Abstract
ERp57 is a lumenal protein of the endoplasmic reticulum (ER) and a member of the protein disulfide isomerase (PDI) family. In contrast to archetypal PDI, ERp57 interacts specifically with newly synthesized glycoproteins. In this study we demonstrate that ERp57 forms discrete complexes with the ER lectins, calnexin and calreticulin. Specific ERp57/calreticulin complexes exist in canine pancreatic microsomes, as demonstrated by SDS-PAGE after cross-linking, and by native electrophoresis in the absence of cross-linking. After in vitro translation and import into microsomes, radiolabeled ERp57 can be cross-linked to endogenous calreticulin and calnexin while radiolabeled PDI cannot. Likewise, radiolabeled calreticulin is cross-linked to endogenous ERp57 but not PDI. Similar results were obtained in Lec23 cells, which lack the glucosidase I necessary to produce glycoprotein substrates capable of binding to calnexin and calreticulin. This observation indicates that ERp57 interacts with both of the ER lectins in the absence of their glycoprotein substrate. This result was confirmed by a specific interaction between in vitro synthesized calreticulin and ERp57 prepared in solution in the absence of other ER components. We conclude that ERp57 forms complexes with both calnexin and calreticulin and propose that it is these complexes that can specifically modulate glycoprotein folding within the ER lumen.
INTRODUCTION
In order to enter the secretory pathway, proteins are cotranslationally translocated across the membrane of the endoplasmic reticulum (ER) as extended polypeptide chains. Upon entering the lumen of the ER the proteins begin to fold, normally with the assistance of molecular chaperones and other folding factors (Gething and Sambrook, 1992). Calnexin, an integral membrane protein, and calreticulin, a soluble lumenal protein, are two such molecular chaperones believed to be involved in this process (reviewed in by Helenius et al., 1997; Trombetta and Helenius, 1998). They share regions of high sequence identity and have similar binding activities, associating transiently with many glycoproteins being synthesized within the ER (Ou et al., 1993; Peterson et al., 1995; Helenius et al., 1997).
Calnexin and calreticulin predominantly act as lectins recognizing proteins carrying one or more monoglucosylated oligosaccharide side chains regardless of the conformation of the polypeptide (Rodan et al., 1996; Zapun et al., 1997). The carbohydrate structure, GlcNAc2Man7–9Glc1, is generated from the initial N-linked glycan, GlcNAc2Man9Glc3, by the action of glucosidases I and II. The presence of monoglucosylated oligosaccharides, and hence calnexin and calreticulin binding, is determined by two enzymes. Glucosidase II removes the final glucose residue generating a deglucosylated side chain, and UDP glucose:glycoprotein glucosyl transferase (UGGT) can add a single glucose residue back onto this structure, thus regenerating the monoglucosylated side chain (Trombetta et al., 1989). Significantly, UGGT recognizes only incompletely folded glycoproteins as substrates (Trombetta and Parodi, 1992; Sousa and Parodi, 1995). Thus, UGGT acts as a folding sensor, detecting nonnative glycoproteins and reglucosylating them, thereby allowing further rounds of binding to calnexin and calreticulin (Hammond and Helenius, 1994; Peterson et al., 1995; van Leeuwen and Kearse, 1996; Helenius et al., 1997).
If calnexin and calreticulin act principally as lectins, then additional ER components may be required to directly influence protein folding during the calnexin/calreticulin cycle. The principal candidate for this role is ERp57. ERp57 (also known as ERp61, ER-60, GRP58, PDI-Q2, and HIP 70) is a member of the protein disulfide isomerase (PDI) family which, in addition to archetypal PDI, also includes ERp72 (CaBP2), P5 (CaBP1) (Freedman et al., 1994; Holtzman, 1997) and the pancreas-specific PDIp (Desilva et al., 1997; Volkmer et al., 1997). ERp57 contains two “thioredoxin motifs” which in PDI constitute the thiol/disulfide oxidoreductase active sites (Freedman et al., 1994). A PDI-like thiol-dependent reductase activity for ERp57 has been demonstrated in vitro by several groups (Srivastava et al., 1993; Bourdi et al., 1995; Hirano et al., 1995), indicating that ERp57 may influence protein folding. ERp57 has also been proposed to be a carnitine palmitoyl transferase (Murthy and Pande, 1994), a cysteine protease (Urade and Kito, 1992), and a hormone-induced protein of the brain (Mobbs et al., 1990a,b).
We have previously shown that the thiol-dependent reductase, ERp57, interacts specifically with glycosylated secretory and membrane proteins imported into canine pancreatic microsomes (Elliott et al., 1997; Oliver et al., 1997) and semipermeabilized mammalian cells (Van der Wal et al., 1998). Like the interactions of calnexin and calreticulin, the interactions of ERp57 with glycoproteins required glucose trimming for both binding and release. We concluded that ERp57 interacted with glycoproteins in tandem with calnexin or calreticulin, most likely as a complex.
Other workers have also provided evidence that ERp57 is an important factor in glycoprotein folding. Zapun et al. (1998) found that when unfolded monoglucosylated ribonuclease B was bound to a soluble version of calnexin, missing the transmembrane and cytosolic portions, its folding was greatly enhanced by the presence of ERp57, but not PDI. This work demonstrated the ability of ERp57 to catalyze the formation of disulfide bonds in a glycoprotein bound to calnexin. Several groups have demonstrated the presence of ERp57, in addition to calnexin and calreticulin, during the assembly of the MHC class I complex (Hughes and Cresswell, 1998; Lindquist et al., 1998; Morrice and Powis, 1998).
In this study, we set out to investigate more directly the interactions of ERp57, calnexin, calreticulin, and PDI in a variety of systems.
MATERIALS AND METHODS
Restriction enzymes were purchased from New England Biolabs (Herts, UK). T7 and T3 RNA polymerases, transcription buffers, rabbit reticulocyte lysate, and Flexi-lysate were supplied by Promega (Southampton, UK). Bismaleimidohexane (BMH) was from Pierce and Warriner (Warrington, UK). Polyclonal rabbit anti-calreticulin serum used for blotting was raised against mature human calreticulin (Roderick et al., 1997). Anti-calreticulin serum for immunoprecipitation was from Affinity BioReagents (Cambridge Bioscience, Cambridge, UK); anti-calnexin was a gift from Dr. Ari Helenius (Yale University, New Haven, CT); anti-ERp57 was a gift from Dr. Tom Wileman (Institute for Animal Health, Woking, UK); anti-PDI was kindly provided by Dr. Neil Bulleid (University of Manchester, Manchester, UK). Chinese hamster ovary and Lec23 cell lines were a gift from Dr. P. Stanley (Albert Einstein College of Medicine, New York, NY). Protein A-Sepharose was supplied by Zymed (Cambridge Bioscience, Cambridge, UK). l-[35S]methionine and the Western blot reagent (Renaissance) were purchased from New England Nuclear DuPont (Stevenage, UK). Digitonin was supplied by Calbiochem (Nottingham, UK). All other chemicals were purchased from Sigma (Dorset, UK) or BDH-Merck (Dorset, UK).
Constructs
Human calreticulin cDNA was isolated by RT-PCR (Roderick et al., 1997) and cloned into pCR3 (Invitrogen, Leek, The Netherlands). Human PDI cDNA in pBluescript SK(−) was kindly provided by Dr. Neil Bulleid (University of Manchester). Human ERp57 (ERp60) cDNA in pVL1392 was a kind gift from Dr. Kari Kivirikko (Koivunen et al., 1996). The ERp57 construct was removed from this vector by EagI and EcoRI digestion and ligated into pBluescript II KS.
All plasmids were linearized at convenient restriction sites after the stop codon of the cDNA sequence. Transcriptions were carried out with T7 (calreticulin and ERp57) or T3 (PDI) RNA polymerase using a standard transcription reaction as described by the manufacturer (Promega). The RNA obtained was used for translation reactions.
Native Gels
The blue native gel electrophoresis method of Schagger and von Jagow was adapted for analysis of the lumenal components of the ER (Schagger and von Jagow, 1991; Schagger et al., 1994). Canine pancreatic microsomes in 750 mM aminocaproic acid, 50 mM BisTris, pH 7, were treated with 0.5% saponin for 30 min at 4°C followed by centrifugation at 100,000 × g for 20 min at 4°C. Sample buffer (final concentration: 15% glycerol, 50 mM BisTris, pH 7) was added to the supernatant, comprising the lumenal contents of the microsomes. The samples were then electrophoresed on 6–16% native polyacrylamide gel with 50 mM BisTris, pH 7, as the anode buffer and 50 mM Tricine, 15 mM BisTris, pH 7, as the cathode buffer at 150 V overnight at 4°C. The resolved proteins were then transferred to PVDF by blotting and probed for the presence of ERp57, calreticulin, and PDI.
Translation and Cross-Linking
Calreticulin, ERp57, and PDI were translated in the presence or in the absence of canine pancreatic microsomes or semipermeabilized mammalian cells as indicated. A standard rabbit reticulocyte lysate translation system was used for translation in the presence of a source of ER membranes (i.e., microsomes or semipermeabilized cells), whereas a Flexi-lysate rabbit reticulocyte translation system was used for translation in the absence of ER membranes.
Translations in the presence of microsomes were carried out in a standard rabbit reticulocyte lysate translation system (Promega) for 45 min at 30°C. Subsequently, 5 mM 7-methylguanosine 5′-monophosphate was added to inhibit initiation, and 5 min later translation was terminated by the addition of 2.5 mM cycloheximide. The microsomal fraction was isolated by centrifugation through a high-salt/sucrose cushion (250 mM sucrose, 500 mM potassium acetate, 5 mM magnesium acetate, 50 mM HEPES-KOH, pH 7.9) for 10 min at 130,000 × g. The microsomal pellet was then resuspended in a low- salt/sucrose buffer (250 mM sucrose, 100 mM potassium acetate, 5 mM magnesium acetate, 50 mM HEPES-KOH, pH 7.9), the cross-linking reagent bismaleimidohexane (BMH) was added to a final concentration of 0.5 mM, and the samples were incubated at 30°C for 10 min. BMH cross-links interacting proteins via the -SH groups of free cysteine residues. The cross-linking reaction was quenched by the addition of 0.1 volumes of 100 mM 2-mercaptoethanol, and the samples were left for 10 min on ice.
Semipermeabilized parental Chinese hamster ovary (CHO) cells and Lec23 CHO cells were prepared as previously described (Van der Wal et al., 1998). After permeabilization of the cells with digitonin at 20 μg/ml and removal of endogenous RNA by micrococcal nuclease treatment, reticulocyte lysate translations were carried out as described above for microsomes.
Translations in the absence of microsomes or semipermeabilized cells were carried out using a Flexi-lysate translation system (Promega). Translations were incubated for 60 min at 30°C in the presence of either [35S]methionine or 1 mM unlabeled methionine. These incubations were similar to the standard reticulocyte lysate translations described above, except that both reducing agents (DTT) and a source of ER (canine pancreatic microsomes or semipermeabilized cells) were absent. Additionally, after incubation with 7-methylguanosine 5′-monophosphate, 1 mM puromycin was added for 5 min before cycloheximide treatment to ensure that all nascent chains were released from ribosomes in addition to inhibiting all protein synthesis. Translation products were mixed as indicated in the figure legend and incubated for 15 min at 30°C. BMH was added to 0.5 mM, and the samples were incubated for 10 min at 30°C and then quenched as described above.
Immunoprecipitation
All samples were subjected to immunoprecipitation after SDS denaturation. SDS was added to 1% and the samples were heated at 95°C for 5 min. At least 4 volumes of IP buffer (10 mM Tris-HCl, pH 7.6, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100) were added. Methionine and PMSF were added to 1 mM each, and the samples were incubated on ice for 15–30 min, followed by centrifugation at 16,000 × g for 5 min. Specific antisera were added to aliquots of the resulting supernatant and the aliquots were then incubated overnight at 4°C with mixing. Protein A-Sepharose, which had been preincubated with 20% BSA for 30 min and then washed 5 times with IP buffer, was added to the samples, and the incubation was continued for 2 h. The protein A-Sepharose–bound material was then isolated by centrifugation at 16,000 × g for 1 min, washed five times with IP buffer, and then heated at 95°C for 5 min in SDS-PAGE sample buffer.
Sample Analysis
The samples were subjected to SDS-PAGE on 8% or 12% gels as indicated and subjected to autoradiography or phosphorimaging using a Fujix Bas 2000 bioimager (Fuji, Kanagawa, Japan). Quantitation was carried out using the Fujix Bas 2000 analysis software.
RESULTS
Cross-Linking in Microsomes
We have previously demonstrated that ERp57, a member of the PDI family of proteins, interacts specifically with both secretory and membrane glycoproteins in association with the ER lectins, calnexin and calreticulin (Elliott et al., 1997; Oliver et al., 1997). In this study we set out to analyze the putative interactions of ERp57 with calnexin and calreticulin directly.
We first investigated the interactions between the endogenous calnexin, calreticulin, ERp57, and PDI present in ER-derived microsomes. Canine pancreatic microsomes were treated with the bifunctional cysteine-specific cross-linking reagent bismaleimidohexane (BMH) or a solvent control (DMSO), and the products were separated by SDS-PAGE. Antibodies to calreticulin, ERp57, calnexin, and PDI were then used to identify these proteins and their cross-linking products after Western blotting. Analysis of the control-treated microsomes with calreticulin-specific antisera revealed a single band at 60 kDa as expected (the predicted molecular mass of calreticulin is 46 kDa; however, on SDS PAGE it usually has an apparent molecular mass of 60 kDa; see Figure 1, lane 1). After cross-linking, two major high-molecular-mass cross-linking products were observed at ∼120–130 kDa, indicating that calreticulin was cross-linked to an ER protein(s) of ∼60–70 kDa (Figure 1, lane 2, indicated by arrows).
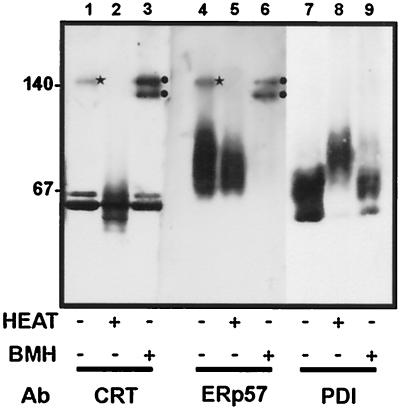
Figure 1.
The association of endogenous ER proteins. Canine pancreatic microsomes were treated with either DMSO (solvent control, lanes 1, 3, 5, and 7) or 1 mM BMH (lanes 2, 4, 6, and 8). The samples were then separated on an 8% SDS polyacrylamide gel and transferred to nitrocellulose. Antisera to calreticulin (CRT, lanes 1 and 2), ERp57 (lanes 3 and 4), calnexin (CNX, lanes 5 and 6), and PDI (lanes 7 and 8) were used for immunodetection.
Blotting with ERp57 antisera reveals microsomal ERp57 to be ∼57 kDa (Figure 1, lane 3). After cross-linking, several high-molecular-weight products were observed, most strikingly two prominent bands with identical mobility to the cross-linking products detected by the calreticulin antisera (Figure 1, lane 4, indicated by arrows). We presume that these products represent ERp57–calreticulin adducts formed between the endogenous proteins present in the ER lumen. The observation of two products with different mobilities probably reflects cross-linking between different cysteines present within the two proteins.
No obvious cross-linking products were observed with the antisera specific for calnexin, and only microsomal calnexin of ∼90 kDa was seen (Figure 1, lanes 5 and 6). PDI, which migrates at 54–57 kDa (Figure 1, lanes 7 and 8), appears to cross-link weakly to several higher molecular weight ER components, but no products comparable to those obtained with ERp57 were observed (Figure 1, lane 8). The small increases in the apparent molecular masses of ERp57 and PDI after treatment with BMH (Figure 1, c.f. lanes 3 and 4, and lanes 7 and 8) probably reflect the modification of all the available cysteine residues present within the proteins. In the case of calreticulin, an intrachain cross-link appears to occur, generating a faster migrating species (Figure 1, lane 2).
Native Gel Analysis of ERp57–Calreticulin Interaction
In order to investigate ERp57–calreticulin complexes in microsomes in the absence of cross-linking, the lumenal contents of microsomes were separated by native gel electrophoresis, with the aim of preserving endogenous protein complexes intact. The lumenal contents were extracted from pancreatic microsomes by saponin treatment and centrifugation, and, after separation by native gel electrophoresis, the presence of protein complexes was investigated by immunoblotting.
Although the estimation of molecular mass from native gels is inaccurate, native gel standards were run to provide some indication of the size of the products. Antisera raised against calreticulin and PDI both detected major bands at 55–70 kDa, which most likely represent the monomers of calreticulin and PDI. The major product detected by ERp57 ran as a smear at ∼75 kDa. However, after immunodetection of calreticulin and ERp57, a further band was observed corresponding to a higher molecular mass product (Figure 2, lanes 1 and 4, star). No higher molecular mass products were observed after immunodetection of PDI. The high-molecular-mass product detected by anti-calreticulin had an identical mobility to the product detected by the anti-ERp57, corresponding to ∼140 kDa, consistent with it being a calreticulin-ERp57 heterodimer.
Figure 2.
Native gel analysis of ER protein complexes. The lumenal contents of canine pancreatic microsomes were separated by blue native gel electrophoresis and then transferred to PVDF. The proteins were detected by immunoblotting with antisera raised against calreticulin (CRT, lanes 1–3), ERp57 (lanes 4–6), and PDI (lanes 7–9). The samples were untreated (lanes 1, 4, and 7); heated to 95°C before electrophoresis (lanes 2, 5 and 8), or treated with 1 mM BMH before isolation of the lumenal contents (lanes 3, 6, and 9).
When the microsomes were treated with the cross-linking reagent BMH before the extraction of the lumenal contents and sample preparation, the results were similar to those observed after SDS-PAGE (see previous section). Two bands were observed at ∼120–140 kDa in both the anti-calreticulin (lane 3, circles) and the anti-ERp57 (lane 6, circles) samples. The upper band of the cross-linked sample (lanes 3 and 6) ran at an identical mobility to the 140-kDa product detected in the untreated samples (lanes 1 and 4, star). Cross-linking enhanced the amount of calreticulin and ERp57 present in the complex, implying that, during saponin extraction, centrifugation, and electrophoresis, some of the complex dissociates in the untreated sample. Heating the sample to 95°C for 5 min before electrophoresis destroyed the association of ERp57 with calreticulin, and only the monomers were detected. Thus the interaction between ERp57 and calreticulin detected by cross-linking represents a genuine protein complex that can also be detected in the absence of cross-linking after native electrophoresis.
Cross-Linking of Calreticulin, ERp57, and PDI to Endogenous Microsomal Proteins
The above data indicated that calreticulin and ERp57 interact within the lumen of pancreatic microsomes. In order to investigate this possibility further, the cDNA constructs for calreticulin, ERp57, and PDI were utilized. RNA was made from the cDNA constructs, enabling the in vitro translation of 35S-labeled calreticulin, ERp57, and PDI. By including microsomes in the translation reaction, these three proteins were translocated across the microsomal membrane with concomitant signal sequence cleavage (demonstrated by resistance to proteinase K digestion [our unpublished observations]). Cross-linking studies were then carried out to determine whether these 35S-labeled proteins interacted with any endogenous microsomal proteins.
Discrete cross-linking products were obtained after the addition of BMH to microsomes into which radiolabeled calreticulin (Figure 3, lane 2 compared with lane 1) or ERp57 (Figure 3, lane 8 compared with lane 7) had been imported. These cross-linking products were identified by immunoprecipitation, and the major cross-linking partner of 35S-labeled calreticulin was found to be microsomal ERp57 (Figure 3, lane 5). The same interaction was reproduced when 35S-labeled ERp57 was imported into microsomes and found to be cross-linked to endogenous calreticulin (Figure 3, lane 11). Three identical major cross-linking products between calreticulin and ERp57 were observed (Figure 3, lanes 5 and 11, circles), most likely due to cross-linking between different cysteine residues within the two proteins. In addition to cross-linking endogenous calreticulin, 35S-labeled ERp57 was also found to cross-link to microsomal calnexin (Figure 3, lane 10, star). In contrast, 35S-labeled calreticulin was not cross-linked to calnexin (Figure 3, lane 4). The specificity of the ERp57/calreticulin and ERp57/calnexin cross-linking products was further underlined by our finding that 35S-labeled PDI did not form any similar cross-linking products (Figure 3, c.f. lanes 13 and 14), and that no PDI cross-linking products were immunoprecipitated with antisera recognizing calnexin, calreticulin, or ERp57 (Figure 3, lanes 16–18).
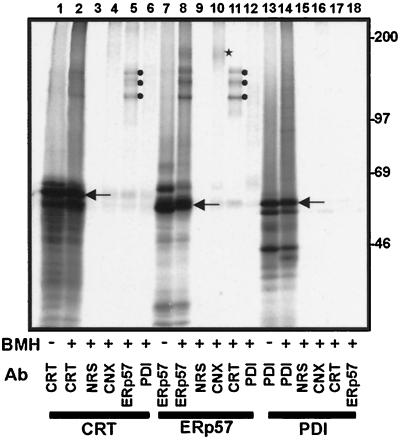
Figure 3.
Interactions of in vitro synthesized calreticulin, ERp57, and PDI with the endogenous ER proteins of microsomes. Calreticulin (CRT, lanes 1–6), ERp57 (lanes 7–12), and PDI (lanes 13–18) RNAs were translated in a rabbit reticulocyte lysate system in the presence of microsomes. After termination of translation, the microsomal fraction was isolated and, where indicated, the samples were treated with the cross-linking reagent BMH. The reaction was quenched, and the samples denatured with 1% SDS before immunoprecipitation with the antisera indicated: CRT, anticalreticulin; NRS, control nonrelated serum; CNX, anti-calnexin; ERp57, anti-ERp57; PDI, anti-PDI. The samples were analyzed on an 8% SDS-polyacrylamide gel. The identity of imported polypeptides with cleaved signal sequences (indicated by arrows) was confirmed by protease protection and comparison with unprocessed polypeptides bearing signal sequences (our unpublished observations).
These results indicated that discrete complexes between calnexin and ERp57, and calreticulin and ERp57, could be reconstituted by importing in vitro translated, 35S-labeled calreticulin or ERp57 into microsomes. None of the newly synthesized proteins (i.e., calreticulin, ERp57, and PDI) are N-glycosylated (our unpublished observations), ruling out any possibility that the interactions observed were due to the ER lectins, calnexin and calreticulin, binding to a glycoprotein substrate. Significantly, the efficiency of cross-linking observed in these experiments was also substantially higher than that which we typically observe for chaperone–substrate interactions (≤30% vs. <5%, respectively).
Cross-Linking of Calreticulin, ERp57, and PDI to Endogenous ER Proteins in Semipermeabilized Mammalian Cells
In the canine pancreatic microsome system employed thus far, it was unclear whether the binding of ERp57 to calnexin and calreticulin required the presence of glucose-trimmed glycoprotein substrates. In order to address this point, a cell line that lacks the glucose-trimmed glycoprotein substrates of these lectins was used. Lec23 cells are derived from CHO cells, but they lack glucosidase I activity and are unable to produce monoglucosylated glycoproteins (Ray et al., 1991). Hence, in Lec23 cells, calnexin and calreticulin do not bind glycoproteins (Ora and Helenius, 1995; Van der Wal et al., 1998). In order to employ parental CHO cells and Lec23 CHO cells in in vitro cross-linking experiments, they were first selectively permeabilized with digitonin to allow the import of 35S-labeled calreticulin, ERp57, and PDI.
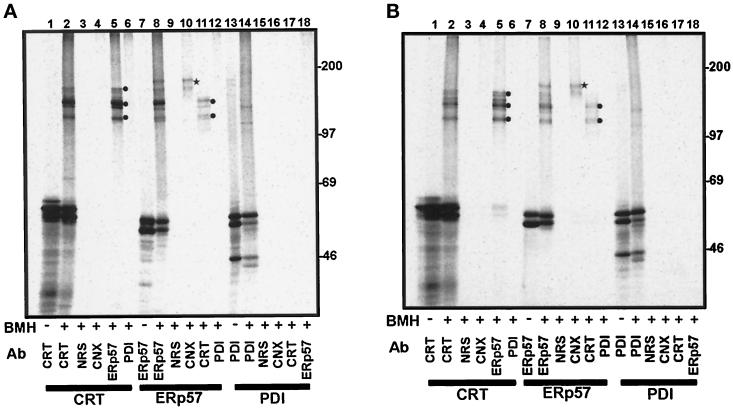
The interactions of the imported radiolabeled components with endogenous ER proteins in semipermeabilized parental CHO cells (Figure 4A) and Lec23 CHO cells (Figure 4B) were detected by cross-linking and were very similar to those observed for canine pancreatic microsomes (c.f. Figure 3). Briefly, 35S-labeled calreticulin interacts with endogenous ERp57 (Figure 4, A and B, lane 5, circles), and 35S-labeled ERp57 interacts with endogenous calnexin (Figure 4, A and B, lane 10, star) and calreticulin (Figure 4, A and B, lane 11, circles). 35S-labeled PDI displays no strong cross-linking products with endogenous proteins (cf. Figure 4, A and B, lanes 13 and 14). These results indicate that interaction of ERp57 with both calnexin and calreticulin does not require the presence of a glucose-trimmed glycoprotein substrate.
Figure 4.
Interactions of in vitro synthesized calreticulin, ERp57, and PDI with the endogenous ER proteins of semipermeabilized cells. Parental CHO cells (panel A) and glucosidase I-deficient Lec23 CHO cells (panel B) were selectively permeabilized with digitonin. Calreticulin (CRT, lanes 1–6), ERp57 (lanes 7–12), and PDI (lanes 13–18) RNAs were translated in a rabbit reticulocyte lysate system in the presence of the semipermeabilized mammalian cells. After termination of translation, the cells were washed and, where indicated, the samples were treated with the cross-linking reagent BMH. The samples were quenched and denatured with 1% SDS before immunoprecipitation with the antisera indicated: CRT, anti-calreticulin; NRS, control nonrelated serum; CNX, anti-calnexin; ERp57, anti-ERp57; PDI, anti-PDI. The samples were analyzed on an 8% SDS-polyacrylamide gel.
ERp57 Forms a Complex with Calreticulin in the Absence of Microsomes
The ability to synthesize calreticulin and ERp57 in vitro enabled us to investigate whether these two proteins could interact in solution in the absence of any other ER proteins. ERp57, calreticulin, and PDI were translated in the Flexi-lysate rabbit reticulocyte translation system supplemented with either unlabeled methionine or [35S]methionine. By mixing “cold” (unlabeled) proteins with radiolabeled proteins, we could cross-link interacting components and define these complexes by immunoprecipitation.
In contrast to the precursors imported into microsomes, the N-terminal signal sequences were still present on the resulting polypeptides. However, although the presence of such signal sequences can slow down the rate of protein folding, the mature region of the protein frequently attains an essentially native conformation that is indistinguishable from that observed after signal sequence cleavage (Park et al., 1988). Hence, in the case of maltose-binding protein, the presence of the N-terminal signal sequence does not prevent the protein binding to its natural ligand, underlining its ability to fold into a native structure (Park et al., 1988).
Since the translation reactions were incubated for 1 h before the different ER precursors were combined, it seemed likely that a substantial amount of the precursor proteins would fold correctly during this time. Indeed, under the experimental conditions used, we found that calreticulin with its N-terminal signal sequence present was able to form a characteristic intrachain disulfide bond (our unpublished observations) indicative of correct protein folding (Matsuoka et al., 1994; c.f. Hebert et al., 1995).
In addition to major radiolabeled products representing the full-length proteins with uncleaved signal sequences (indicated by the arrowheads in Figure 5A–C), several products with slightly different mobilities were also observed (Figure 5, A and C). These may be the result of premature chain termination, ribosome stacking, proteolytic cleavage, or other posttranslational events and are not unusual in such assays (e.g., Lutcke et al., 1992).
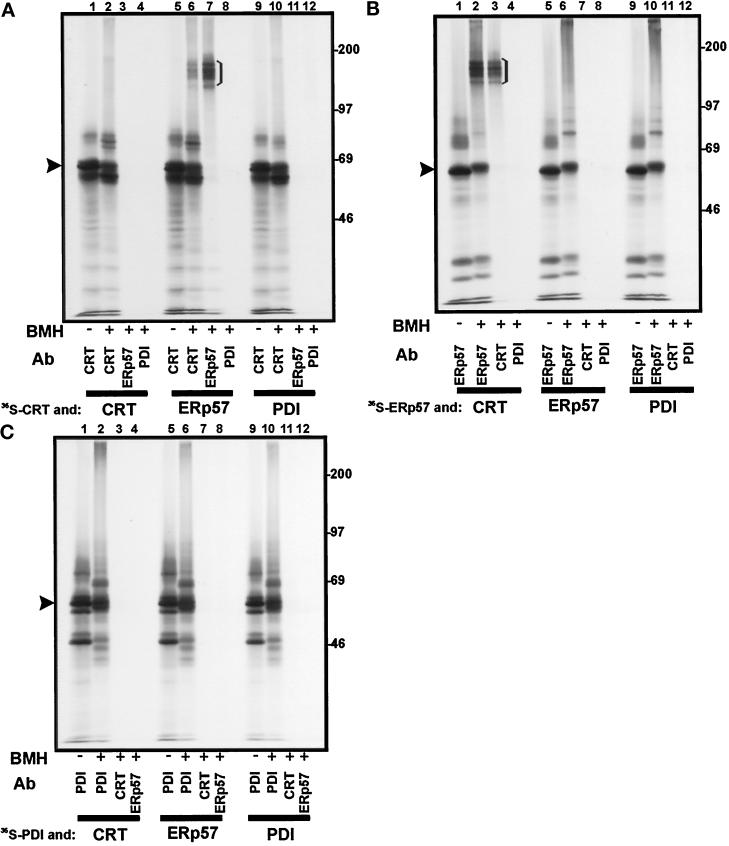
Figure 5.
Interactions between in vitro translated calreticulin, ERp57, and PDI in solution. The products of a translation reaction carried out in the presence of [35S]methionine were mixed with the products of a translation reaction carried out with unlabeled methionine, and the resulting protein complexes were cross-linked with BMH. 35S-labeled calreticulin (panel A), ERp57 (panel B), and PDI (panel C) were mixed with unlabeled calreticulin (CRT, lanes 1–4), ERp57 (lanes 5–8), and PDI (lanes 9–12). After cross-linking and SDS denaturation, immunoprecipitation was carried out with the antisera indicated (see Figure 3 for key). The samples were analyzed on an 8% SDS-polyacrylamide gel. The identity of full-length precursor proteins (i.e., with signal sequences; indicated by arrowheads) was experimentally confirmed (our unpublished observations).
Mixing 35S-calreticulin with unlabeled ERp57 led to an interaction that could be stabilized by cross-linking, and the resulting adduct was immunoprecipitated by anti-calreticulin (Figure 5A, lane 6) and anti-ERp57 (Figure 5A, lane 7) sera. This product was not observed when unlabeled ERp57 was replaced by unlabeled calreticulin or PDI (Figure 5A, lanes 3 and 11). When the reciprocal experiment was performed, and 35S-ERp57 was mixed with unlabeled calreticulin, a similar product was observed (Figure 5B, lanes 2 and 3). Once again, PDI did not form any cross-linking products with either calreticulin (Figure 5A, lane 12; Figure 5C, lane 3) or ERp57 (Figure 5B, lane 12; Figure 5C, lane 8). Since the cross-linking reactions in these experiments were carried out in the complete in vitro translation system, and therefore in the presence of the many cytosolic proteins present in the reticulocyte lysate, this result indicates that the cross-linking observed between ERp57 and calreticulin is due to a very specific protein–protein interaction.
DISCUSSION
The exact nature and importance of the calnexin–calreticulin pathway during glycoprotein folding and quality control remain controversial (see Helenius et al., 1997; Trombetta and Helenius, 1998). The view that calnexin and calreticulin bind to the carbohydrate moiety of a glycoprotein substrate as an initial recognition event, and that this enables subsequent protein–protein interactions to occur (Arunachalam and Cresswell, 1995; Ware et al., 1995; Zhang et al., 1995), has now largely been superseded. There is very good evidence that calnexin and calreticulin function as lectins and recognize only carbohydrate, and not protein, determinants. Several groups have recently demonstrated that calnexin and calreticulin alone do not distinguish between folded and unfolded polypeptides and will bind to a substrate as long as it is monoglucosylated (Rodan et al., 1996; Allen and Bulleid, 1997; Zapun et al., 1997). These data also suggest that calnexin and calreticulin do not influence glycoprotein folding directly.
While calnexin and calreticulin may not recognize protein determinants directly, it is possible that these molecules recruit other ER components that do exert a direct effect on protein folding. The best candidate for such a component is ERp57, a member of the PDI family of proteins. PDI-like proteins contain two (PDI, ERp57, P5, PDIp), or three (ERp72) copies of the sequence motif -Cys-Gly-His-Cys-. In PDI this motif is the active site for disulfide formation and isomerization activities (Vuori et al., 1992; LaMantia and Lennarz, 1993). ERp57 has been ascribed a variety of names and functions, and elucidating its role in vivo has proved complex (see Elliott et al. [1997] and references therein).
Evidence that ERp57 can interact with newly synthesized glycoproteins originated from a study of the biosynthesis of the Glut 1 glucose transporter (Oliver et al., 1996). A 60-kDa ER lumenal protein was found to interact with calnexin and its glycoprotein substrate Glut 1. After observing a similar 60- kDa protein associated with nascent secretory glycoproteins, we were able to identify it as ERp57 (Oliver et al., 1997). Subsequently, ERp57 was also shown to associate with the membrane protein glycophorin C and was formally identified as a cross-linking partner of Glut 1 (Elliott et al., 1997). Throughout these studies it was striking that the substrate-binding properties of ERp57 mimicked those of calnexin and calreticulin, i.e., both glycosylation and glucose trimming were required for binding to occur. In addition, ERp57-glycoprotein cross-linking products were coimmunoprecipitated with calnexin and calreticulin if the samples were not first denatured by heating with SDS. These findings suggested that ERp57 bound to nascent glycoproteins as part of discrete complexes formed with both calnexin and calreticulin (Elliott et al., 1997; Oliver et al., 1997; Van der Wal et al., 1998).
In order to understand better the relationship between ERp57 and the ER lectins, calnexin and calreticulin, we have studied their interactions with one another directly. When the interactions between endogenous ER lumenal proteins were stabilized by cross-linking, before SDS-PAGE and immunoblotting, evidence for specific ERp57–calreticulin adducts was obtained. Likewise, when the soluble components present inside ER microsomes were separated by native gel electrophoresis, an ERp57/calreticulin complex could still be identified in the absence of any cross-linking. This confirmed the presence of ERp57/ER lectin complexes within ER-derived pancreatic microsomes.
To increase the sensitivity of our assay, we next synthesized radiolabeled calreticulin, ERp57, and PDI in vitro, imported these 35S-labeled proteins into microsomes, and studied their interactions with endogenous ER lumenal components. Very strong cross-linking of labeled calreticulin to endogenous ERp57 was observed, and in the converse experiment labeled ERp57 was found to cross-link strongly to endogenous calreticulin. In addition, labeled ERp57 was cross-linked to endogenous calnexin. Thus, it was possible to form complexes of ERp57 with either calnexin or calreticulin in vitro. Since ERp57 is not glycosylated, these complexes are clearly not the result of “typical” interactions between calnexin/calreticulin and a glycoprotein substrate.
As ERp57 is a member of the PDI family of proteins, we chose archetypal PDI as a control for these experiments. Archetypal PDI has 29% sequence identity and 56% sequence similarity with ERp57 (Koivunen et al., 1996). The cross-linking experiments employed a homobifunctional reagent, BMH, which specifically cross-links proteins to one another via their cysteine residues. Both mature proteins contain six cysteine residues, while PDI contains an additional cysteine in the signal sequence. Furthermore, PDI had successfully been cross-linked to a cys-containing mutant of glycophorin C (Elliott et al., 1997) and to procollagen chains (Wilson et al., 1998) using BMH. Hence, although clearly capable of BMH-dependent cross-linking, no adducts of PDI with calnexin or calreticulin were observed with this reagent. In addition, no complexes were detected between calreticulin and PDI after native gel electrophoresis.
The cross-linking experiments were repeated substituting semipermeabilized mammalian cells for microsomes. Once again, complexes were observed between ERp57 and calreticulin or calnexin, both in parental CHO cells capable of typical glycoprotein processing, and in Lec23 CHO cells in which glucosidase I activity is defective. Calnexin and calreticulin do not bind to glycoproteins in Lec23 cells (Ora and Helenius, 1995; Van der Wal et al., 1998), and hence we could conclude that ERp57–calreticulin and ERp57–calnexin complexes are formed in the absence of glycoprotein substrates for the ER lectins. This observation was verified by showing that ERp57 could interact with calreticulin in solution. The products of two separate in vitro translation reactions were simply mixed, and the ability of the two translation products to interact with one another was determined. In these experiments both proteins are present at a much lower effective concentration than inside the ER lumen, and no monoglucosylated glycoprotein substrates would be present. Remarkably, efficient and specific cross-linking of ERp57 to calreticulin was observed in this system.
We conclude that the ERp57 present in the ER lumen normally functions as a subunit of discrete complexes formed with calreticulin and calnexin, and that the formation of these complexes is not dependent upon the presence of glycoprotein substrates. These data add substantial weight to our original proposal that ERp57 functions in combination with calnexin or calreticulin in order to modulate glycoprotein folding within the ER lumen (Elliott et al., 1997; Oliver et al., 1997). We have now shown that ERp57 forms discrete, stable complexes with both calnexin and calreticulin.
Our data are in good agreement with the recent study of Corbett et al. (1999) which showed that purified recombinant calreticulin and ERp57 interact specifically in solution. Our current study shows that this interaction reflects the situation occurring inside an intact ER. This may be contrasted with the previously reported interaction between calreticulin and PDI (Baksh et al., 1995). We detect no such interaction when using canine pancreatic microsomes, semi-intact mammalian cells, or mixing in vitro translation products. It has now been shown that calreticulin associates with PDI only at very low Ca2+ concentrations, i.e., below 100 μM (Corbett et al., 1999). The steady state Ca2+ concentration in the ER lumen is reported to be 500–800 μM, and this reduces to 200–300 μM after Ca2+ release in response to 10 μM histamine (Alonso et al., 1999). Our results were obtained using intact ER-derived microsomes and semi-intact mammalian cells, and they underline the fact that, under the conditions observed in vivo (Alonso et al., 1999), ERp57 forms a specific complex with the ER lectins, calnexin and calreticulin, while we find no evidence that PDI is associated with these components. The very low Ca2+ concentration needed to observe an interaction between PDI and calreticulin (Corbett et al., 1999) would require an almost complete emptying of the ER calcium store such as that observed in response to treatment with 50 mM caffeine (Alonso et al., 1999).
We propose that ERp57 is recruited to interact with newly synthesized, incompletely folded, glycoproteins via its association with the lectins, calreticulin and calnexin. Thus, the perturbation of glycoprotein folding that results from preventing calnexin and calreticulin binding (see Helenius et al. [1997] and references therein) most likely results from interfering with ERp57 function. Once exposed to a newly synthesized glycoprotein, ERp57 may function as a glycoprotein-specific thiol/disulfide oxidoreductase (see Figure 6) and thereby have a direct influence on protein folding.
Figure 6.
A proposed model of glycoprotein folding. Calreticulin (and calnexin, its integral membrane protein homologue; not shown) acts as a lectin by binding to monoglucosylated oligosaccharide chains present on newly synthesized glycoproteins. By virtue of its association with calreticulin, ERp57 is brought into contact with the glycoprotein. ERp57 modulates the folding of the glycoprotein, illustrated here as disulfide bond formation, but possibly by other mechanisms. The glycoprotein is released from calreticulin upon glucose trimming by glucosidase II. If the folding has been successful, the native glycoprotein is free to continue along the secretory pathway. If the glycoprotein is incompletely folded, the folding sensor UDP-glucose:glycoprotein glucosyl transferase (UGGT) adds back a single glucose residue, and the glycoprotein undergoes another round of lectin binding.
The initial studies of ERp57 thiol-reductase activity (Srivastava et al., 1993; Bourdi et al., 1995; Hirano et al., 1995) were all carried out in the absence of any ER lectins and used a nonglycosylated precursor (insulin). Since the results described above and our previous work (Elliott et al., 1997; Oliver et al., 1997; Van der Wal et al., 1998) all indicate that ERp57 functions in combination with calnexin and calreticulin to interact exclusively with glycoproteins, these early studies of ERp57 activity are unlikely to reflect its true capacity. A recent study by Zapun et al. (1998) has now addressed these deficiencies. The authors found that the PDI activity of ERp57 on the refolding of monoglucosylated ribonuclease B is greatly enhanced by the presence of calreticulin or a soluble form of calnexin. In contrast, the activity of PDI in the same assay was decreased upon the addition of ER lectins (Zapun et al., 1998). This observation provides the first direct evidence that ERp57 can indeed specifically modulate glycoprotein folding in combination with the ER lectins, calnexin and calreticulin (c.f. Figure 6). On the basis of the work presented here, we propose that ERp57 performs this function as a subunit of discrete, stable protein complexes formed with calnexin and calreticulin. Significantly, ERp57 also binds to glycoproteins that lack any cysteine residues (Elliott et al., 1997; Oliver et al., 1997), and that do not, therefore, require any intramolecular disulfide exchange. Thus, it remains possible that, like PDI (Freedman et al., 1994; Kemmink et al., 1997; Yao et al., 1997; Wilson et al., 1998), ERp57 may also play a more general role as a molecular chaperone within the ER lumen.
ACKNOWLEDGMENTS
We thank Dr. Pamela Stanley, Dr. Neil Bulleid, Dr. Ari Helenius, Dr. Kari Kivirikko, Dr. Peppi Koivunen, and Dr. Tom Wileman for providing materials and Dr. Viki Allan, Dr. Neil Bulleid, and Dr. Phil Woodman for their critical comments during preparation of the manuscript. This work was supported by funding from the Biotechnology and Biological Sciences Research Council and the Wellcome Trust.
Abbreviations used:
- BMH
bismaleimidohexane
- CHO
Chinese hamster ovary
- ER
endoplasmic reticulum
- PDI
protein disulfide isomerase
- UGGT
UDP glucose:glycoprotein glucosyl transferase
REFERENCES
- Allen S, Bulleid NJ. Calnexin and calreticulin bind to enzymically active tissue-type plasminogen activator during biosynthesis and are not required for folding to the native conformation. Biochem J. 1997;328:113–119. doi: 10.1042/bj3280113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MT, Barrero MJ, Michelena P, Carnicero E, Cuchillo I, Garcia AG, Garcia-Sancho J, Montero M, Alvarez J. Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J Cell Biol. 1999;144:241–254. doi: 10.1083/jcb.144.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam B, Cresswell P. Molecular requirements for the interaction of class II major histocompatibility complex molecules and invariant chain with calnexin. J Biol Chem. 1995;270:2784–2790. doi: 10.1074/jbc.270.6.2784. [DOI] [PubMed] [Google Scholar]
- Baksh S, Burns K, Andrin C, Michalak M. Interaction of calreticulin with protein disulfide isomerase. J Biol Chem. 1995;270:31338–31344. doi: 10.1074/jbc.270.52.31338. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Demady D, Martin JL, Jabbour SK, Martin BM, George JW, Pohl LR. cDNA cloning and baculovirus expression of the human liver endoplasmic reticulum P58: characterization as a protein disulfide isomerase isoform, but not as a protease or a carnitine acyltransferase. Arch Biochem Biophys. 1995;323:397–403. doi: 10.1006/abbi.1995.0060. [DOI] [PubMed] [Google Scholar]
- Corbett EF, Oikawa K, Francois P, Tessier DC, Kay C, Bergeron JJ, Thomas DY, Krause KH, Michalak M. Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J Biol Chem. 1999;274:6203–6211. doi: 10.1074/jbc.274.10.6203. [DOI] [PubMed] [Google Scholar]
- Desilva MG, Notkins AL, Lan MS. Molecular characterization of a pancreas-specific protein disulfide isomerase, PDIp. DNA Cell Biol. 1997;16:269–274. doi: 10.1089/dna.1997.16.269. [DOI] [PubMed] [Google Scholar]
- Elliott JG, Oliver JD, High S. The thiol-dependent reductase ERp57 interacts specifically with N-glycosylated integral membrane proteins [published erratum appears in J Biol Chem 1997 Aug 8;272(32):20312] J Biol Chem. 1997;272:13849–13855. doi: 10.1074/jbc.272.21.13849. [DOI] [PubMed] [Google Scholar]
- Freedman RB, Hirst TR, Tuite MF. Protein disulphide isomerase: building bridges in protein folding. Trends Biochem Sci. 1994;19:331–336. doi: 10.1016/0968-0004(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A, Trombetta ES, Hebert DN, Simons JF. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997;7:193–200. doi: 10.1016/S0962-8924(97)01032-5. [DOI] [PubMed] [Google Scholar]
- Hirano N, Shibasaki F, Sakai R, Tanaka T, Nishida J, Yazaki Y, Takenawa T, Hirai H. Molecular cloning of the human glucose-regulated protein ERp57/GRP58, a thiol-dependent reductase. Identification of its secretory form and inducible expression by the oncogenic transformation. Eur J Biochem. 1995;234:336–342. doi: 10.1111/j.1432-1033.1995.336_c.x. [DOI] [PubMed] [Google Scholar]
- Holtzman JL. The roles of the thiol:protein disulfide oxidoreductases in membrane and secretory protein synthesis within the lumen of the endoplasmic reticulum. J Investig Med. 1997;45:28–34. [PubMed] [Google Scholar]
- Hughes EA, Cresswell P. The thiol oxidoreductase ERp57 is a component of the MHC class I peptide-loading complex. Curr Biol. 1998;8:709–712. doi: 10.1016/s0960-9822(98)70278-7. [DOI] [PubMed] [Google Scholar]
- Kemmink J, Darby NJ, Dijkstra K, Nilges M, Creighton TE. The folding catalyst protein disulfide isomerase is constructed of active and inactive thioredoxin modules. Curr Biol. 1997;7:239–245. doi: 10.1016/s0960-9822(06)00119-9. [DOI] [PubMed] [Google Scholar]
- Koivunen P, Helaakoski T, Annunen P, Veijola J, Raisanen S, Pihlajaniemi T, Kivirikko KI. ERp60 does not substitute for protein disulphide isomerase as the beta-subunit of prolyl 4-hydroxylase. Biochem J. 1996;316:599–605. doi: 10.1042/bj3160599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMantia ML, Lennarz WJ. The essential function of yeast protein disulfide isomerase does not reside in its isomerase activity. Cell. 1993;74:899–908. doi: 10.1016/0092-8674(93)90469-7. [DOI] [PubMed] [Google Scholar]
- Lindquist JA, Jensen ON, Mann M, Hammerling GJ. ER-60, a chaperone with thiol-dependent reductase activity involved in MHC class I assembly. EMBO J. 1998;17:2186–2195. doi: 10.1093/emboj/17.8.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutcke H, High S, Romisch K, Ashford AJ, Dobberstein B. The methionine-rich domain of the 54 kDa subunit of signal recognition particle is sufficient for the interaction with signal sequences. EMBO J. 1992;11:1543–1551. doi: 10.1002/j.1460-2075.1992.tb05199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Seta K, Yamakawa Y, Okuyama T, Shinoda T, Isobe T. Covalent structure of bovine brain calreticulin. Biochem J. 1994;298:435–442. doi: 10.1042/bj2980435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs CV, Fink G, Pfaff DW. HIP-70: a protein induced by estrogen in the brain and LH-RH in the pituitary. Science. 1990a;247:1477–1479. doi: 10.1126/science.247.4949.1477. [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Fink G, Pfaff DW. HIP-70: an isoform of phosphoinositol-specific phospholipase C-alpha. Science. 1990b;249:566–567. doi: 10.1126/science.2382136. [DOI] [PubMed] [Google Scholar]
- Morrice NA, Powis SJ. A role for the thiol-dependent reductase ERp57 in the assembly of MHC class I molecules. Curr Biol. 1998;8:713–716. doi: 10.1016/s0960-9822(98)70279-9. [DOI] [PubMed] [Google Scholar]
- Murthy MS, Pande SV. A stress-regulated protein, GRP58, a member of thioredoxin superfamily, is a carnitine palmitoyltransferase isoenzyme. Biochem J. 1994;304:31–34. doi: 10.1042/bj3040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD, Hresko RC, Mueckler M, High S. The glut 1 glucose transporter interacts with calnexin and calreticulin. J Biol Chem. 1996;271:13691–13696. doi: 10.1074/jbc.271.23.13691. [DOI] [PubMed] [Google Scholar]
- Oliver JD, van der Wal FJ, Bulleid NJ, High S. Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science. 1997;275:86–88. doi: 10.1126/science.275.5296.86. [DOI] [PubMed] [Google Scholar]
- Ora A, Helenius A. Calnexin fails to associate with substrate proteins in glucosidase-deficient cell lines. J Biol Chem. 1995;270:26060–26062. doi: 10.1074/jbc.270.44.26060. [DOI] [PubMed] [Google Scholar]
- Ou WJ, Cameron PH, Thomas DY, Bergeron JJ. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Park S, Liu G, Topping TB, Cover WH, Randall LL. Modulation of folding pathways of exported proteins by the leader sequence. Science. 1988;239:1033–1035. doi: 10.1126/science.3278378. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Ora A, Van PN, Helenius A. Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Mol Biol Cell. 1995;6:1173–1184. doi: 10.1091/mbc.6.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MK, Yang J, Sundaram S, Stanley P. A novel glycosylation phenotype expressed by Lec23, a Chinese hamster ovary mutant deficient in alpha-glucosidase I. J Biol Chem. 1991;266:22818–22825. [PubMed] [Google Scholar]
- Rodan AR, Simons JF, Trombetta ES, Helenius A. N-linked oligosaccharides are necessary and sufficient for association of glycosylated forms of bovine RNase with calnexin and calreticulin. EMBO J. 1996;15:6921–6930. [PMC free article] [PubMed] [Google Scholar]
- Roderick HL, Campbell AK, Llewellyn DH. Nuclear localization of calreticulin in vivo is enhanced by its interaction with glucocorticoid receptors. FEBS Lett. 1997;405:181–185. doi: 10.1016/s0014-5793(97)00183-x. [DOI] [PubMed] [Google Scholar]
- Schagger H, Cramer WA, von Jagow G. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- Sousa M, Parodi AJ. The molecular basis for the recognition of misfolded glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. EMBO J. 1995;14:4196–4203. doi: 10.1002/j.1460-2075.1995.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SP, Fuchs JA, Holtzman JL. The reported cDNA sequence for phospholipase C alpha encodes protein disulfide isomerase, isozyme Q-2 and not phospholipase-C. Biochem Biophys Res Commun. 1993;193:971–978. doi: 10.1006/bbrc.1993.1720. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol. 1998;8:587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- Trombetta SE, Bosch M, Parodi AJ. Glucosylation of glycoproteins by mammalian, plant, fungal, and trypanosomatid protozoa microsomal membranes. Biochemistry. 1989;28:8108–8116. doi: 10.1021/bi00446a022. [DOI] [PubMed] [Google Scholar]
- Trombetta SE, Parodi AJ. Purification to apparent homogeneity and partial characterization of rat liver UDP-glucose:glycoprotein glucosyltransferase. J Biol Chem. 1992;267:9236–9240. [PubMed] [Google Scholar]
- Urade R, Kito M. Inhibition by acidic phospholipids of protein degradation by ER-60 protease, a novel cysteine protease, of endoplasmic reticulum. FEBS Lett. 1992;312:83–86. doi: 10.1016/0014-5793(92)81415-i. [DOI] [PubMed] [Google Scholar]
- Van der Wal FJ, Oliver JD, High S. The transient association of ERp57 with N-glycosylated proteins is regulated by glucose trimming. Eur J Biochem. 1998;256:51–59. doi: 10.1046/j.1432-1327.1998.2560051.x. [DOI] [PubMed] [Google Scholar]
- van Leeuwen JE, Kearse KP. Calnexin associates exclusively with individual CD3 delta and T cell antigen receptor (TCR) alpha proteins containing incompletely trimmed glycans that are not assembled into multisubunit TCR complexes. J Biol Chem. 1996;271:9660–9665. doi: 10.1074/jbc.271.16.9660. [DOI] [PubMed] [Google Scholar]
- Volkmer J, Guth S, Nastainczyk W, Knippel P, Klappa P, Gnau V, Zimmermann R. Pancreas specific protein disulfide isomerase, PDIp, is in transient contact with secretory proteins during late stages of translocation. FEBS Lett. 1997;406:291–295. doi: 10.1016/s0014-5793(97)00288-3. [DOI] [PubMed] [Google Scholar]
- Vuori K, Myllyla R, Pihlajaniemi T, Kivirikko KI. Expression and site-directed mutagenesis of human protein disulfide isomerase in Escherichia coli. This multifunctional polypeptide has two independently acting catalytic sites for the isomerase activity. J Biol Chem. 1992;267:7211–7214. [PubMed] [Google Scholar]
- Ware FE, Vassilakos A, Peterson PA, Jackson MR, Lehrman MA, Williams DB. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995;270:4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- Wilson R, Lees JF, Bulleid NJ. Protein disulfide isomerase acts as a molecular chaperone during the assembly of procollagen. J Biol Chem. 1998;273:9637–9643. doi: 10.1074/jbc.273.16.9637. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhou Y, Wang C. Both the isomerase and chaperone activities of protein disulfide isomerase are required for the reactivation of reduced and denatured acidic phospholipase A2. EMBO J. 1997;16:651–658. doi: 10.1093/emboj/16.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapun A, Darby NJ, Tessier DC, Michalak M, Bergeron JJ, Thomas DY. Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J Biol Chem. 1998;273:6009–6012. doi: 10.1074/jbc.273.11.6009. [DOI] [PubMed] [Google Scholar]
- Zapun A, Petrescu SM, Rudd PM, Dwek RA, Thomas DY, Bergeron JJ. Conformation-independent binding of monoglucosylated ribonuclease B to calnexin. Cell. 1997;88:29–38. doi: 10.1016/s0092-8674(00)81855-3. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Tector M, Salter RD. Calnexin recognizes carbohydrate and protein determinants of class I major histocompatibility complex molecules. J Biol Chem. 1995;270:3944–3948. doi: 10.1074/jbc.270.8.3944. [DOI] [PubMed] [Google Scholar]