Abstract
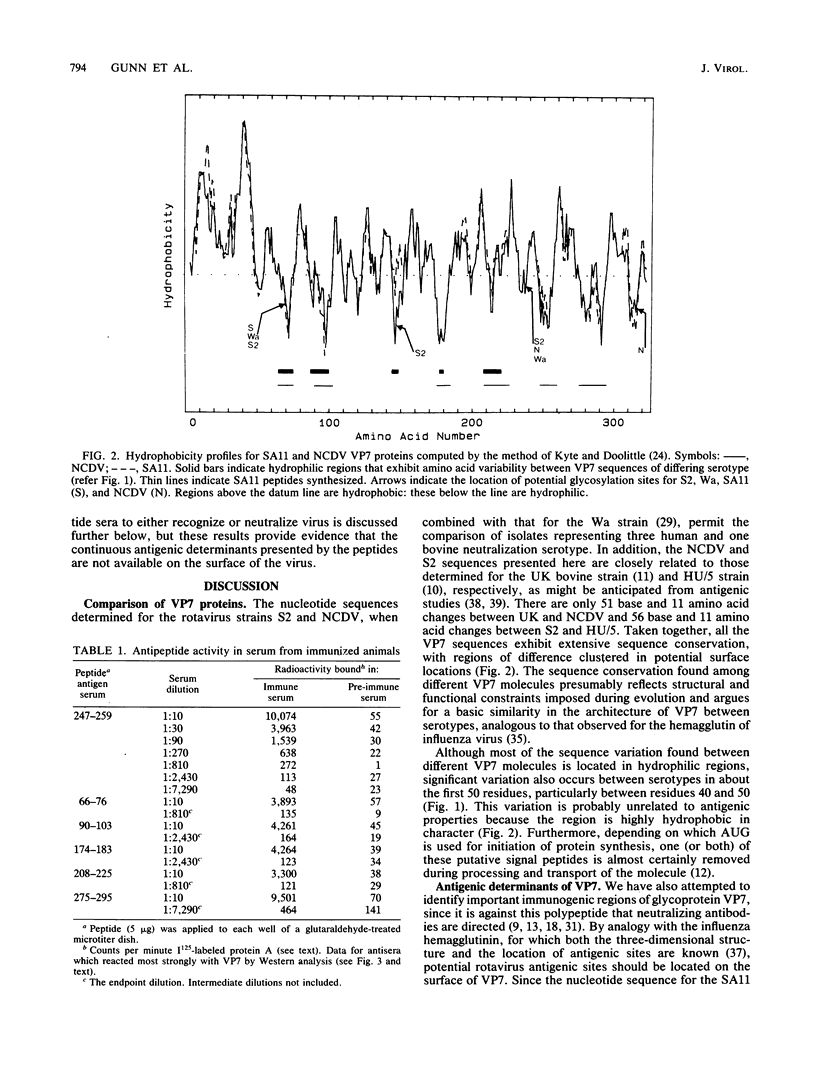
The rotavirus neutralizing antigen, VP7, is a 37,000-molecular-weight glycoprotein which is a major component of the outer shell of the virion. The amino acid sequence of VP7 for strain S2 (human serotype 2) and Nebraska calf diarrhea virus (bovine serotype) has been inferred from the nucleic acid sequence of cloned copies of genomic segment nine. Comparison of the amino acid sequences of these two VP7 proteins with those already determined for other rotavirus strains reveals extensive sequence conservation between serotypes with clusters of amino acid differences sited predominantly in hydrophilic domains of the protein. Six peptides have been synthesized that span the hydrophilic regions of the molecule. Antisera to these peptides both recognize the respective homologous peptides in a solid-phase radioimmunoassay and bind to denatured VP7 in a Western blot. However, none of the antisera either recognize virus or exhibit significant neutralizing activity, indicating that these peptide sequences are not available on the surface of the virus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias C. F., López S., Bell J. R., Strauss J. H. Primary structure of the neutralization antigen of simian rotavirus SA11 as deduced from cDNA sequence. J Virol. 1984 May;50(2):657–661. doi: 10.1128/jvi.50.2.657-661.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Bastardo J. W., McKimm-Breschkin J. L., Sonza S., Mercer L. D., Holmes I. H. Preparation and characterization of antisera to electrophoretically purified SA11 virus polypeptides. Infect Immun. 1981 Dec;34(3):641–647. doi: 10.1128/iai.34.3.641-647.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Bellamy A. R., Street J. E., Siegman L. J. A general strategy for cloning double-stranded RNA: nucleotide sequence of the Simian-11 rotavirus gene 8. Nucleic Acids Res. 1982 Nov 25;10(22):7075–7088. doi: 10.1093/nar/10.22.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann A. J., Stanway G., Hauptmann R., Minor P. D., Schild G. C., Clarke L. D., Mountford R. C., Almond J. W. Poliovirus type 3: molecular cloning of the genome and nucleotide sequence of the region encoding the protease and polymerase proteins. Nucleic Acids Res. 1983 Mar 11;11(5):1267–1281. doi: 10.1093/nar/11.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R. Human viral gastroenteritis. Microbiol Rev. 1984 Jun;48(2):157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Sequence homology between human and animal rotavirus serotype-specific glycoproteins. Nucleic Acids Res. 1984 May 11;12(9):3973–3982. doi: 10.1093/nar/12.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Dyall-Smith M. L., Holmes I. H., Azad A. A. Nucleotide sequence of the gene encoding the serotype-specific glycoprotein of UK bovine rotavirus. Nucleic Acids Res. 1983 Jul 25;11(14):4689–4701. doi: 10.1093/nar/11.14.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Hanssen H. H., Estes M. K. Two types of glycoprotein precursors are produced by the simian rotavirus SA11. Virology. 1983 Jun;127(2):320–332. doi: 10.1016/0042-6822(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding D. R., Battersby J. E., Husbands D. R., Hancock W. S. Letter: Synthesis of a protein with the properties of the apolipoprotein C-I (ApoLP-Ser). J Am Chem Soc. 1976 Apr 28;98(9):2664–2665. doi: 10.1021/ja00425a044. [DOI] [PubMed] [Google Scholar]
- Ihara T., Samejima T., Kuwahara H., Tajima M. Isolation of new serotypes of bovine rotavirus. Brief report. Arch Virol. 1983;78(1-2):145–150. doi: 10.1007/BF01310870. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kouvelos K., Petric M., Middleton P. J. Comparison of bovine, simian and human rotavirus structural glycoproteins. J Gen Virol. 1984 Jul;65(Pt 7):1211–1214. doi: 10.1099/0022-1317-65-7-1211. [DOI] [PubMed] [Google Scholar]
- Kouvelos K., Petric M., Middleton P. J. Oligosaccharide composition of calf rotavirus. J Gen Virol. 1984 Jul;65(Pt 7):1159–1164. doi: 10.1099/0022-1317-65-7-1159. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lentz M. R., Air G. M., Laver W. G., Webster R. G. Sequence of the neuraminidase gene of influenza virus A/Tokyo/3/67 and previously uncharacterized monoclonal variants. Virology. 1984 May;135(1):257–265. doi: 10.1016/0042-6822(84)90135-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M. A., Iwamoto A., Ikegami N., Nomoto A., Furuichi Y. Nucleotide sequence of the gene encoding the serotype-specific antigen of human (Wa) rotavirus: comparison with the homologous genes from simian SA11 and UK bovine rotaviruses. J Virol. 1984 Sep;51(3):860–862. doi: 10.1128/jvi.51.3.860-862.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S., Breschkin A. M., Holmes I. H. The major surface glycoprotein of simian rotavirus (SA11) contains distinct epitopes. Virology. 1984 Apr 30;134(2):318–327. doi: 10.1016/0042-6822(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Street J. E., Croxson M. C., Chadderton W. F., Bellamy A. R. Sequence diversity of human rotavirus strains investigated by northern blot hybridization analysis. J Virol. 1982 Aug;43(2):369–378. doi: 10.1128/jvi.43.2.369-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Alexander H., Houghten R. A., Olson A. J., Lerner R. A., Hendrickson W. A. The reactivity of anti-peptide antibodies is a function of the atomic mobility of sites in a protein. Nature. 1984 Nov 8;312(5990):127–134. doi: 10.1038/312127a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Greenberg H. B., James W. D., Pittman A. L., Kalica A. R., Flores J., Chanock R. M., Kapikian A. Z. Definition of human rotavirus serotypes by plaque reduction assay. Infect Immun. 1982 Jul;37(1):110–115. doi: 10.1128/iai.37.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]