacteriophage phi 105 and SP02 deoxyribonucleic acids in Bacillus subtilis. J Bacteriol. 1980 Aug;143(2):879–886. doi: 10.1128/jb.143.2.879-886.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauël C., Karamata D. Prophage induction in thermosensitive DNA mutants of Bacillus subtilis. Mol Gen Genet. 1984;194(3):451–456. doi: 10.1007/BF00425557. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Marmur J. Conversion of Bacillus subtilis DNA to phage DNA following mitomycin C induction. J Mol Biol. 1968 Jun 28;34(3):429–437. doi: 10.1016/0022-2836(68)90170-8. [DOI] [PubMed] [Google Scholar]
- Poluéktova E. U., Prozorov A. A. Nalichie plazmidy pBD12 v golovkakh defektnogo faga PBSX Bacillus subtilis. Genetika. 1982 Dec;18(12):2050–2051. [PubMed] [Google Scholar]
- Ruhfel R. E., Robillard N. J., Thorne C. B. Interspecies transduction of plasmids among Bacillus anthracis, B. cereus, and B. thuringiensis. J Bacteriol. 1984 Mar;157(3):708–711. doi: 10.1128/jb.157.3.708-711.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer-Abramowitz J., Gryczan T. J., Dubnau D. Origin and mode of replication of plasmids pE194 and pUB110. Plasmid. 1981 Jul;6(1):67–77. doi: 10.1016/0147-619x(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Stewart G. C., Wilson F. E., Bott K. F. Detailed physical mapping of the ribosomal RNA genes of Bacillus subtilis. Gene. 1982 Sep;19(2):153–162. doi: 10.1016/0378-1119(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Thurm P., Garro A. J. Isolation and characterization of prophage mutants of the defective Bacillus subtilis bacteriophage PBSX. J Virol. 1975 Jul;16(1):184–191. doi: 10.1128/jvi.16.1.184-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. D., Krumlauf R. A computer program for the calculation of sedimentation coefficients and molecular weights of nucleic acids. Anal Biochem. 1981 Jul 15;115(1):97–101. doi: 10.1016/0003-2697(81)90530-3. [DOI] [PubMed] [Google Scholar]